Abstract
Purpose
Although aromatase inhibitors (AIs) prolong survival in post-menopausal breast cancer (BC) patients, AI-associated arthralgia can lead to discontinuation. Obese patients have higher rates of AI arthralgia than non-obese patients, but treatment options are limited. Omega-3 fatty acid (O3-FA) treatment for AI arthralgia has produced mixed results.
Methods
We performed an exploratory analysis of SWOG S0927, a multicenter randomized placebo-controlled trial of O3-FA use for AI arthralgia. Post-menopausal women with stage I–III BC taking an AI were randomized to 24 weeks of O3-FAs or placebo. Brief Pain Inventory (BPI) questionnaires and fasting serum were collected at baseline, 12, and 24 weeks. The BPI assessment included worst pain, average pain, and pain interference scores (range 0–10).
Results
Among the 249 participants, 139 had BMI < 30 kg/m2 (56%) and 110 had BMI ≥ 30 kg/m2 (44%). Among obese patients, O3-FA use was associated with significantly lower BPI worst pain scores at 24 weeks compared with placebo (4.36 vs. 5.70, p = 0.02), whereas among non-obese patients, there was no significant difference in scores between treatment arms (5.27 vs. 4.58, p = 0.28; interaction p = 0.05). Similarly, O3-FA use was associated with lower BPI average pain and pain interference scores at 24 weeks compared with placebo among obese patients, but no significant difference between treatment arms in non-obese patients (interaction p = 0.005 and p = 0.01, respectively).
Conclusions
In obese BC patients, O3-FA use was associated with significantly reduced AI arthralgia compared to placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In post-menopausal women with hormone receptor-positive breast cancer (BC), aromatase inhibitors (AIs) are superior to tamoxifen as adjuvant treatment, prolonging survival and improving outcomes [1,2,3,4]. While AIs are associated with lower risk of thromboembolic events and endometrial cancer compared with tamoxifen, they are associated with substantially greater rates of arthralgia [5]. AI arthralgia has been estimated to affect 45–60% of patients, many of whom report severe or continuous pain [6, 7]. Due to AI arthralgia pain, patients may have decreased adherence or prematurely discontinue taking these potentially life-saving therapies. One study reported an overall AI discontinuation rate of 32.4% within 2 years due to adverse effects, 24.3% specifically due to musculoskeletal symptoms, which was the primary reason for discontinuation [8]. Self-reported Brief Pain Inventory (BPI) worst joint pain score of 4 or greater is a significant predictor of discontinuation [9].
Obesity has been linked to increased AI arthralgia. A cross-sectional study found that normal weight and obese patients were more likely to have AI arthralgia than overweight patients with body mass index (BMI) 25–30 kg/m2 (57% and 54% vs. 34%, respectively) [10]. In a retrospective analysis of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial, patients with BMI > 30 kg/m2 reported significantly more joint symptoms than patients with BMI ≤ 30 kg/m2 [11]. A retrospective analysis of the Intergroup Exemestane Study (IES) trial showed similar results, with weight ≥ 80 kg at the time of randomization associated with significantly greater risk of developing AI arthralgia [12].
There is no optimal treatment regimen for AI arthralgia. In meta-analyses, omega-3 fatty acids (O3-FAs) have shown benefit in patients with rheumatoid arthritis, reducing joint pain intensity, number of affected joints, stiffness, and NSAID use [13, 14]. In a small pilot study, O3-FAs were found to be well tolerated in breast cancer patients; pain scores for AI arthralgia were secondary outcomes [15]. SWOG S0927 was a randomized double-blind placebo-controlled trial of O3-FA use for AI arthralgia which found significant improvements in AI arthralgia in both the placebo and O3-FA groups but no significant difference between the groups [16]. Given that obese patients are at higher risk of AI arthralgia, we performed a retrospective, exploratory analysis of SWOG S0927 data to evaluate whether the effects of O3-FAs on AI arthralgia are associated with BMI.
