Abstract
Transient receptor transient receptor potential vanilloid 1 (TRPV1) is a Ca2+-permeable channel gated by oxidative stress and capsaicin (CAP) and modulated by melatonin (MEL) and capsazepine (CPZ). A combination of doxorubicin (DOX) and MEL may offer a potential therapy for breast cancer by exerting antitumor and anti-apoptotic effects and modulating Ca2+ influx and TRPV1 activity. We aimed to investigate the effects of MEL and DOX on the oxidative toxicity of MCF-7 human breast cancer cells, in addition to the activity of the TRPV1 channel and apoptosis. The MCF-7 cells were divided into the following six treatment groups: control, incubated with MEL (0.3 mM), incubated with 0.5 μM DOX, incubated with 1 μM DOX, incubated with MEL + 0.5 μM DOX, or incubated with MEL + 1 μM DOX. The intracellular free Ca2+ concentration was higher in the DOX groups than in the control, and the concentration was decreased by MEL. The intracellular free Ca2+ concentration was further increased by treatment with the TRPV1 channel activator CAP (0.01 mM), and it was decreased by the CPZ (0.1 mM). The intracellular production of reactive oxygen species, mitochondrial membrane depolarization, apoptosis level, procaspase 9 and PARP activities, and caspase 3 and caspase 9 activities were higher in the DOX and MEL groups than in the control. Apoptosis and the activity of caspase 9 were further increased in the DOX plus MEL groups. Taken together, the findings indicate that MEL supported the effects of DOX by activation of TRPV1 and apoptosis, as well as by inducing MCF-7 cell death. As the apoptosis and caspase activity of cancer cells increase because of their elevated metabolism, MEL may be useful in supporting their apoptotic capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death, known as apoptosis, occurs in numerous biochemical and physiological pathways and involves the activation of a series of cytosolic cysteine proteases (Shi 2002). The activation of caspases by cytosolic cysteine proteases results in apoptosis (Shi 2002; Uğuz et al. 2009). The caspase enzyme mammalian family contains 14 enzymes. Of these, caspase 3, 8, and 9 have important roles in the induction of apoptotic cell death. The activities of caspase 3 are important in the Fas/CD95 and executioner caspase-activated pathways, respectively, and the activity of caspase 9 is important in the mitochondrial apoptotic pathway (Shi 2002; Espino et al. 2013). The cell-surface death receptor-dependent extrinsic pathway and the mitochondria-dependent intrinsic pathway are well-known apoptotic pathways (Shi 2002). In addition to these pathways, apoptosis is induced by endoplasmic reticulum oxidative stress, which is mainly caused by an increase in the intracellular free Ca2+ concentration ([Ca2+]c) (Uğuz et al. 2012). Recently, we observed that an increase in the free Ca2+ concentration-induced proliferative and cell injury effects in different cells through oxidative stress and the activation of cation channels (Nazıroğlu et al. 2012; Çiğ and Nazıroğlu 2015; Köse and Nazıroğlu 2015).
The intracellular [Ca2+]c attenuates a variety of basic cellular mechanisms, including cell proliferation and cell death (Nazıroğlu 2012; Kumar et al. 2014). The mechanisms contributing to the regulation of the cytosolic Ca2+ concentration include the entry of Ca2+ from outside of cells following the activation of cation channels. The transient receptor potential (TRP) family is an important nonselective cation channel (Nazıroğlu 2007, 2012). TRPV1 is a nonselective cation channel, and it is a member of the TRP family that was first reported in sensory neurons (Caterina et al. 1997; Susankova et al. 2006). TRPV1 responds to various stimuli, including oxidative stress and capsaicin (CAP) (Caterina et al. 1997; Susankova et al. 2006). The presence of TRPV1 channels with expressions were reported in cancer cells, including MCF-7 breast cancer cells (Vercelli et al. 2014).
Melatonin (MEL) is a strong antioxidant, which is synthesized by the pineal gland (Ekmekcioglu 2006; Reiter et al. 2000). Many studies have reported the anti-apoptotic role of MEL in the inhibition or stimulation of oxidative stress in healthy cells. However, there are conflicting reports on the role of MEL in cancer cells (Dziegiel et al. 2008; Radogna et al. 2009; Bejarano et al. 2011; Nemeth et al. 2011; Uğuz et al. 2012). Some recent studies have reported that MEL modulated apoptosis, oxidative stress, mitochondrial membrane depolarization, and the intracellular [Ca2+]c in TRP channels of healthy neurons and cells (Reuss et al. 2010; Nazıroğlu et al. 2012; Celik and Nazıroğlu 2012; Gupta and Sharma 2014; Yürüker et al. 2015). It is possible that MEL may exert the same effects in MCF-7 cancer cells. Doxorubicin (DOX) is one of the most potent and frequently used chemotherapeutic agents in modern oncology (Sag et al. 2011). It is used to treat various types of cancer, such as breast, ovary, and lung (Sag et al. 2011). Reactive oxygen species (ROS) play an important role in the pathogenesis of DOX-related cell injury because they affect noncancerous cell functions by directly acting on cellular components, such as DNA and lipids, destroying their structure (Koçkar et al. 2010; Uguz et al. 2012). In cells, MEL protects against DOX-induced ROS production through different redox systems, including those involving mitochondrial oxidation or NADPH oxidase (Uğuz et al. 2012; Sag et al. 2013; González-Flores et al. 2014). TRPV1 channels are activated by oxidative stress. Hence, DOX-induced overload of intracellular [Ca2+]c via activation of TRPV1 channels may induce apoptosis and excessive ROS production through mitochondrial membrane depolarization. However, no study has examined the relationship of ROS production with MEL- and DOX-related apoptosis and oxidative stress in the MCF-7 breast cancer cell line.
