Abstract
Background/aims
Bronchiolitis is the most common lower respiratory illness that characteristically affects the children below 2 years of age accounting about 2–3% of patients admitted to hospital each year [1,2,3,4]. We compared the effect of racemic epinephrine (RE) and 3% hypertonic saline (HS) nebulization on the length of stay (LOS) in the hospital.
Methods
We looked at the infants with moderate bronchiolitis, from October 2013 to March 2014. Out of eighty cases, 16 in HS and 18 in RE groups were enrolled. At the time of admission, 0.2 ml of RE added to 1.8 ml of distilled water was nebulized to RE group, as compared with 2 ml of 3% HS in nebulized form. RE was re-administered if needed on 6 h in comparison with 3% HS at the frequency of 1 to 4 h.
Results
One infant from RE group and three infants from HS group were excluded due to progression towards severe bronchiolitis. The LOS in RE group ranged between 18 and 160 h (mean 45 h), while in HS group, LOS was 18.50–206 h (mean 74.3 h). The LOS was significantly short in RE group (p value 0.015) which was statistically significant.
Conclusion
Racemic epinephrine nebulization as first-line medication may significantly reduce the length of hospital stay in infants with moderate bronchiolitis in comparison with nebulized HS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchiolitis is the most common lower respiratory illness that characteristically affects the children below 2 years of age. Annually, around 2–3% of the patients are admitted in the hospital with this condition [1,2,3,4]. Of the cases, 50–80% are caused by respiratory syncytial virus [1, 3,4,5,6].
With exception of infants at risk, the illness is self-limiting, and therefore the treatment is supportive [2, 6]. Supportive care includes individualized supplementary oxygen and hydration in moderate cases as mainstay of treatment as per various guidelines [2, 4, 7, 8] (http://www.rch.org.au/clinicalguide/guideline_index/Bronchiolitis/).
Typical signs and symptoms include respiratory distress and feeding issues which are an important cause of parental anxiety [9,10,11,12]. Nevertheless, the length of hospital stay and the cost are additional causes of concern.
Contemporary research trials studied effects of epinephrine in comparison with traditional bronchodilators and various concentrations of saline. The emphasis in those trials was focused towards requirement of admission after emergency visit, efficacy of certain nebulized medicine leading to discharge preventing hospital admission from outpatient settings, the drug effect on supplementary oxygen requirement, and total length of hospital stay. Variable results have been demonstrated in those trials while looking at the above variables [13,14,15,16,17,18,19,20].
Therefore, the aim of our study was to explore role of racemic epinephrine versus 3% hypertonic saline, both in nebulized form in order to reduce the length of stay in the hospital within our safety remit.
Furthermore, our vision was to build further evidence via this trail on the comprehensive work already performed in this active research area in an effort to deploy epinephrine as a reliable supportive care for these infants in the hospital setting.
Our primary outcome was to look at the length of hospital stay of children admitted with moderate bronchiolitis aged between 0 and 24 months.
Our secondary outcome was to assess the requirement of supplementary oxygen, feeding support, or parenteral fluids.
Method
A quasi-randomized unblinded trial was conducted with alternate allocation between October 2013 and April 2014. A quasi-randomized trial is one in which participants are allocated to different arms of the trial (to receive the study medicines) using a method of allocation that is not truly random. Each infant diagnosed with moderate bronchiolitis was enrolled either in racemic epinephrine (RE) or hypertonic saline (HS) group on alternative basis. Ethical approval was obtained from Clinical Research Ethics Committee of the Cork University Hospital.
Our study group included all children suffering from moderate bronchiolitis who presented to Paediatric Emergency Department (ED) of University Hospital Kerry during study period.
All the infants were between day 1 of life to 24 months of age with symptoms and signs of moderate bronchiolitis. The mild severity of bronchiolitis was defined as infant with normal behavior, respiratory rate, no or minimal accessory muscle use, feeding normally, oxygen saturation ≥ 94% without oxygen supplementation, and without apneic episodes.