Methods
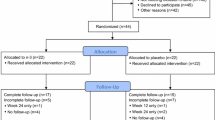
We performed a post hoc analysis of SWOG S0927 (Clinicaltrials.gov NCT01385137), which accrued patients from February 2012 to February 2013 across 52 sites. Informed consent was obtained from all individual participants included in the study. The study was approved by each site’s Institutional Review Board and was conducted in compliance with the provisions of the Declaration of Helsinki.
Post-menopausal women with stage I–III breast cancer taking an AI for ≥ 90 days were screened with the Brief Pain Inventory (BPI)-Short Form. Patients with a BPI worst pain score of ≥ 5 out of 10 on the form, who reported that their pain started or worsened after initiating AI therapy and who had a Zubrod performance score of 0–2, were eligible for inclusion in the study. Patients were excluded if they had taken O3-FAs in the 3 months prior to enrollment, topical analgesics or oral narcotics in the 14 days prior to enrollment, oral steroids or intra-articular steroid injections in the 28 days prior to enrollment, or if there was a history of fracture or surgery in the affected joint(s) in the 6 months prior to enrollment.
Subjects were randomized to 24 weeks of O3-FA (3.3 g per day; 560 mg eicosapentanoic acid plus docosahexaenoic acid in a 40:20 ratio) or placebo (soybean-corn oil blend). Clinical and demographic information were collected at baseline, including comorbid conditions and medication use. Weight and height were measured at baseline and were used to calculate body-surface area and BMI in kg/m2 [17]. Patients were categorized into BMI < 30 kg/m2 (non-obese) or ≥ 30 kg/m2 (obese). Since there are no validated scales specifically for the measurement of AI arthralgia, a generalized pain scale was used. The BPI-Short Form, a 14-item questionnaire for the assessment of cancer pain, was administered at baseline, 6, 12, and 24 weeks. Using this form, subjects were asked to rate their worst pain (item 2), average pain (item 4), and pain interference with general activities (item 8) over the prior week on a 0–10 scale (10 being the worst pain or interference). The Global Ratings of Change questionnaire regarding joint pain and stiffness was assessed at 6, 12, and 24 weeks with scores ranging from − 3 for “very much worse” to + 3 for “very much better” than baseline. Three other scales were also used to assess patients’ symptoms at the same time points, including the Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (M-SACRAH), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and the Functional Assessment of Cancer Therapy–Endocrine Symptoms (FACT-ES).
Fasting serum was collected at baseline, 12, and 24 weeks. Lipid profile components were measured with the Roche Cobas Integra 400 plus automated analyzer using individual enzymatic colorimetric methods. For cholesterol, inter-assay precision was 1.90%, intra-assay precision was 0.51%, and the quantification limit was 0.116 mg/dL. For high-density lipoprotein (HDL), inter-assay precision was 1.00%, intra-assay precision was 1.13%, and the quantification limit was 3 mg/dl. For triglycerides, inter-assay precision was 1.90%, intra-assay precision was 1.60%, and the quantification limit was 8.85 mg/dL. LDL was calculated using the Friedewald equation.
Statistical methods
Participant characteristics at baseline were compared between patients with BMI < 30 kg/m2 and patients with BMI ≥ 30 kg/m2 using t tests for continuous measures and Chi-square tests for categorical measures. Raw means of each lipid profile component, BPI scores, and Global Ratings of Change were calculated at each time point, separately by treatment arm and BMI group. Linear regression was used to calculate p values comparing mean lipid profile components and pain scores between treatment arms, separately by BMI group. Interaction tests were derived as the product of the indicator terms for BMI group and treatment within the regression models, and reflected whether patterns of lipid profiles, BPI scores, or Global Ratings of Change by arm differed between BMI groups (< 30 vs. ≥ 30 kg/m2). p values comparing baseline measures are unadjusted, and p values comparing week 12 and week 24 measures were adjusted for baseline measures. Statistical analysis was performed using SAS 9.4 (SAS, Cary, NC). All tests were 2-sided, and p < 0.05 was considered significant.