A study of the impact of MEL on cellular survival and death may help clarify how ROS formation and apoptosis occur following DOX-induced injury. With the aim of developing a new adjuvant therapy for breast cancer, the present study examined the effects of DOX on oxidative damage of breast cancer cells, apoptosis, and ROS production, as well as the possible protective effects of MEL against these changes. We analyzed apoptosis, caspase activities, mitochondrial membrane depolarization, cytosolic ROS production, and oxidative stress induced by the intracellular [Ca2+]c.
Materials and Methods
Cells and Reagents
The Michigan Cancer Foundation-7 breast cancer cell line (MCF-7) was used in this study. The cell line was originally obtained from ‘The Leibniz Institute- German Collection of Microorganisms and Cell Cultures’ Cell Lines Bank (Braunschweig, Germany). Ethylene glycol-bis(2-aminoethyl-ether)-N,N,N′,N′-tetraacetic acid (EGTA), and dimethyl sulfoxide (DMSO) and Roswell Park Memorial Institute (RPMI) 1640 medium were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). Fura-2/AM was obtained from Calbiochem (Darmstadt, Germany). Dihydrorhodamine-123 (DHR 123) and Tris–glycine gels were purchased from Molecular Probes (Eugene, OR, USA). Caspase substrates [N-acetyl-Leu-Glu- His-Asp-7-amino-4-methylcoumarin (AC-LEHD-AMC) and N-acetyl-Leu-Glu-His-Asp-7-amino-4-methylcoumarin (AC-LEHD-AMC)] were purchased from Bachem (Bubendorf, Switzerland). Capsazepine (CPZ) and CAP were purchased from (Santa Cruz Inc, İstanbul, Turkey). A mitochondrial stain 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) was purchased from Santa Cruz (Dallas, TX, USA). All organic solvents were also purchased from Santa Cruz (Dallas, TX, USA). The reagents were equilibrated at room temperature for 30 min before an analysis.
Cell Culture
The MCF-7 cells were cultured in RPMI 1640 medium supplemented with fetal bovine serum in a humidified incubator at 37 °C, 5 % CO2, and 95 % air. The cells were counted daily by removing a small volume from the tissue culture flask (filter cap, sterile, 250 ml, 75 cm2), diluting it with an equal volume of trypan blue (0.4 %), and tallying viable cells (trypan blue excluding) with a cell counter (Casy Model TT, Roche, Germany). Cultures were maintained as a suspension without shaking or stirring at a density of 1 × 106 cells per ml by dilution with fresh media. Cultures were transferred once a week.
Groups
Cells were seeded in 8–10 flasks at a density of 1 × 106 cells per flask (filter cap, sterile, 250 ml, 75 cm2). All cells were cultured in the same culture medium (37 °C) and for an identical time (1 h). The cells were divided into six main groups:
-
Group 1 (control group) The cells were not incubated with DOX, CPZ, and MEL but were kept in flask containing the same cell culture medium and conditions for 24 h.
-
Group 2 (MEL group) Cells in the group were incubated with MEL (0.3 mM) for periods of 2 h (Celik and Nazıroğlu 2012).
-
Group 3 (0.5 μM DOX group) Cells in the group were incubated with 0.5 μM DOX for periods of 24 h (Inoue et al. 2014).
-
Group 4 (1 μM DOX group) Cells in the group were incubated with 1 μM DOX for periods of 24 h (Inoue et al. 2014).
-
Group 5 (MEL + 0.5 μM DOX group) Cells in the group were pre-incubated with MEL (0.3 mM) for periods of 2 h, and then they were incubated with 0.5 μM DOX for periods of 24 h.
-
Group 6 (MEL + 1 μM DOX group) Cells in the group were pre-incubated with MEL (0.3 mM) for periods of 2 h, and then they were incubated with 1 μM DOX for periods of 24 h.
For the intracellular [Ca2+]c assays, the cells were further treated with CAP (0.01 mM) for activation of TRPV1 channel and they were also inhibited the TRPV1 blocker CPZ (0.1 mM). The CAP and CPZ were dissolved in DMSO, and the pH adjustment was performed with HCl.
