Abstract
Purpose
The prognosis of AML patients with chemotherapy is poor, especially those who are insensitive to and resistant to chemotherapy drugs. To clarify the underlying pathogenesis of AML and provide new therapeutic targets for clinical treatment, we explore the role of circRNA in leukemia.
Methods
High-throughput circRNA sequencing analysis was performed in patients with leukemia and healthy donors. RT-qPCR and western blot analysis were used to determine expression of GSK3β. RNA pull-down assay was used to detect miRNAs pulled down by hsa_circ_0121582. RNA immunoprecipitation assay was performed to evaluate the binding capacity between TET1 and hsa_circ_0121582.
Results
A new and highly stable circRNA was found, which was derived from the reverse splicing of GSK3β exon 1 to exon 7, and hsa_circ_0121582 was down-regulated in leukemia cells. In gain-of-function experiments, the up-regulated hsa_circ_0121582 inhibited the proliferation of leukemia cells in vitro and in vivo. In the cytoplasm, hsa_circ_0121582 could act as a sponge for miR-224, attenuate the inhibiting effect of miR-224 on GSK3β, and thus up-regulate the expression level of GSK3β. In addition, hsa_circ_0121582 could bind to GSK3β promoter in the nucleus, and recruit DNA demethylase TET1 to ensuring the transcription of GSK3β. The upregulated GSK3β inhibited the Wnt/β-catenin signaling pathway, and reduced the aggregation of β-catenin in the nucleus, thus inhibited the proliferation of leukemia cells.
Conclusions
This study found that hsa_circ_0121582 was involved in the inhibition of tumor proliferation, and the restoration of hsa_circ_0121582 could be an effective treatment strategy for patients with leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a highly invasive and heterogeneous malignant tumor derived from bone marrow hematopoietic stem cells or progenitor cells [1]. AML is the most common type of acute leukemia in adults. Currently, the treatment of AML mainly depends on chemotherapy and hematopoietic stem cell transplantation [2, 3]. The prognosis of AML patients with chemotherapy is poor, especially those who are insensitive to and resistant to chemotherapy drugs [4, 5]. The cost of hematopoietic stem cell transplantation is huge and the patient’s economic burden is heavy [6]. Therefore, it is necessary to clarify the underlying pathogenesis of AML and provide new therapeutic targets for clinical treatment.
Circular RNAs (circRNAs) are non-coding RNAs that do not contain 5′ cap or 3′ poly-A-tail, and form a covalent closed-loop structure through head–tail splicing [7]. CircRNAs are more stable and resistant to nucleic acid exonuclease than linear non-coding RNA. CircRNAs are enriched in the cytoplasm of eukaryotic cells and have a certain organization, timing, and disease specificity [8, 9]. With the development of high-throughput sequencing, large amounts of circRNAs have been discovered. Numerous studies have confirmed that circRNAs take part in the development and progression of human diseases, especially tumors [10, 11]. CircRNAs could regulate the expression of oncogenes or tumor suppressor genes, thus alter tumor proliferation, invasion, and metastasis [12, 13]. However, it is unclear whether circRNAs are involved in the malignancy of AML. The mechanism of circRNAs depends mainly on their subcellular localization [14]. Many studies have found that circRNAs contain miRNA response elements that could act as miRNA sponges to up-regulate the expression of target genes [15, 16].
In this study, a novel circRNA (hsa_circ_0121582) associated with AML was identified by high-throughput sequencing. Through bioinformatics analysis, it was found that hsa_circ_0121582 could act as a sponge for miR-224, thus inhibiting the expression of GSK3β. Therefore, we discussed the expression, biological function, and mechanism of hsa_circ_0121582 in AML.
Materials and methods
AML samples and cell lines
The clinical samples were from the Hunan Provincial People’s Hospital, the First-affiliated Hospital of Hunan Normal University. Each patient was provided a written informed consent and this study was approved by the ethics committee of the First-affiliated Hospital of Hunan Normal University. Human AML cell line (BDCM, HL-60, Kasumi-3, KG-1), and normal peripheral blood mononuclear cells (PBMC) were purchased from Chinese academy of sciences (Shanghai).