Results
Overall, 249 participants were included in the analyses; the characteristics of this cohort have been previously reported [16]. Patient characteristics are shown in Table 1. The median age was 59 years; 8% of patients were black and 7% were Hispanic. Overall, the median BMI in the entire group was 28.8 kg/ m2; 139 (56%) patients had BMI < 30 kg/m2 while 110 (44%) had BMI ≥ 30 kg/m2. The most common self-reported comorbid conditions were hypertension (41%) and hypercholesterolemia (31%); 33% of the cohort had ≥ 2 cardiovascular risk factors. There were significant differences between those with BMI < 30 kg/m2 versus those with BMI ≥ 30 kg/m2 in the prevalence of diabetes (8% vs. 19%, respectively, p = 0.009), hypertension (31% vs. 53%, respectively, p = 0.0005), and osteoarthritis (16% vs. 32%, respectively, p = 0.003). Patients with BMI < 30 kg/m2 were less likely to have ≥ 2 cardiovascular risk factors (27% vs. 42%, p = 0.01). Statin (HMG-CoA reductase inhibitor) use, however, was similar between the non-obese and obese groups (22% vs. 26%, respectively, p = 0.38).
Patient-reported outcomes
Patterns of BPI pain scores at 24 weeks between O3-FA and placebo arm patients differed between BMI groups. In particular, there were no differences in BPI worst pain score between O3-FA versus placebo arm patients among those with BMI < 30 kg/m2 (5.27 vs. 4.58, p = 0.28), but among those with BMI ≥ 30 kg/m2, O3-FA use was associated with a significantly lower BPI worst pain score at 24 weeks (4.36 vs. 5.70, p = 0.02, interaction p = 0.05; Table 2). Similarly, there were no significant differences in scores between O3-FA and placebo arm patients among those with BMI < 30 kg/m2, but among those with BMI ≥ 30 kg/m2, O3-FA use was associated with significantly lower BPI average pain scores (3.36 vs. 4.65, p = 0.002, interaction p = 0.005) and pain interference scores (2.15 vs. 3.49, p = 0.009, interaction p = 0.01) at week 24 compared to placebo. In terms of change in BPI scores over time, patients with BMI ≥ 30 kg/m2 taking O3-FAs had significantly greater reductions in BPI worst pain, average pain, and interference from baseline to week 24 (p = 0.02, 0.002, and 0.05, respectively) compared to those taking placebo (Fig. 1).
Analogous trends were found in both Global Ratings of Change in joint pain and joint stiffness scores from baseline, although in both cases the statistically significant differences were observed at 12 weeks rather than at 24 weeks. Among patients with BMI ≥ 30 kg/m2, Global Rating of Change in joint pain from baseline to 12 weeks was significantly improved among those taking O3-FAs compared to placebo (+ 0.98 vs. + 0.48, p = 0.05; Table 2), whereas there was no significant difference in those with BMI < 30 kg/m2 (p = 0.80). Global Ratings of Change in joint stiffness scores from baseline to 12 weeks were also significantly improved in patients with BMI ≥ 30 kg/m2 taking O3-FAs compared to placebo (+ 0.93 vs. + 0.30, p = 0.02), with no observed difference in those with BMI < 30 kg/m2 (p = 0.94). The interaction trended toward but did not achieve statistical significance (p = 0.10). M-SACRAH and WOMAC scores for pain were also significantly lower at 24 weeks among patients with BMI ≥ 30 kg/m2 taking O3-FAs compared to placebo but not significantly different between the treatment arms in those with BMI < 30 kg/m2 (Supplemental Table 1). No significant differences were observed with O3-FAs and placebo with quality of life (FACT-ES) and analgesic use based upon BMI.