All the exposures at the different distances were repeated 4–6 times. At the end of the 1-h incubation, the control and treated cells were used for the analyses of intracellular [Ca2+]c, cell viability, immunohistochemistry, caspase, and intracellular reactive ROS assays.
Intracellular Free Calcium Ion Concentration ([Ca2+]c) Analysis
Fura-2/AM (4 μM) fluorescence was utilized to determine intracellular [Ca2+]c concentrations. The MCF-7 cells were loaded with the fura-2/AM in loading buffer with 1 × 106 cells per ml for 45 min at 37 °C in the dark, washed twice with phosphate buffer and then incubated for an additional 30 min at 37 °C to complete probe de-esterification, and re-suspended in loading buffer at a density of 1 × 106 cells per ml according to a procedure published elsewhere (Çiğ and Nazıroğlu 2015). All groups were immediately exposed to CAP (CAP and 0.1 mM) for stimulation of Ca2+ influx. Fluorescence in the six groups was recorded from 2-ml aliquots of a magnetically stirred cell suspension at 37 °C by a spectrofluorometer (Carry Eclipsys; Varian Inc, Sydney, Australia) with excitation wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. Changes in intracellular [Ca2+]c were monitored by the fura-2 340/380 nm fluorescence ratio, and they were calibrated according to the method of Grynkiewicz et al. (1985). We performed a total of 6 experiments (n = 6) to measure the intracellular [Ca2+]c. The data were expressed in terms of the fold increase, relative to the control, after the pretreatment level.
The release of Ca2+ was estimated using the integral of the rise in intracellular [Ca2+]c for 100 s after addition of CAP (Köse and Nazıroğlu 2015; Özdemir et al. 2015). The release is reported as nanomolar concentration (nM) with sampling at 10-s intervals, as previously described (Heemskerk et al. 1997). All experiments were carried out at 37 °C.
Cell Viability (MTT) Assay
To assess MEL protective effects on cell viability, we evaluated the mitochondrial activity of living cells by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) quantitative colorimetric assay. For these assays, distance differentiated the breast cancer cells that were cultured in 96-well culture plates before DOX and MEL incubations. The optical density in each well was evaluated by measurement of absorbance at 490 and 650 nm using a microplate reader (Infinite pro200; Tecan Austria GmbH, Groedig, Austria) (Yürüker et al. 2015). We performed a total of 6 cell viability experiments (n = 6) were performed in the current study. The data are presented as fold increase over the pretreatment level (experimental/control).
Assay for Apoptosis, Caspase 3 and 9 Activities
The apoptosis assay was performed with a commercial kit according to the instructions provided by Biocolor Ltd. (Northern Ireland) and elsewhere (Özdemir et al. 2015). The APO Percentage assay is a dye-uptake assay, which stains only the apoptotic cells with a red dye. When the membrane of an apoptotic cell loses its asymmetry, the APO Percentage dye is actively transported into cells, staining apoptotic cells red, thus allowing detection of apoptosis by spectrophotometry.
The determination of caspase 3 and caspase 9 activities was based on a method previously reported (Uğuz et al. 2012; Özdemir et al. 2015) with minor modifications. Caspase 3 substrate (AC-DEVD-AMC) and caspase 9 substrate (AC-LEHD-AMC) cleavages were measured with the microplate reader (Infinite pro200) with excitation wavelength of 360 nm and emission at 460 nm. The data were calculated as fluorescence units/mg protein and presented as fold increase over the pretreatment level (experimental/control).
Intracellular Reactive Oxygen Species (ROS) Measurement
DHR 123 is a nonfluorescent, noncharged dye that easily penetrates cell membrane. Once inside the cell, DHR 123 becomes fluorescent upon oxidation to yield rhodamine 123 (Rh 123), the fluorescence being proportional to ROS generation. The fluorescence intensity of Rh123 was measured in the microplate reader (Infinite Pro200). Excitation was set at 488 nm and emission at 543 nm (Espino et al. 2010). We performed a total of 6 experiments (n = 6) for the intracellular ROS assay. The data were presented as fold increase over the pretreatment level (experimental/control).
Mitochondrial Membrane Potential Determination
Cells were incubated with 1 μM JC-1 for 15 min at 37 °C as previously described (Uğuz et al. 2012; Özdemir et al. 2015). The cationic dye, JC-1, exhibits potential-dependent accumulation in the mitochondria. It indicates mitochondria depolarization by a decrease in the red to green fluorescence intensity ratio. The green JC-1 signal was measured at the excitation wavelength of 485 nm and the emission wavelength of 535 nm, the red signal at the excitation wavelength of 540 nm, and the emission wavelength of 590 nm. Fluorescence changes were analyzed using the in the microplate reader (Infinite Pro200). The data are presented as fold increase over the pretreatment level (experimental/control).