Polymerase chain reaction (PCR)
DNA extraction kit (Beyotime, D6001, China) was used to extract total DNA. Total RNA was extracted using an RNA extraction kit and quantified on NanoDrop 2000 (Invitrogen). Cytoplasmic and nuclear RNA were separated according to the instructions using the mirVana™ kit (Thermo, AM1556, US). RNA was synthesized into cDNA using a reverse transcription kit. PCR was then performed and nucleic acid electrophoresis was performed on 2% agarose gel. The primers used in this study are shown in Table S1.
Cell transfection and preparation of stable hsa_circ_0121582 expressed cell lines
Lipo8000™ (Beyotime, C0533, China) was used to transfect cells. Control miRNA or miR-224 mimics, negative control small interfering RNA (si-NC), si-TET1, or si-GSK3β were all from Shanghai sangon biotechnology co., LTD. The full-length TET1 sequence was inserted into pcDNA 3.0 vector (Invitrogen) to construct the overexpressed plasmid. The transfection efficiency was detected by qRT-PCR after 48 h transfection.
To stabilize the overexpression of hsa_circ_0121582, the full-length sequence of hsa_circ_0121582 was embedded into the lentivirus vector (GeneChem, China). Lentiviruses were then infected with BDCM and kasumi-3 cells, and stable cells were screened with 1.5 μg/ml purinomycin. The infection efficiency was detected by fluorescence microscopy and RT-PCR.
Cell proliferation assay
Proliferation of BDCM and kasumi-3 cells was detected by CCK-8, EdU DNA incorporation, and cell cycle assay. In the CCK-8 experiment, after cell culture and treatment, 10 μl CCK-8 reagent (Beyotime, C0037, China) was added and treated for 2 h, and the absorbance was measured at 450 nm. The DNA incorporation rate was tested using BeyoClick™ EdU-488 kit (Beyotime, C0071, China) in accordance with the instructions. To evaluate cell cycle, BDCM and kasumi-3 cells were stained with propiazine iodide (Beyotime, ST512, China) solution, and the ratio of cells in G0/G1, S, and G2/M phases was determined by flow cytometry.
Animal studies
The 20 g nude mice were obtained from SJA laboratory animal company, and the animal experiments were conducted in accordance with the procedures of animal care committee of hunan provincial people's hospital. To detect the role of hsa_circ_0121582 in vivo, we established a xenograft tumor model by subcutaneous injection of control or stable hsa_circ_0121582 overexpressed BDCM cells. Before subcutaneous injection, the BDCM cells (4 × 107 cells in 50 μl PBS) were mixed with 50 μl matrigel (356237, BD) to promote cell adhesion.
Western blot analysis
Total proteins from cells and tumor tissues were separated by SDS-PAGE, then transferred to PVDF membrane and sealed with 5% skim milk powder solution. Anti-GSK3β (Abcam, ab93926, USA), anti-phosphorylated-β-catenin (Abcam, ab27798, USA), anti-TET1 (Abcam, ab191698, USA), and anti-β-catenin (Abcam, ab32572, USA) were incubated with PVDF membrane. Anti-GAPDH (Abcam, ab181602, USA) and anti-Lamin B1 (Abcam, ab133741, USA) were used as internal parameters for total protein and nuclear protein, respectively. Finally, horseradish peroxidase-labeled secondary antibodies were incubated and imaging was performed using an enhanced chemiluminescence solution (Beyotime, P0018, China).
RNA pull-down assay
To detect miRNAs pulled down by hsa_circ_012158, BDCM and Kasumi—3 cell lysis solution was incubated with biotin labeled control (5′- AGCGAGACCATCTCTGCCCTCGGGTCATCATACCAAATCG-3′) or hsa_circ_012158 probe (5′-TTAATGCCCTGGGTTTACAGTTTGATGGTTTACCCGC-3′) over night. Then, the lysates were incubated with magnetic beads (Invitrogen) at room temperature for 3 h.
To detect miR-224 could pull down hsa_circ_0121582, biotin wild or mutant miR-224 mimics were synthesized and transfected into BDCM and kasumi-3 cells using Lipo8000™ (Beyotime, C0533, China). The cells were incubated with streptavidin magnetic beads, and the expression of hsa_circ_0121582 was detected by qRT-PCR.