Lipid profile data
Lipid profile data were available for 175 patients. At baseline, 52% of patients had cholesterol ≥ 200 mg/dL, 13% had HDL < 40 mg/dL, 32% had LDL ≥ 130 mg/dL, and 36% had triglycerides ≥ 150 mg/dL. There were no observed differences in patterns of intervention effects (O3-FAs vs. placebo) by BMI categories for either cholesterol or LDL levels (Table 3). Among patients with BMI < 30 kg/m2, patients taking O3-FAs had significantly higher HDL levels at 24 weeks compared to placebo (69.5 vs. 58.6, p = 0.002). In contrast, among patients with BMI ≥ 30 kg/m2, HDL levels at 24 weeks were similar by arm (50.6 vs. 51.0, p = 0.48). These differences by arm between BMI groups were statistically significant (interaction p = 0.003). Patients randomized to the O3-FA arm had lower triglyceride levels at 12 weeks than placebo arm patients in both BMI groups (BMI < 30 kg/m2, 101.4 vs. 145.3, p = 0.02; BMI ≥ 30 kg/m2, 131.2 vs. 157.3, p = 0.02; interaction p = 0.01). At 24 weeks, there was no significant difference in triglyceride levels between treatment arms among those with BMI < 30 kg/m2 (101.0 vs. 134.2, p = 0.24), while there was a trend toward significantly lower levels in the O3-FA arm compared to placebo in those with BMI ≥ 30 kg/m2 (141.8 vs. 159.3, p = 0.09; interaction p = 0.06).
Discussion
In this exploratory analysis of SWOG S0927, we found that among obese patients, BPI worst pain, average pain, and pain interference scores after 24 weeks of treatment were significantly lower among patients who received O3-FAs than those who received placebo, but this treatment effect was not seen among non-obese patients. Treatment with O3-FAs was also associated with a statistically significant improvement in Global Ratings of Change scores for both joint pain and joint stiffness at 12 weeks from baseline and improved joint-specific symptoms in the hands (M-SACRAH) and knees/hips (WOMAC) compared to placebo in obese patients, but not in non-obese patients.
There is no consensus on the best treatment for AI arthralgia. The most commonly used therapy is NSAIDs, although there are no formal data testing their efficacy for AI arthralgia, and they are associated with potentially severe adverse effects such as renal failure, gastrointestinal bleeding, and increased risk of cardiovascular events [7, 18,19,20]. Randomized controlled trials have shown benefits from exercise (aerobic and strength training exercises), acupuncture, and duloxetine compared to placebo for AI arthralgia [21,22,23]. Studies have also demonstrated improvement in symptoms over time even among patients receiving placebo, although no BMI effect has been reported [23, 24]. In our study, there was a considerable placebo effect among patients with BMI < 30 kg/m2.
The use of O3-FAs for joint pain and stiffness in rheumatoid arthritis has been extensively studied. A meta-analysis of rheumatoid arthritis patients found that 3 months of dietary fish oil supplementation was associated with significantly decreased number of tender joints and morning stiffness compared to controls [13]. Another meta-analysis of 17 randomized controlled trials including patients with rheumatoid arthritis or joint pain related to inflammatory bowel disease found that supplementation with O3-FAs for 3–4 months was associated with decreased intensity of joint pain and fewer number of painful joints; the effect size was greater in studies that lasted longer than 5 months [14]. In the present study, we found significantly lower pain scores at 24 weeks (6 months) and significant changes in BPI scores at 24 weeks from baseline in those taking O3-FAs but not at 12 weeks (3 months), suggesting that long-term use of O3-FAs may be necessary for patients to experience the effects on joint pain and stiffness. It has been shown that BPI worst pain scores of 4 or greater can predict discontinuation of AI use [9]. Although at 24 weeks, average BPI worst pain score among obese patients was above 4 in both the O3-FA and placebo groups, the average score among patients taking O3-FAs of 4.36 (near the threshold) compared to 5.70 with placebo suggests that over time, this subset of patients may be more likely to persist with AI therapy.