Moderate bronchiolitis was labeled as infants with some or intermittent irritability, tachypnea, nasal flaring, moderate chest wall retraction, trachea tug, having some feeding difficulty, mildly hypoxemic (SaO2 90–93%) requiring less 2 l/min oxygen, and brief apneas lasting 10 sec.
Severe intensity was defined as infants with increasing irritability/lethargy/fatigue, markedly increased or decreased respiratory rate, nasal flaring, marked chest wall retraction, marked tracheal tug, saturating less than 90%, requiring more than 2 l/min oxygen, and having frequent prolonged apneas.
We also included premature infants without cardiopulmonary comorbidities.
Exclusion criteria were infants with mild or severe bronchiolitis as defined above; infants given systemic or inhaled corticosteroids within the last 4 weeks; infants having significant cardiac condition, previous severe or persisting (> 4 weeks) respiratory disease, and neurologic, immunologic, and oncologic condition; and infants with more than one previous similar wheezy episode in the past.
Nebulization with salbutamol and/or ipratropium prior to admission was not an exclusion criterion as these agents do not change the course of illness [3, 7].
Patients were clinically diagnosed by a senior clinical team member on the basis of recognized signs and symptoms of bronchiolitis, namely, cough, tachypnea, increased work of breathing, chest hyperinflation, wide spread wheeze with or without crepitation, reduced oxygen saturation, pyrexia, and signs of dehydration. Further assessment was performed to categorize the severity as defined above.
In order to accurately define and confirm the degree of severity, the clinical signs and symptoms were looked upon by two senior clinicians.
Chest X-rays and blood tests were performed only where clinical diagnosis was unclear.
Written informed consent was obtained after formal information was provided to parents of eligible children prior to their enrolment in the study. Enrolled infants were allocated to either HS group or RE group on alternate basis.
The variables studied include age, gender, gestation at birth, number of days with wheezy episode at the time of presentation in ED, type of parental feed, and any form of nebulized medication given prior to presentation to ED either by GP or ambulance crew or caregivers at home. Once initial clinical parameters were noted, the first nebulization was given in ED.
Patients allocated to racemic adrenaline (RE) arm of study were nebulized only with racemic adrenaline solution. The nebulizing solution was composed of 0.2 ml of racemic epinephrine inhalation solution (USP 2.25%) added to 1.8 ml of distilled water. First nebulization was given on admission and then 6 h as required.
Patients allocated to 3% hypertonic saline (HS) arm of study were nebulized exclusively with 2.0 ml of 3% hypertonic saline solution on admission and then 1 to 4 h as required.
It was preordained that if the child had received same medication by nebulization as he/she was enrolled to, prior to presenting to A + E (GP/ambulance), then dose would be repeated appropriate interval depending upon the arm of study allocated as described above.
If the child received a different medication (RE or HS) as enrolled, it was decided to be kept in the same medication group as given prior to admission. However, no patient had received hypertonic saline or epinephrine nebulization prior to presentation.
Infants were monitored for vital signs, oxygen monitoring, parenteral fluid monitoring, and nebulization requirement at least every 4 h. Severity of the condition was reevaluated on the basis of criteria as stated above. At any stage, if a patient showed clinical signs of severe illness, infant was excluded from the study for further escalation of care. Record of excluded patients was kept until time of exclusion.
Length of stay was calculated from the date of admission to the date of discharge.
Results
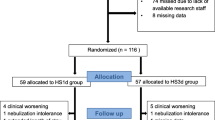
A total of eighty infants with bronchiolitis presented to the emergency department. Initially, 50 (62.5%) babies were enrolled. These babies were allocated racemic epinephrine or hypertonic saline groups on alternate basis. Each group had 25 babies. Further rundown of enrollment showed some cases with mild severity of disease. So to avoid the contamination of results, the mild cases were excluded post enrollment with remaining 16 (47%) patients in HS and 18 (53%) patients in RE group.