Luciferase reporter and TOPFlash/FOPFlash reporter assay
To detect the transcriptional activity of the Wnt/β-catenin signaling pathway, BDCM and Kasumi-3 cells with high hsa_circ_0121582 expression were co-transfected with TOPFlash/FOPFlash repoter and miR-224 or si-TET1. Then, luciferase activity was evaluated according to the ratio of TOPFlash to FOPFlash.
RNA immunoprecipitation (RIP)
RNA immunoprecipitation assay was performed using ChIP Assay Kit (Beyotime, P2078, China) and anti-TET1 antibody to evaluate the binding capacity between TET1 and hsa_circ_0121582. In brief, BDCM and kasumi-3 cell lysates were collected and incubated with anti-TET1 antibodies overnight, then incubated with protein A/G magnetic beads at 25 °C for 2 h. Then, TET1-bound RNA was isolated from the complex by TRIzol solution, and the expression of hsa_circ_0121582 was detected by qRT-PCR.
Statistical analysis
All data were showed as mean ± standard deviation (SD). SPSS 18.0 software was used to analyze the statistical differences. Differences between groups were used by ANOVA and subsequent Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Characterization of hsa_circ_0121582 in DLBCL
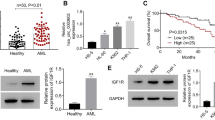
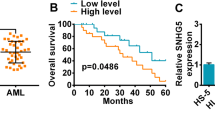
To find the circRNAs with significant differences in AML, high-throughput sequencing technology was used to analyze the circRNAs expression profile of three pairs of bone marrow mononuclear cells from AML patients and healthy donors (Fig. 1a). It was found that the expression of hsa_circ_0121582 in AML was the most different, which was considered as a further research object. Meanwhile, fluorescence quantitative PCR (qRT-PCR) results indicated that hsa_circ_0121582 was significantly down-regulated in mononuclear cells of AML patients, consistent with the results of high-throughput sequencing of circRNA (Fig. 1b). hsa_circ_0121582 expression in four AML cell lines (BDCM, HL-60, kasumi-3, KG-1) was also lower than that in normal peripheral blood mononuclear cells (Fig. 1c).
hsa_circ_0121582 in AML. a Up-regulated and down-regulated circRNAs in AML and normal tissues. b, c hsa_circ_0121582 in AML tissues and cell lines. d, e hsa_circ_0121582 and GSK3β mRNA were determined by qRT-PCR after treatment with actinomycin D or RNase R. f Schematic diagram of the origin of hsa_circ_0121582. g Analysis of hsa_circ_0121582 with convergent and divergent primers for cDNA or gDNA. h The distribution of hsa_circ_0121582 in cytoplasm and nucleus. *p < 0.05, **p < 0.01, ***p < 0.001
To detect the stability of hsa_circ_0121582, we treated cells with transcription inhibitor actinomycin D for 0, 4, 8, 12, and 24 h. Total cell RNA was collected for quantitative detection of GSK3β mRNA and hsa_circ_0121582. As shown in Fig. 1d, the half-life of GSK3β mRNA was less than 4 h, while the half-life of hsa_circ_0121582 is more than 24 h, indicating that hsa_circ_0121582 is highly stable. In addition, after treating with RNase R, linear GSK3β mRNA was degraded, but the ring structure hsa_circ_0121582 was not affected (Fig. 1e).
Through sequence alignment analysis, it was found that hsa_circ_0121582 was generated by the reverse splicing of the linear GSK3β exon 1 to exon 7, and the sequence length was 769 bp (Fig. 1f), which was further verified by RT-PCR using divergent primers on the hsa_circ_0121582 junction sequence (Fig. 1g).
The distribution of hsa_circ_0121582 in cytoplasm and nucleus was also analyzed by qRT-PCR, and it was found that hsa_circ_0121582 was evenly distributed between cytoplasm and nucleus (Fig. 1h). These results indicate that hsa_circ_0121582 is highly stable, which may be involved in the development of leukemia.