Obesity is a significant risk factor for osteoarthritis in post-menopausal women; two possible mechanisms include greater dynamic stress on joints caused by increased weight leading to cartilage disruption, and greater bone mass among obese patients which may increase bone stiffness and promote cartilage breakdown in joints [25, 26]. Systemic inflammation is also thought to play a role; obese individuals have higher levels of circulating leptin and pro-inflammatory cytokines which promote local joint inflammation and cartilage degradation [27]. Estrogen has protective effects in osteoarthritis and thus in states of estrogen depletion, cartilage turnover and surface erosion are accelerated [28]. The exact mechanism of AI arthralgia is unknown, but is thought to be mediated by both estrogen depletion and increased systemic inflammation; women taking AIs who report arthralgia have higher serum levels of inflammatory biomarkers [29]. Additionally, obese patients taking anastrozole have higher plasma concentrations of the drug than normal weight patients, suggesting that they may also be at increased risk for developing the adverse effect of AI arthralgia [30].
The mechanism by which O3-FAs may work for obese patients but not for non-obese patients is unclear. O3-FAs are thought to work through various mechanisms. Polyunsaturated fatty acid supplementation results in increased production of eicosanoids formed from eicosapentanoic acid and decreased production of eicosanoids formed from arachidonic acid [31]. The mediators that arise from eicosapentanoic acid are thought to be significantly less potent in inducing inflammatory mediators than those that arise from arachidonic acid. Polyunsaturated fatty acid consumption in the form of dietary fish oil is also associated with decreased leukocyte chemotaxis, production of reactive oxygen species, and production of pro-inflammatory cytokines [32]. Thus, O3-FA use decreases production of inflammatory mediators and, as a result, leads to less joint pain and stiffness in inflammatory conditions. Given that adipose tissue is a source of inflammatory cytokines in obese individuals and is thought to play a role in joint inflammation, the anti-inflammatory properties of O3-FAs may more selectively affect obese patients than their non-obese counterparts, resulting in significantly improved joint symptoms.
It is well known that O3-FA use decreases triglyceride levels [33]. Our results are consistent with known effects of O3-FAs on triglyceride levels, with lower levels at 12 weeks in both non-obese and obese patients, and at 24 weeks a trend toward lower triglyceride levels in those taking O3-FAs compared with placebo in the BMI ≥ 30 kg/m2 group. These findings also suggest medication compliance in the O3-FA treatment arm. O3-FAs are also known to have a positive effect on HDL levels, which was interestingly seen particularly in non-obese patients in our study [33]. While a recent meta-analysis did not demonstrate any significant cardiovascular benefit to O3-FA use, elevated triglyceride levels are associated with increased risk of cardiovascular disease although the extent of causality is a subject of controversy [34,35,36]. Further studies with increased power are certainly needed to clarify the interaction between treatment and BMI in effects on lipid profile components, but given that O3-FA use may result in symptomatic benefit in joint pain and inflammation for obese patients, the extra benefit of decreased triglyceride levels at 24 weeks may provide an additional incentive for O3-FA administration in these patients. On the other hand, since O3-FA use does not result in symptom improvement in non-obese patients, lower triglyceride levels with O3-FA use in these patients may not have clinical significance.
This study had numerous strengths. SWOG 0927 was a large multicenter randomized controlled trial and as such required uniform protocol-specific monitoring and therapy, and the patient-reported symptoms including pain scores were collected prospectively. The inclusion criteria were broad and required moderate to severe symptoms at baseline, so the results are generalizable to many patients with AI arthralgia. We also acknowledge a few limitations. The original study was not specifically designed to examine differences in pain scores by BMI, and the intervention was limited to 24 weeks. This was a post hoc secondary analysis and we did not account for multiple comparisons. However, the improvement in all patient-reported outcomes for joint symptoms with O3-FAs among obese women was consistent.
In conclusion, we found that joint symptoms decreased after 24 weeks of treatment with O3-FAs as compared with placebo in obese patients, but not in non-obese patients. These results were consistent among multiple measures. These data suggest that O3-FA use may reduce AI arthralgia and improve quality of life, which could lead to improved AI adherence in this subset of breast cancer patients.