Western Blot Analyses
All of the Western Blotting analyses were performed using the standard procedures. To detect β-actin, PARP, and caspase 3 and caspase 9 protein expression, the frozen cells were homogenized in lysis buffer; the supernatant was removed and conserved after centrifuge at 16,000×g, 20 min. The total protein was assessed using Bradford reagent at 595 nm. For immunoblotting, equal amounts of proteins (30 μg from each sample) were loaded into 12 % sodium dodecyl sulfate-polyacrylamide gel, and after electrophoresis, they were transferred to a nitrocellulose membrane. The blot was blocked for 1 h at room temperature with 5 % nonfat dry milk in tris buffered saline with 0.1 % Tween 20. Afterwards, the membrane was incubated with primary antibodies (Rabbit mAb caspase 3, 1:1000; Mouse mAb caspase-9, 1:1000; mouse monoclonal β-actin, 1:1000, Cell Signaling Technology, USA) and a secondary antibody (Anti-mouse IgG and Anti-rabbit IgG antibodies conjugated with horseradish peroxidase, 1:5000, GE Healthcare, Amersham, UK). Bands were visualized using ECL Western HRP Substrate (Millipore Luminate Forte, USA), and visualization was achieved through Syngene G:Box Gel Imagination System (UK) and normalized against β-actin protein. The data are presented as relative density (fold increase) over the pretreatment level (experimental/control).
Statistical Analyses
Data were analyzed using the SPSS statistical program (version 17.0, software, SPSS. Chicago, IL, USA). All results are expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) and an unpaired Mann–Whitney U test were performed p values of <0.01 were regarded as significant. Significant values were assessed with the least significance difference test.
Results
Effects of DOX and Melatonin on the Intracellular Free Ca2+ Concentration
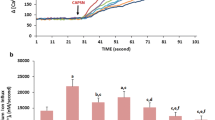
Figures 1 and 2 show the results of the effects of MEL and DOX on the intracellular [Ca2+]c in the MCF-7 cells. MCF-7 cells contain a CAP-sensitive TRPV1 ion channel (Vercelli et al. 2014). Compared with the control, the MCF-7 cells in the 0.5 and 1 μM DOX groups were more sensitive to Ca2+ entry. The intracellular [Ca2+]c was significantly (p < 0.001) higher in the 0.5 and 1 μM DOX groups compared to the control and MEL groups. However, the pretreatment with MEL induced TRPV1 channel inhibitor effects. Thus, the intracellular [Ca2+]c was significantly (p < 0.001) lower in the MEL + 0.5 DOX and MEL + 1 μM DOX groups than in the 0.5 DOX and 1 μM DOX groups. The intracellular [Ca2+]c was also significantly (p < 0.05) higher in and 1 μM DOX groups than in the 0.5 DOX with and without MEL groups.
In vitro effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on the intracellular free Ca2+ concentration ([Ca2+]c) in MCF-7 cells (n = 3 and mean ± SD). The cells were stimulated with capsaicin (CAP) 0.1 mM for 10 min and inhibited with capsazepine (CPZ) 0.1 mM for 30 min
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on the free intracellular Ca2+ concentration ([Ca2+]c) in MCF-7 cells incubated with DOX (n = 3 and mean ± SD). The cells were stimulated with capsaicin (CAP) 0.1 mM for 10 min and inhibited with capsazepine (CPZ) 0.1 mM for 30 min. a p < 0.001, b p < 0.01, and c p < 0.05 versus the control. d p < 0.05 and e p < 0.001 versus the 0.5 µM DOX group. f p < 0.05 and g p < 0.001 versus the 1 µM DOX group. h p < 0.05 versus the MEL + 0.5 µM DOX and MEL + 1 µM DOX groups
Effects of CPZ on the Intracellular [Ca2+]c
CPZ is a specific TRPV1 channel antagonist (Susankova et al. 2006). To clarify the TRPV1 channel activity-dependent Ca2+ entry in the DOX and MEL groups, some of the cells were pre-incubated with CPZ before incubation with DOX and MEL. CPZ reduced the intracellular [Ca2+]c, and it was significantly (p < 0.001) lower in the MEL + 0.5 DOX + CPZ and MEL + 1 μM DOX + CPZ groups than in the MEL + 0.5 DOX and MEL + 1 μM DOX groups.
Cell Viability (MTT)
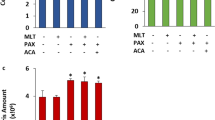
The mean MTT values in the control, MEL, 0.5 μM DOX, 1 μM DOX, MEL + 0.5 μM DOX, and MEL + 1 μM DOX groups are shown in Fig. 3. The MTT values were significantly (p < 0.05) lower in the 0.5 μM DOX, 1 μM DOX, MEL + 0.5 μM DOX, and MEL + 1 μM DOX groups than in the control group and MEL-only group. The addition of MEL did not further decrease the cell viability.