The exogenous expressed hsa_circ_0121582 inhibited the proliferation of leukemia cells in vivo and in vitro
To investigate the biological function of hsa_circ_0121582, a stable overexpressed hsa_circ_0121582 leukemia cell line was constructed by lentiviral vector. qRT-PCR result showed that hsa_circ_0121582 expression in BDCM and kasumi-3 cell lines was up-regulated by about 16 times (Fig. 2a).
hsa_circ_0121582 inhibits AML cell proliferation in vitro and in vivo. a Expression of hsa_circ_0121582 by RT-PCR in AML cells. b Cell viability by CCK8 asssay. c DNA synthesis rate by EdU assay in AML cells. d Cell cycle (G0/G1, S, G2/M phase) in control or hsa_circ_0121582-overexpressing BDCM and kasumi-3 cells. e Tumors in the control and hsa_circ_0121582-overexpressing mice. f Expression of hsa_circ_0121582 by FISH in tumors. g HE staining of tumors. *p < 0.05, **p < 0.01, ***p < 0.001
The effect of hsa_circ_0121582 on the proliferation of leukemia cells was evaluated by CCK-8 assay. CCK-8 assay showed that the survival rate of hsa_circ_0121582 overexpressed BDCM and kasumi-3 cells was significantly reduced (Fig. 2b). Similarly, EdU assay showed that the overexpressed hsa_circ_0121582 also significantly inhibited DNA synthesis (Fig. 2c). In addition, hsa_circ_0121582 overexpression elevated the number of cells blocked in the G0/G1 phase (Fig. 2d).
To verify whether hsa_circ_0121582 can inhibit the proliferation of leukemia cells in vivo, we subcutaneously inoculated BDCM cells in nude mice to prepare a xenograft model. The volume and weight of tumors in the overexpressed hsa_circ_0121582 group were obviously smaller than those in the control group (Fig. 2e). In the FISH assay, hsa_circ_0121582 was highly expressed in hsa_circ_0121582 group (Fig. 2f). Furthermore, HE staining result indicated that overexpressed hsa_circ_0121582 tumor tissue had loose structure (Fig. 2g). Therefore, in vivo and in vitro experiments indicated that hsa_circ_0121582 was a proliferation-inhibiting regulator of AML.
GSK3β was up-regulated by hsa_circ_0121582 sponging miR-224 in vivo and in vitro
CircRNAs can change the expression of host genes and thus participate in the development of disease, thus we verified the regulatory effect of hsa_circ_0121582 on GSK3β. Overexpressed hsa_circ_0121582 significantly increased mRNA and protein of GSK3β in BDCM and kasumi-3 cells (Fig. 3a, b). Importantly, in tumor-bearing mice injected with overexpressed hsa_circ_0121582 BDCM cells, GSK3β expression was also significantly up-regulated (Fig. 3c, d).
hsa_circ_0121582 increases GSK3β expression by sponging miR-224 in AML. a, b GSK3β in control or hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells. a Lv-GFP, b Lv-hsa_circ_0121582. c, d GSK3β expression in the subcutaneous tumors of nude mice with control or hsa_circ_0121582-overexpressing BDCM cells. a Lv-GFP, b Lv-hsa_circ_0121582. e Cell proliferation in hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with si-GSK3β. f 14 miRNAs for both hsa_circ_0121582 and GSK3β were identified by CircInteractome and miRanda softwares. g Schematic diagram of the hsa_circ_0121582 luciferase reporter vector with a wild or mutant miR-224 binding site. h Luciferase reporter assay in BDCM and Kasumi-3 cells with the wild or mutant hsa_circ_0121582 luciferase vector and control miRNA or miR-224. i hsa_circ_0121582 expression was determined by qRT-PCR in RNA pull-down assay in BDCM and Kasumi-3 cells transfected with wild or mutant miR-224. j Analysis of miR-224 after hsa_circ_0121582 overexpression. k Cell viability in hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with wild-type or mutant miR-224. l Luciferase reporter assay in control or hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells co-transfected with the wild or mutant GSK3β 3′-UTR luciferase vector and control miRNA or miR-224. m Analysis of GSK3β in control or hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with control miRNA or miR-224. *p < 0.05, **p < 0.01, ***p < 0.001
To detect whether the inhibitory effect of hsa_circ_0121582 on cell proliferation was dependent on its ability to up-regulate GSK3β, we knocked out GSK3β in BDCM and kasumi-3 cells. GSK3β silence almost completely reversed the inhibitory effect of hsa_circ_0121582 on cell proliferation (Fig. 3e). These results indicate that hsa_circ_0121582 has a positive regulatory effect on GSK3β expression in AML.