References
Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366(9484):455–462
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M et al (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25(5):486–492
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369(9561):559–570
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Mouridsen HT (2006) Incidence and management of side effects associated with aromatase inhibitors in the adjuvant treatment of breast cancer in postmenopausal women. Curr Med Res Opin 22(8):1609–1621
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639
Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, Ebrahimi B, Yeon C, Howard F (2007) Aromatase inhibitor-associated arthralgia and/ or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer 7(10):775–778
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942
Chim K, Xie SX, Stricker CT, Li QS, Gross R, Farrar JT, DeMichele A, Mao JJ (2013) Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer 13:401
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25(25):3877–3883
Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, Buzdar AU, Group AT (2008) Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol 9(9):866–872
Mieog JS, Morden JP, Bliss JM, Coombes RC, van de Velde CJ, Committee IESS (2012) Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2–3 years of tamoxifen: a retrospective analysis of the Intergroup Exemestane Study. Lancet Oncol 13(4):420–432
Fortin PR, Lew RA, Liang MH, Wright EA, Beckett LA, Chalmers TC, Sperling RI (1995) Validation of a meta-analysis: the effects of fish oil in rheumatoid arthritis. J Clin Epidemiol 48(11):1379–1390
Goldberg RJ, Katz J (2007) A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 129(1–2):210–223
Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, Cole R, Logan A, Layman R, Ramaswamy B et al (2018) Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat 167(3):709–718
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, Greenlee H, Lew DL, Minasian LM, Till C et al (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol 33(17):1910–1917
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989 5(5):303–311; discussion 312–303
Griffin MR, Yared A, Ray WA (2000) Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol 151(5):488–496
Walt R, Katschinski B, Logan R, Ashley J, Langman M (1986) Rising frequency of ulcer perforation in elderly people in the United Kingdom. Lancet 1(8479):489–492
Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, Sorensen HT (2005) Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case–control study. Arch Intern Med 165(9):978–984
Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y et al (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 33(10):1104–1111
Crew KD, Capodice JL, Greenlee H, Brafman L, Fuentes D, Awad D, Yann Tsai W, Hershman DL (2010) Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol 28(7):1154–1160
Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, Sharer CW, Burton GV, Kuzma CS, Moseley A et al (2018) Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 36(4):326–332
Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ (2011) Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat 129(1):107–116
Wright NC, Riggs GK, Lisse JR, Chen Z, Women’s Health I (2008) Self-reported osteoarthritis, ethnicity, body mass index, and other associated risk factors in postmenopausal women-results from the Women’s Health Initiative. J Am Geriatr Soc 56(9):1736–1743
Cimmino MA, Parodi M (2004) Risk factors for osteoarthritis. Semin Arthritis Rheum 34(6):29–34
Sowers MR, Karvonen-Gutierrez CA (2010) The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 22(5):533–537
Hoegh-Andersen P, Tanko LB, Andersen TL, Lundberg CV, Mo JA, Heegaard AM, Delaisse JM, Christgau S (2004) Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther 6(2):R169–R180
Bauml J, Chen L, Chen J, Boyer J, Kalos M, Li SQ, DeMichele A, Mao JJ (2015) Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res (BCR) 17:89
Hubalek M, Oberguggenberger A, Beer B, Meraner V, Sztankay M, Oberacher H, Schubert B, Wildt L, Seeber B, Giesinger J et al (2014) Does obesity interfere with anastrozole treatment? Positive association between body mass index and anastrozole plasma levels. Clin Breast Cancer 14(4):291–296
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52(8):885–897
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83(6 Suppl):1505S–1519S
Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J (2006) Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189(1):19–30
Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P et al (2018) Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 3(3):225–234
Hokanson JE, Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 3(2):213–219
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM et al (2011) Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123(20):2292–2333
Acknowledgements
This study was supported by BCRF, NCI, and SWOG.
Funding
This study was funded by the Breast Cancer Research Foundation; National Cancer Institute, Division of Cancer Prevention, NCORP Research Base grant 1UG1CA189974-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, S., Unger, J.M., Crew, K.D. et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res Treat 172, 603–610 (2018). https://doi.org/10.1007/s10549-018-4946-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4946-0