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on the levels of cell viability (MTT) in MCF-7 breast cancer cells. The values are presented as the mean ± SD of six separate experiments and expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 versus the control and MEL groups
Effects of DOX and MEL on Apoptosis, Caspase 3, and Caspase 9
We investigated the protective effects of MEL against the rate of programmed cell death, as indicated by caspase values in the DOX-incubated cancer cells. The rate of apoptosis, caspase 3 activity, and caspase 9 activity in the six groups are shown in Figs. 4, 5, and 6, respectively. The apoptosis (p < 0.05 and p < 0.01), caspase 3 (p < 0.05), and caspase 9 (p < 0.05 and p < 0.01) values in the 0.5 μM DOX, 1 μM DOX, MEL + 0.5 μM DOX, and MEL + 1 μM DOX groups were significantly higher than in the control and MEL groups. Furthermore, the apoptosis and caspase 9 values in the MEL + 0.5 μM DOX and MEL + 1 μM DOX groups were significantly higher (p < 0.05) compared to those in the 0.5 μM DOX and 1 μM DOX groups.
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on the level of apoptosis in MCF-7 breast cancer cells (mean ± SD and n = 6). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 and b p < 0.01 versus the control and MEL groups, respectively. c p < 0.05 versus the 0.5 μM DOX group. d p < 0.05 versus the 1 μM DOX group
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on caspase 3 activity in MCF-7 breast cancer cells (mean ± SD and n = 6). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 versus the control and MEL groups
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on caspase 9 activity in MCF-7 breast cancer cells (mean ± SD and n = 6). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 and b p < 0.01 versus the control and MEL groups, respectively. c p < 0.05 versus the 0.5 μM DOX group. d p < 0.05 versus the 1 μM DOX group
Intracellular ROS Production and Mitochondrial Depolarization (JC-1) Values
Some reports have indicated that MEL increased the production of excessive amounts of intracellular ROS in tumor cells (Bejarano et al. 2011; Uğuz et al. 2012). To determine whether the potentiating effect of MEL was due to modulation of the production of intracellular ROS, oxidation-sensitive intracellular probes loaded with DHR123 were treated with MEL and three chemotherapeutic agents, both separately and together, for 24 h.
The mean cytosolic ROS production and mitochondrial depolarization (JC-1 level) in the six groups are shown in Figs. 7 and 8, respectively. The results showed that the levels of cytosolic ROS production and JC-1 were markedly (p < 0.05) higher in the 0.5 μM DOX and 1 μM DOX groups than in the MEL and control groups. Pretreatment with MEL did not increase the production of ROS or the level of JC-1 in the MEL + 0.5 μM DOX and MEL + 1 μM DOX groups.
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on intracellular reactive oxygen species (ROS) production in MCF-7 breast cancer cells (mean ± SD and n = 6). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 and b p < 0.01 versus the control and MEL groups, respectively. c p < 0.05 versus the 0.5 μM DOX group. d p < 0.05 versus the 1 μM DOX group
Effect of melatonin treatment (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) and (0.5 and 1 μM for 24 h) on the mitochondrial membrane depolarization (JC-1) level in MCF-7 cells (mean ± SD and n = 6). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 versus the control and MEL groups
Procaspase 3 and Procaspase 9 Results
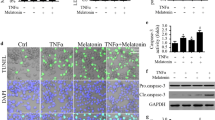
Caspase 9 activity is important in the mitochondrial apoptotic pathway, and caspase 3 activity has an important role in executioner caspase-activated pathways (Shi 2002; Espino et al. 2013). Upon apoptotic stimulation, cytochrome c released from mitochondria was associated with procaspase 3 and 9/Apaf 1 (Liu et al. 1996). Procaspase further processes other caspase members, including caspase 3, to initiate caspase cascade (Liu et al. 1996; Li et al. 1997). In other words, procaspase 3 and 9 are reduced by apoptotic process because they are converted to caspase 3 and 9 in the apoptotic processes. We assayed procaspase 3, procaspase 9, and cleaved caspase 3 and 9 as indicators of apoptosis (Fig. 9). The activities of cleaved caspase 9 were significantly (p < 0.05) higher in the 0.5 μM DOX, 1 μM DOX, MEL + 0.5 μM DOX, and MEL + 1 μM DOX groups than in the control and MEL groups.
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on procaspase 3 and procaspase 9 activities in MCF-7 cells (mean ± SD and n = 3). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 versus groups 1 and 2
Poly (ADP-Ribose) Polymerase (PARP) Results
Poly (ADP-ribose) polymerase (PARP) is an abundant enzyme in cells that indicators and signals DNA damage to repair mechanisms. It is activated in response to single-strand DNA breaks and subsequently attaches to regions of injured DNA (Nazıroğlu 2007). In addition to caspase activities, we also assayed PARP activities as indicators of apoptosis (Fig. 10). The activities of PARP were significantly (p < 0.05) higher in the 0.5 μM DOX, 1 μM DOX, MEL + 0.5 μM DOX, and MEL + 1 μM DOX groups as compared to the control and MEL groups.