CircRNAs in the cytoplasm can competitively regulate endogenous gene expression by adsorption of miRNA. Previous experiments found that about 50% of hsa_circ_0121582 was located in the cytoplasm, and we speculated that hsa_circ_0121582 might regulate the expression of GSK3β through this mechanism. Using CircInteractome and miRanda analysis software, we found that 14 miRNAs (miR-1180, miR-1304, miR-145, miR-1825, miR-194, miR-203, miR-224, miR-331-3p and miR-338-3p, miR-494, miR-507, miR-557, miR-766, and miR-889) had binding sites with hsa_circ_0121582 and GSK3β (Fig. 3f).
To determine which miRNA is involved in the regulation of hsa_circ_0121582/GSK3β, RNA pull-down experiments were performed using biotin labeled hsa_circ_0121582 probes. In BDCM and kasumi-3 cells, miR-224 content in the drop-down was the highest (Figure S1). The luciferase reporter gene was then determined by inserting the wild or mutant hsa_circ_0121582 sequence in the downstream of luciferase (Fig. 3g). As shown in Fig. 3h, overexpressed miR-224 obviously decreased luciferase activity of wild-type reporter gene, but did not influence luciferase activity of mutant reporter gene. To confirm the binding between hsa_circ_0121582 and miR-224, we transfected biotin labeled wild-type or mutant mir-224 mimics into BDCM and kasumi-3 cells and then conducted RNA pull-down experiments. It was found that wild but not mutant miR-224 could effectively enrich hsa_circ_0121582 (Fig. 3i).
miR-224 was significantly up-regulated in AML. However, the overexpressed hsa_circ_0121582 significantly reduced the expression of miR-224 (Fig. 3j). To determine whether the anti-proliferation effect of hsa_circ_0121582 in AML cells was dependent on its ability to down-regulate miR-224, the hsa_circ_0121582 overexpressed BDCM and Kasumi-3 cells were transfected with either wild or mutant miR-224 mimics. The heterotopic expression of wild miR-224 (but not the mutant) partially reversed the inhibitory effect of hsa_circ_0121582 on the proliferation of AML cells (Fig. 3k).
Next, luciferase reporter gene assay was used to detect whether GSK3β was the target of miR-224. Overexpressed miR-224 did not alter the luciferase activity of the mutant GSK3β 3′-UTR reporter, but significantly decreased the activity of the wild GSK3β 3′-UTR reporter (Fig. 4l). In BDCM and kasumi-3 cells, overexpressed hsa_circ_0121582 completely blocked this inhibitory effect (Fig. 4l). Meanwhile, qRT-PCR analysis showed that overexpressed miR-224 decreased the expression of GSK3β, while the expression of exogenous hsa_circ_0121582 effectively restored the expression of GSK3β (Fig. 4m). The above results showed that in AML, hsa_circ_0121582 increased the expression of GSK3β by adsorption and inhibition of miR-224 expression.