None of the participants had received either of hypertonic saline or racemic epinephrine prior to presentation to A + E. The demographics of infants are shown in Table 1.
The demographics of two groups were not significantly different: age (p = 0.509), birth weight (p = 0.072), birth gestation (p = 1.00), and gender (p = 0.715).
During the study, one infant from RE group and three infants from HS group were excluded as they progressed to severe disease.
Primary outcome
In RE group, out of 17 remaining infants, the length of stay ranged between 18 and 160 h (mean, 45 h).
In HS group, out of remaining 13 infants, the length of stay ranged between 18.50 and 206 h (mean, 74.3 h).
The length of hospital stay was significantly short in epinephrine group (p value 0.015 by Mann-Whitney U test).
Secondary outcome
None of the infants in RE group required supportive oxygen, while in HS group, four patients required oxygen with mean duration of 30 h.
One patient in RE group required intravenous fluids for 24 h, while two patients in HS group required intravenous fluids for the mean duration of 15 h.
There were no adverse effects recorded between the two groups during the whole duration of the study.
Discussion
Despite self-resolving nature, bronchiolitis remains cause of concern owing to stress of infants, parental anxiety, and hospitalization. Hospitalization per se is a stressful event for infants and parents [9,10,11,12].
Due to broncho-obstructive pathophysiology, traditional bronchodilators, namely, salbutamol, ipratropium, and steroids have commonly been used to reduce the symptoms with no proven efficacy.
International guidelines do not recommend use of albuterol or salbutamol to infants with bronchiolitis [1, 2].
In a Cochrane review by Gadomski and Scribani, salbutamol or albuterol did not show improvement in oxygen saturation, hospital admission reduction after outpatient treatment, shortening the duration of hospitalization or increase the resolution phase of illness. They suggested weighing small improvements in clinical scores for outpatients against the costs and adverse effects of bronchodilators [7].
Until present, the international guidelines do not recommend use of epinephrine [1, 2, 7, 8].
Use of 3% hypertonic saline is recommended by the American Association of Pediatrics (AAP) [1, 2], while the National Institute of Clinical Excellence (NICE) disapprove its use [8].
Auxiliary research however has shown epinephrine as promising treatment in achieving symptomatic relief of affected infants.
In a Cochrane systematic review conducted by Hartling et al., the effectiveness and superiority of adrenaline for outcomes of most clinical relevance among outpatients with acute bronchiolitis in the first 24 h were showed [14].
Plint et al. demonstrated significant reduction in hospital admission of infants with bronchiolitis in a large randomized placebo control trial by giving combination of nebulized epinephrine and dexamethasone in the emergency department [16].
In a Norwegian trial, Skjerven et al. found that the “on-demand” dosing of epinephrine led to shorter length of hospital stay even though the clinical score remained the same among different groups [13].
Tal G and colleagues compared the effect of mixtures of epinephrine with normal saline and epinephrine with hypertonic saline on hospital admission length and found the second combination being more effective in reducing the duration hospital admission [17].
Miraglia et al. found similar results with same combinations and regarded the combination of HS and adrenaline to be effective in reducing length of stay (LOS) [18].
However, there are some studies where researchers did not find convincing difference in the effectiveness of hypertonic saline and epinephrine [6, 11, 12].
Our study which is the first of its kind on the Republic of Ireland adds to the research already carried out internationally by the above researchers and signifies role of adrenaline in reducing symptoms of these infants, providing relief to parents and reducing the hospital stay.
The limitation of the study is smaller cohort of infants. However, owing to fact that this was the first comparative trial in Ireland between these two groups, more hospitals can use this tool to enhance the strength of Irish data in order to look at the efficacy further.