Effect of treatment with melatonin (MEL) (0.3 mM for 2 h) and doxorubicin (DOX) (0.5 and 1 μM for 24 h) on poly (ADP-ribose) polymerase (PARP) activity in MCF-7 cells (mean ± SD and n = 3). The values are expressed as a fold increase over the pretreatment level (experimental/control). a p < 0.05 versus groups 1 and 2
Discussion
The results of the current study indicate that a combination of MEL and DOX enhances apoptotic effects when compared with MEL or DOX alone. These results suggest that chemotherapy, combined with MEL treatment, may increase the therapeutic effect of anticancer drugs. In the present study, MEL exerted a synergetic effect with DOX, increasing the apoptosis of MCF-7 cancer cells. To our knowledge, this is the first study to report the relationship between MEL, the TRPV1 channel, and apoptosis in MCF-7 cells. The results of this study indicated that that MEL may have potential as a supplementary drug in the treatment of MCF-7 cells.
Apoptosis, also known as programmed death, is mediated by specific proteinases, namely caspases. Apoptosis is initiated by two molecular pathways: extrinsic and intrinsic (González-Flores et al. 2014). The intrinsic pathway is activated by stress and involves mitochondrial-mediated caspase 9 activation (Shi, 2002). The antitumor and apoptotic effects of MEL are an ongoing area of study. Recent studies have shown that it is a potent therapeutic agent, when used in combination with other drugs, with some studies supporting its efficacy as a conventional anticancer agent, with the ability to reduce their side effects (Uğuz et al. 2012; Govender et al. 2014; Ekmekcioglu 2014). In the current study, the results of a Western blot and caspase analyses indicated that the synergism was correlated with the induction of apoptosis in the MCF-7 cells. DOX-induced apoptosis and caspase 9 activity in the cells was further augmented by MEL. The MTT assays and apoptosis, PARP, caspase 3, and caspase 9 analyses demonstrated that pretreatment with MEL supported DOX-induced breast cancer cell toxicity, as well as decreasing the number of viable cells. These data suggest that MEL can be used in combination with DOX to increase the apoptosis of MCF-7 cancer cells undergoing mitochondrial depolarization. Reports that the intracellular [Ca2+]c is increased in breast cancer cells via the activation of TRP cation channels, such as TRPV1 and TRPM2, and the role of calcium signaling in proliferation and apoptosis have led researchers to examine the role of calcium channel inhibitors as potential therapies for some cancers (Sung et al. 2012; Chen et al. 2014; Wu et al. 2014). Some studies have proposed the use of TRPV1 channel activators to stimulate the death of cancer cells (Çiğ and Nazıroğlu 2015). However, the risk of these activators promoting cancer cell proliferation and the importance of the intracellular [Ca2+]c are unclear (Wu et al. 2014). The depolarization activity of mitochondria depends on the intracellular [Ca2+]c, which is fueled by Ca2+ entry from the extracellular space via TRP channels in response to cancer cell proliferation (González-Flores et al. 2014; Chen et al. 2014). Some cation channels, such as TRPM2 and voltage-gated Ca2+ channels in cancer cells, are gated by electromagnetic radiation-induced ROS production (Sag et al. 2013; Çiğ and Nazıroğlu 2015). Studies have demonstrated that the repeated activation of TRPV1 increased the intracellular [Ca2+]c, oxidative stress, and apoptotic cell injury (Özdemir et al. 2015; Çiğ and Nazıroğlu 2015). Another study found that CAP-induced activation of TRPV1 in cancer cells substantially increased following mitochondrial oxidative stress (Ip et al. 2012). A recent study reported that the activation of CAP and TRPV1 channels induced apoptosis and oxidative stress in colorectal cancer cells and that CPZ, a TRPV1 antagonist, potentiated these effects (Sung et al. 2012). Further, Wu et al. (2014) reported that CAP-induced necrotic cell death in MCF-7 cells was associated with up-regulation of c-Fos.
No previous studies examined the effects of MEL on DOX-induced mitochondrial depolarization, oxidative stress, and apoptosis via the activation of the TRPV1 channel in cancer cells. In the current study, we found that DOX increased the intracellular [Ca2+]c in the MCF-7 cells and that MEL prevented the death of cultured MCF-7 cells exposed to DOX. MEL also enhanced DOX-induced increases in caspase 9 activity and apoptosis. González-Flores et al. (2014) reported that the antitumor effects of TRPV1 channel activators, vanilloids, and in oral squamous cell carcinoma were independent of TRPV1 activation but most likely due to ROS induction and subsequent apoptosis. Their findings on ROS production are similar to those of the present study.