hsa_circ_0121582 recruits TET1 to the GSK3β promoter to enhance the transcription of GSK3β in AML. a GSK3β expression in BDCM and Kasumi-3 cells treated with 5-Aza-dC. b GSK3β expression in BDCM and Kasumi-3 cells transfected with a TET1, TET2 or TET3 overexpression vector. c The probability of binding between hsa_circ_0121582 and TET1 by RPISeq tool predicting. d TET1 expression was determined by western blot after RNA pull-down assay with a control or hsa_circ_0121582 probe. e hsa_circ_0121582 expression was determined by qRT-PCR after RNA immunoprecipitation assay with an IgG or TET1 antibody. f The GSK3β promoter region was determined by qRT-PCR after ChIRP assay with a control or hsa_circ_0121582 probe. g, h The GSK3β promoter region was determined by qRT-PCR after ChIP assay with the indicated transfection. i GSK3β expression in hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with si-TET1. j TOPFlash/FOPFlash reporter assay in hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with miR-224 or si-TET1. k, l Proteins were analysed by western blot in hsa_circ_0121582-overexpressing BDCM and Kasumi-3 cells transfected with miR-224 or si-TET1. a Lv-GFP, b Lv-hsa_circ_0121582, c Lv-hsa_circ_0121582 + miR-224, d Lv-hsa_circ_0121582 + si-TET1. *p < 0.05, **p < 0.01, ***p < 0.001
hsa_circ_0121582 binds to the GSK3β promoter and recruits the DNA demethylase TET1
GSK3β is a common tumor suppressor gene, which could be inactivated by hypermethylation in the promoter region, which was confirmed by treating AML cells with the DNA methyltransferase inhibitor 5-Aza-dC. As shown in Fig. 4a, 5-Aza-dC increased GSK3 expression in BDCM and kasumi-3 cells in a dose-dependent manner.
Since DNA hypermethylation may be due to decreased activity of demethylase, we then investigated whether decreased demethylase caused inhibition of GSK3β expression. There were three members: TET1, TET2, and TET3 in ten–eleven translocation (TET) demethylase family. The overexpressed TET1 rather than TET2 or TET3 significantly up-regulated the expression of GSK3β mRNA (Fig. 4b).
Recent studies have shown that circRNAs can regulate gene expression by binding with proteins. Therefore, we want to know whether hsa_circ_0121582 can be directly combined with TET1. It was predicted that there was high interaction between hsa_circ_0121582 and TET1 by RPISeq online tool (RF classifier = 0.92, SVM classifier = 0.89, the prediction probability of > 0.5 was positive) (Fig. 4c). To confirm this prediction, RNA pull-down experiments were performed. Compared with the control probe, the hsa_circ_0121582 probe enriched TET1, which was more obvious in hsa_circ_0121582 overexpressed BDCM and kasumi-3 cells (Fig. 4d). Meanwhile, in the RNA immunoprecipitation test, there was much TET1 antibody co-precipitated with hsa_circ_0121582 (Fig. 4e).
In view of the fact that hsa_circ_0121582 can be combined with TET1, we want to know whether hsa_circ_0121582 can recruit TET1 to the GSK3β promoter. First, we conducted ChIRP analysis in BDCM and Kasumi-3 cells, and the results indicated that hsa_circ_0121582 could directly bind to the GSK3β promoter (Fig. 4f). Then, the occupancy of TET1 in the GSK3β promoter was evaluated, and exogenous hsa_circ_0121582 promoted the expression of TET1 (Fig. 4g). When TET1 oxidizes 5mC to 5hmC, the level of 5hmC on the GSK3β promoter was also evaluated. Similarly, in hsa_circ_0121582 overexpressed BDCM and kasumi-3 cells, the level of 5hmC on the GSK3β promoter was significantly increased, and this effect was completely eliminated when TET1 was silenced (Fig. 4h). In addition, TET1 knockout partially offsets the increased GSK3 caused by overexpressed hsa_circ_0121582 (Fig. 4i). These results indicated that TET1-induced DNA demethylation promoted the expression of hsa_circ_0121582 and up-regulated GSK3β expression in AML.
GSK3β is a key inhibitor of the Wnt/β-catenin pathway and increases the phosphorylation and proteolytic degradation of β-catenin, thereby reducing its accumulation in the nucleus. Therefore, we inferred that hsa_circ_0121582 might inhibit Wnt/β-catenin signals by up-regulating the expression of GSK3β. To test this hypothesis, we performed TOPFlash/FOPFlash reporter gene experiments using wild and mutant TCF-4 consistent binding sites. As shown in Fig. 4j, the exogenous hsa_circ_0121582 significantly decreased the transcriptional activity of TOP/FOP, while overexpressed miR-224 or knocked β-catenin significantly increased the transcriptional activity. Overexpressed hsa_circ_0121582 also significantly increased the expression of GSK3β and phosphorylation of β-catenin, but reduced the concentration of β-catenin in the nucleus (Fig. 4k, l). Exogenous expression of miR-224 or GSK3β knockout inhibited the above effects (Fig. 4k, l). These results suggested that cytoplasmic and nuclear hsa_circ_0121582 can be combined with miR-224 and TET1, respectively, to jointly promote GSK3β expression and ultimately inhibit oncogenic Wnt/β-catenin signaling in AML.