A valid argument can be made that we enrolled infants with first or second wheezy episodes only to exclude early asthmatics with recurrent wheezy episodes. Therefore, our results do not necessarily imply on asthmatics but are applicable to infants with typical viral bronchiolitis.
Both medications were tolerated well, and no adverse effects were observed.
In conclusion, this trial demonstrated beneficial effect of racemic epinephrine in reducing the mean length of stay significantly compared with hypertonic saline group in our cohort. With growing evidence in this area, racemic epinephrine may potentially be one of the first-line agents in the treatment of moderate bronchiolitis. More favorable results are observed with epinephrine, possibly due to its dual effects of bronchodilation and mucous reduction.
In the ongoing research in form of randomized controlled multicenter trials, systematic reviews would assist to consolidate the evidence in this regard.
References
American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis (2006) Diagnosis and management of bronchiolitis. Pediatrics 118(4):1774–1793
American Academy of Pediatrics (2014) Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 134(5):e1474–e1502. https://doi.org/10.1542/peds.2014-2742
Bush A, Thomson AH. Acute bronchiolitis. BMJ. 2007;335(7628):1037-41. 3
Zorc JJ, Hall CB (2010) Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 125(2):342–349
Mansbach JM, McAdam AJ, Clark S et al (2008) Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med 15:111–118
Friedman JN, Rieder MJ, Walton JM, Canadian Paediatric Society, Acute Care Committee, Drug Therapy and Hazardous Substances Committee (2014) Bronchiolitis: recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr Child Health 19(9):485–498
Gadomski AM, Scribani MB (2014) Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 6:CD001266
Bronchiolitis in children: diagnosis and management. NICE guideline [NG9] Published date: June 2015
Leidy NK, Margolis MK, Marcin JP et al (2005) The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 115(6):1536–1546
Lapillonne A, Regnault A, Gournay V, Gouyon JB, Benmedjahed K, Anghelescu D, Arnould B (2013) Moriette G Development of a questionnaire to assess the impact on parents of their infant’s bronchiolitis hospitalization. BMC Health Services Research 13:272
Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E (2005) A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med 31:1248–1254
Graves JK, Ware ME (1990) Parents’ and health professionals’ perceptions concerning parental stress during a child’s hospitalization. Child Health Care 19:37–42
Skjerven HO, Hunderi JO, Brügmann-Pieper SK et al (2013) Racemic adrenaline and inhalation strategies in acute bronchiolitis. N Engl J Med. 368(24):2286–2293
Hartling L, Bialy LM, Vandermeer B, Tjosvold L et al (2011) Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 6:CD003123
Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N (2010) High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol. 45(1):41–47
Plint AC, Johnson DW, Patel H et al (2009) Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med 360(20):2079–2089
Tal G, Cesar K, Oron A et al (2006) Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Isr Med Assoc J 8(3):169–173
Miraglia Del Giudice M, Saitta F, Leonardi S et al (2012) Effectiveness of nebulized hypertonic saline and epinephrine in hospitalized infants with bronchiolitis. Int J Immunopathol Pharmacol 25(2):485–491
Gupta N, Puliyel A, Manchanda A, Puliyel J (2012) Nebulized hypertonic-saline vs epinephrine for bronchiolitis; proof of concept study of cumulative sum (CUSUM) analysis. Indian Pediatr 49(7):543–547
Grewal S, Ali S, McConnell DW, Vandermeer B, Klassen TP (2009) A randomized trial of nebulized 3% hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department. Arch Pediatr Adolesc Med. 163(11):1007–1012
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
was obtained from the Clinical Research Ethics Committee of the Cork University Hospital. Written informed consent was obtained after formal information was provided to parents of eligible children prior to their enrolment in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yasin, F., Afridi, Z.S., Mahmood, Q. et al. Role of nebulized epinephrine in moderate bronchiolitis: a quasi-randomized trial. Ir J Med Sci 190, 239–242 (2021). https://doi.org/10.1007/s11845-020-02293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-020-02293-5