It is well known that DOX generates free radicals primarily in two ways: by the NADPH-mitochondria pathway and forming complexes with iron (González-Flores et al. 2014). The exposure of mitochondria to DOX seems to initiate a disastrous cascade of events, leading to ROS generation, which subsequently induces the inhibition of the mitochondrial complex and generates excessive ROS production (Sag et al. 2011; Gilliam et al. 2013). The excessive production of ROS radicals induces further oxidation of mitochondrial membranes and DNA damage, which can impair important components of the mitochondrial apparatus, such as the mitochondrial respiratory chain (Nazıroğlu et al. 2004; Reiter et al. 2000). The latter it leads to more oxidative stress, generating a vicious cycle, which culminates in cell death (Gilliam et al. 2013). In the current study, the intracellular production of ROS increased in the 0.5 μM DOX and 1 μM DOX groups due to increased mitochondrial membrane depolarization. In healthy cells, MEL supports the antioxidant defense system of cells and the activities of various cellular antioxidants (Ekmekcioglu 2006; Reiter et al. 2000). Reports of the effect of MEL on DOX-induced toxicity are conflicting, with some reporting that it suppressed DOX-induced mitochondrial depolarization and excessive ROS production (Dziegiel et al. 2008; Song et al. 2012). However, others reported that MEL increased intracellular ROS production in tumor cells (Radogna et al. 2009; Bejarano et al. 2011; Uğuz et al. 2012). In the current study, MEL did not alter the level of mitochondrial depolarization or intracellular ROS production in the MCF-7 cells.
In conclusion, the results of the present study indicate that MEL, in combination with DOX, exerts an apoptotic effect in MCF-7 breast cancer cells and that this effect may be related to down-regulation of Ca2+ entry and up-regulation of apoptosis and activation of caspase 9. The findings suggest that MEL has an inhibitory effect on the activity of the TRPV1 channel, consistent with our hypothesis that down-regulation of the activity of the TRPV1 channel by DOX exposure is responsible for apoptosis and oxidative stress in MCF-7 breast cancer cells. It seems also that MEL has not a risk on the effectiveness of DOX in the cancer cells. Therefore, when used in combination with DOX, MEL may a useful chemotherapeutic agent to improve the therapeutic effect of chemotherapy in MCF-7 breast cancer cells. MEL may be an adjuvant drug in breast cancer treatment in the future.
Abbreviations
- [Ca2+]c :
-
Intracellular free Ca2+ concentration
- CAP:
-
Capsaicin
- CPZ:
-
Capsazepine
- DMSO:
-
Dimethyl sulfoxide
- DOX:
-
Doxorubicin
- EGTA:
-
Ethylene glycol-bis[2-aminoethyl-ether]-N,N,N,N-tetraacetic acid
- JC-1,1,1′,3,3′:
-
Tetraethylbenzimidazolylcarbocyanine iodide
- PARP:
-
Poly (ADP-ribose) polymerase
- ROS:
-
Reactive oxygen species
- TRP:
-
Transient receptor potential
- TRPM2:
-
Transient receptor potential melastatin 2
- TRPV1:
-
Transient receptor potential vanilloid 1
References
Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2011) Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol 108:14–20
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Celik O, Nazıroğlu M (2012) Melatonin modulates apoptosis and TRPM2 channels in transfected cells activated by oxidative stress. Physiol Behav 107:458–465
Chen SJ, Hoffman NE, Shanmughapriya S, Bao L, Keefer K, Conrad K, Merali S, Takahashi Y, Abraham T, Hirschler-Laszkiewicz I, Wang J, Zhang XQ, Song J, Barrero C, Shi Y, Kawasawa YI, Bayerl M, Sun T, Barbour M, Wang HG, Madesh M, Cheung JY, Miller BA (2014) A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem 289:36284–36302
Çiğ B, Nazıroğlu M (2015) Investigation of the effects of distance from sources on apoptosis, oxidative stress and cytosolic calcium accumulation via TRPV1 channels induced by mobile phones and Wi-Fi in breast cancer cells. Biochim Biophys Acta 1848:2756–2765
Dziegiel P, Podhorska-Okolow M, Zabel M (2008) Melatonin: adjuvant therapy of malignant tumors. Med Sci Monit 14:RA64–RA70
Ekmekcioglu C (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 60:97–108
Ekmekcioglu C (2014) Expression and putative functions of melatonin receptors in malignant cells and tissues. Wien Med Wochenschr 164:472–478
Espino J, Bejarano I, Redondo PC, Rosado JA, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2010) Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: evidence for the involvement of mitochondria and Bax activation. J Membr Biol 233:105–118
Espino J, Rodríguez AB, Pariente JA (2013) The inhibition of TNF-α-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J Pineal Res 54:442–452
Gilliam LA, Fisher-Wellman KH, Lin CT, Maples JM, Cathey BL, Neufer PD (2013) The anticancer agent doxorubicin disrupts mitochondrial energy metabolism and redox balance in skeletal muscle. Free Radic Biol Med 65:988–996
González-Flores D, Rodríguez AB, Pariente JA (2014) TNFα-induced apoptosis in human myeloid cell lines HL-60 and K562 is dependent of intracellular ROS generation. Mol Cell Biochem 390:281–287