Discussion
As a special non-coding RNA, circRNAs opened a new direction in cancer research [17]. Many studies have found that circRNAs played an important role in the occurrence, development, and progression of tumors [18, 19]. Through high-throughput sequencing of circRNA in AML and normal tissues, circRNAs with dysregulated expression were found, suggesting that circRNAs may be involved in the occurrence and development of AML.
We then focused on hsa_circ_0121582, a previously undescribed circRNA derived from the reverse splicing of GSK3β exon 1 to exon 7. hsa_circ_0121582 was significantly down-regulated in AML, which was associated with clinical invasiveness and poor prognosis. Exogenous high expression of hsa_circ_0121582 inhibited the proliferation of AML in vitro and in vivo. At the same time, through different mechanisms, hsa_circ_0121582 in cytoplasm and nucleus jointly promoted the expression of GSK3β, thus inhibiting the Wnt/β-catenin signaling pathway to inhibit the proliferation of AML cells. These results illustrated the important role of hsa_circ_0121582 in the pathogenesis of AML.
Cellular sublocalization is critical to the biological function of non-coding RNAs [20]. Like long non-coding RNAs, circRNAs can broadly sponge miRNAs into the cytoplasm [21]. However, due to the high stability of circRNAs, their effect on miRNAs may be longer than long non-coding RNAs [22]. hsa_circ_0121582 was evenly split between cytoplasm and nucleus. hsa_circ_0121582 in the cytoplasm effectively adsorbed miR-224, reducing the inhibition of miR-224 on GSK3β. However, considering that half of hsa_circ_0121582 is located in the nucleus, we speculated that hsa_circ_0121582 may also up-regulate the expression of GSK3β in the nucleus. It has been demonstrated that circRNA in the nucleus could regulate the expression of host genes by binding to proteins. In this study, it was found that hsa_circ_0121582 could bind directly to TET1 and introduce it into the GSK3β promoter, thereby reducing the methylation level of GSK3β and enhancing the transcription of GSK3β. Therefore, hsa_circ_0121582 up-regulated the expression of GSK3β in AML at both transcriptional and post-transcriptional levels. hsa_circ_0121582 inhibited the Wnt/β-catenin signaling pathway by up-regulating the expression of GSK3β, thereby inhibiting the proliferation of AML cells.
Conclusions
In summary, our experimental evidence suggested that hsa_circ_0121582 inhibited the proliferation of AML by inhibiting the Wnt/β-catenin signaling pathway. Therefore, restoring hsa_circ_0121582 may be a viable treatment for AML.
References
Abdul-Aziz AM, Sun Y, Hellmich C, Marlein CR, Mistry J, Forde E, et al. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133:446–56.
Shallis RM, Boddu PC, Bewersdorf JP, Zeidan AM. The golden age for patients in their golden years: The progressive upheaval of age and the treatment of newly-diagnosed acute myeloid leukemia. Blood Rev. 2019;2019:100639.
Chen X, Pan J, Wang S, Hong S, Hong S, He S. The epidemiological trend of acute myeloid leukemia in childhood: a population-based analysis. J Cancer. 2019;10:4824–35.
Li G, Zhou Z, Yang W, Yang H, Fan X, Yin Y, et al. Long-term cardiac-specific mortality among 44,292 acute myeloid leukemia patients treated with chemotherapy: a population-based analysis. J Cancer. 2019;10:6161–9.
Patel HP, Perissinotti AJ, Patel TS, Bixby DL, Marshall VD, Marini BL. Incidence and risk factors for breakthrough invasive mold infections in acute myeloid leukemia patients receiving remission induction chemotherapy. Open Forum Infect Dis. 2019;6:176.
Ricci A, Jin Z, Broglie L, Bhatia M, George D, Garvin JH, et al. Healthcare utilization and financial impact of acute-graft-versus host disease among children undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;55(2):384.
Liu J, Li D, Luo H, Zhu X. Circular RNAs: The star molecules in cancer. Mol Aspects Med. 2019;70:141–52.
Li Z, Ruan Y, Zhang H, Shen Y, Li T, Xiao B. Tumor-suppressive circular RNAs: mechanisms underlying their suppression of tumor occurrence and use as therapeutic targets. Cancer Sci. 2019;110:3630–8.
Ruan H, Xiang Y, Ko J, Li S, Jing Y, Zhu X, et al. Comprehensive characterization of circular RNAs in ~ 1000 human cancer cell lines. Genome Med. 2019;11:55.
Cao S, Ma T, Ungerleider N, Roberts C, Kobelski M, Jin L, et al. Circular RNAs add diversity to androgen receptor isoform repertoire in castration-resistant prostate cancer. Oncogene. 2019;38:7060–72.
Kolling M, Haddad G, Wegmann U, Kistler A, Bosakova A, Seeger H, et al. Circular RNAs in urine of kidney transplant patients with acute T cell-mediated allograft rejection. Clin Chem. 2019;65:1287–94.
Wu Z, Sun H, Li J, Jin H. Circular RNAs in leukemia. Aging. 2019;11:4757–71.
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin XM, et al. Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 2019;9:3526–40.
Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, et al. ncx1A circular transcript of gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8:5855–69.
Ma N, Pan J, Ye X, Yu B, Zhang W, Wan J. Whole-transcriptome analysis of APP/PS1 mouse brain and identification of circRNA-miRNA-mRNA networks to investigate AD pathogenesis. Mol Ther Nucleic Acids. 2019;18:1049–62.
Wang G, Guo X, Cheng L, Chu P, Chen M, Chen Y, et al. An integrated analysis of the circRNA-miRNA-mRNA network reveals novel insights into potential mechanisms of cell proliferation during liver regeneration. Artif Cells Nanomed Biotechnol. 2019;47:3873–84.
Yin Y, Long J, He Q, Li Y, Liao Y, He P, et al. Emerging roles of circRNA in formation and progression of cancer. J Cancer. 2019;10:5015–21.
Ma X, Liu C, Gao C, Li J, Zhuang J, Liu L, et al. circRNA-associated ceRNA network construction reveals the circRNAs involved in the progression and prognosis of breast cancer. J Cell Physiol. 2019;235(4):3973.
Li HM, Dai YW, Yu JY, Duan P, Ma XL, Dong WW, et al. Comprehensive circRNA/miRNA/mRNA analysis reveals circRNAs protect against toxicity induced by BPA in GC-2 cells. Epigenomics. 2019;11:935–49.
Raveendra BL, Swarnkar S, Avchalumov Y, Liu XA, Grinman E, Badal K, et al. Long noncoding RNA GM12371 acts as a transcriptional regulator of synapse function. Proc Natl Acad Sci USA. 2018;115:E10197–E1020510205.
Lu G, Zhang J, Liu X, Liu W, Cao G, Lv C, et al. Regulatory network of two circRNAs and an miRNA with their targeted genes under astilbin treatment in pulmonary fibrosis. J Cell Mol Med. 2019;23:6720–9.
Wang J, Yin J, Wang X, Liu H, Hu Y, Yan X, et al. Changing expression profiles of mRNA, lncRNA, circRNA, and miRNA in lung tissue reveal the pathophysiological of bronchopulmonary dysplasia (BPD) in mouse model. J Cell Biochem. 2019;120:9369–80.
Author information
Authors and Affiliations
Contributions
All authors have participated in the analysis and interpretation of data, drafting of the first version of the manuscript, revising critically subsequent versions, and approving the fnal version of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
All patients involved consented to participate under the Clinical Patient ethical statement. The study was approved by the Institutional Ethical Board of The First-Affiliated Hospital of Hunan Normal University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, JJ., Lei, P. & Zhou, M. hsa_circ_0121582 inhibits leukemia growth by dampening Wnt/β-catenin signaling. Clin Transl Oncol 22, 2293–2302 (2020). https://doi.org/10.1007/s12094-020-02377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02377-9