Govender J, Loos B, Marais E, Engelbrecht AM (2014) Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin. J Pineal Res 57:367–380
Grynkiewicz C, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gupta S, Sharma B (2014) Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol Biochem Behav 122:122–135
Heemskerk JW, Feijge MA, Henneman L, Rosing J, Hemker HC (1997) The Ca2+-mobilizing potency of alpha-thrombin and thrombin receptor-activating peptide on human platelets concentration and time effects of thrombin-induced Ca2+ signalling. Eur J Biochem 249:547–555
Inoue S, Setoyama Y, Odaka A (2014) Doxorubicin treatment induces tumor cell death followed by immunomodulation in a murine neuroblastoma model. Exp Ther Med 7:703–708
Ip SW, Lan SH, Lu HF, Huang AC, Yang JS, Lin JP, Huang HY, Lien JC, Ho CC, Chiu CF, Wood W, Chung JG (2012) Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum Exp Toxicol 31(6):539–549
Koçkar MC, Nazıroğlu M, Celik O, Tola HT, Bayram D, Koyu A (2010) N-acetylcysteine modulates doxorubicin-induced oxidative stress and antioxidant vitamin concentrations in liver of rats. Cell Biochem Funct 28(8):673–677
Köse SA, Nazıroğlu M (2015) N-acetyl cysteine reduces oxidative toxicity, apoptosis, and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Free Radic Res 49:338–346
Kumar VS, Gopalakrishnan A, Nazıroğlu M, Rajanikant GK (2014) Calcium ion: the key player in cerebral ischemia. Curr Med Chem 21:2065–2075
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Nazıroğlu M, Karaoğlu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Nazıroğlu M, Çelik Ö, Özgül C, Çiğ B, Doğan S, Bal R, Gümral N, Rodríguez AB, Pariente JA (2012) Melatonin modulates wireless (2.45 GHz)-induced oxidative injury through TRPM2 and voltage gated Ca(2+) channels in brain and dorsal root ganglion in rat. Physiol Behav 105:683–692
Nemeth C, Humpeler S, Kallay E, Mesteri I, Svoboda M, Rögelsperger O, Klammer N, Thalhammer T, Ekmekcioglu C (2011) Decreased expression of the melatonin receptor 1 in human colorectal adenocarcinomas. J Biol Regul Homeost Agents 25:531–542
Özdemir ÜS, Nazıroğlu M, Şenol N, Ghazizadeh V (2015) Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 23:1–12
Radogna F, Paternoster L, De Nicola M, Cerella C, Ammendola S, Bedini A, Tarzia G, Aquilano K, Ciriolo M, Ghibelli L (2009) Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol 239:37–45
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress: a review. J Biomed Sci 7:444–458
Reuss S, Disque-Kaiser U, Binzen U, Greffrath W, Peschke E (2010) ‘TRPing’ synaptic ribbon function in the rat pineal gland: neuroendocrine regulation involves the capsaicin receptor TRPV1. Neuroendocrinology 92:133–142
Sag CM, Köhler AC, Anderson ME, Backs J, Maier LS (2011) CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol 51:749–759
Sag CM, Wagner S, Maier LS (2013) Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med 63:338–349
Shi Y (2002) Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 2002(9):459–470
Song N, Kim AJ, Kim HJ, Jee HJ, Kim M, Yoo YH, Yun J (2012) Melatonin suppresses doxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J Pineal Res 53:335–343
Sung B, Prasad S, Ravindran J, Yadav VR, Aggarwal BB (2012) Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic Biol Med 53(10):1977–1987
Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V (2006) Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol 70:383–394
Uğuz AC, Nazıroğlu M, Espino J, Bejarano I, Gonzalez D, Rodriguez AB, Pariente JA (2009) Selenium modulates oxidative stress induced cell apoptosis in human myeloid HL-60 cells via regulation of caspase-3, -9 and calcium influx. J Membr Biol 232:15–23
Uğuz AC, Cig B, Espino J, Bejarano I, Nazıroğlu M, Rodríguez AB, Pariente JA (2012) Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res 53:91–98
Vercelli C, Barbero R, Cuniberti B, Racca S, Abbadessa G, Piccione F, Re G (2014) Transient receptor potential vanilloid 1 expression and functionality in mcf-7 cells: a preliminary investigation. J Breast Cancer 17:332–338
Wu TT, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR (2014) Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium 56(2):59–67
Yürüker V, Nazıroğlu M, Şenol N (2015) Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: possible involvement of TRPM2 channels. Metab Brain Dis 30:223–231
Acknowledgments
MN formulated the present hypothesis and was responsible for writing the report. BÇ, ISÖ, and PAK were responsible for the analysis of the data.
Financial Support
This study received no financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Koşar, P.A., Nazıroğlu, M., Övey, İ.S. et al. Synergic Effects of Doxorubicin and Melatonin on Apoptosis and Mitochondrial Oxidative Stress in MCF-7 Breast Cancer Cells: Involvement of TRPV1 Channels. J Membrane Biol 249, 129–140 (2016). https://doi.org/10.1007/s00232-015-9855-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9855-0