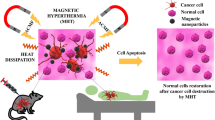
Abstract
Cancer stem cells (CSCs) have been identified as the main center of tumor therapeutic resistance. They are highly resistant against current cancer therapy approaches particularly radiation therapy (RT). Recently, a wide spectrum of physical methods has been proposed to treat CSCs, including high energetic particles, hyperthermia (HT), nanoparticles (NPs) and combination of these approaches. In this review article, the importance and benefits of the physical CSCs therapy methods such as nanomaterial-based heat treatments and particle therapy will be highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the main challenging disease worldwide. From statistical point of view, cancer is rapidly becoming a leading cause of death and disability in many countries. By advances in biological and molecular techniques, a great progress has been made in cancer research from diagnosis to treatment [1, 2].
Radiotherapy is one of the most feasible approaches to treat cancer. More than 50% of all cancer patients receive radiation in their treatment course. In recent years, radiotherapy has undergone a remarkable improvement in terms of both tumor control and normal tissues complication probabilities thanks to the implementation of the new physical and biological techniques [3]. However, radiotherapy encounters with serious drawbacks including tumor hypoxia, radiation-associated side effects and radioresistant cancer stem cells (CSCs) which result in unsatisfactory therapeutic outcome [4].
As the newly emerging challenge in radiotherapy, CSCs have gained increasing interest among the researchers and clinicians. The molecular biology of CSCs, their therapy resistance and new treatment approaches to break them have been the subjects of recent researches in this area. Given that there is no comprehensive review regarding the physical CSCs therapy, we herein aim to review and discuss the recent progresses in the application of physical approaches to eliminate CSCs.
Radiation therapy
Cancer stem cells and radiation resistance
CSCs or tumor-initiating/maintaining cells or cancer stem-like cells represent one of the most interesting topics of radiation oncology in recent years. Studies have indicated that CSCs are highly resistant against current radiotherapy approaches. There are several challenges with regard to CSCs identification for diagnostic and therapeutic purposes. Based on the tissue being studied, a range of biomarkers are available to identify CSCs. These markers have different patterns in different histological types of CSCs and are necessary to isolate and analyze the main biological and physiological characteristics of CSCs. These markers belong to two main groups including CD Molecules and ATP-binding cassette transporters, although there are several new biomarkers [5]. In first group, CD44, CD133, and CD24 and in second group, ABCG2, ABCB5, EpCAM, ALDH, CXCR4, Telomerase, SP Cells are most well-known markers. DCAMKL-1, Podocalyxin, Piwil2, Nestin, LRCs are also identified as new biomarkers. Several groups have reported different CSCs markers differentially expressed on CSCs cell surface which are main issues for CSCs targeting. At first these markers have been isolated from acute myeloid leukemia (AML) [6]. In this cancer, CD34+ and CD38 subpopulations were available and able to propagate AML in a xenograft transplant system. In regard to solid tumors, expressing specific biomarkers such as CD44 and CD133 were widely used for isolating CSC from solid tumors. As a well identified marker, CD44 has a great impact on tumorigenesis in several cancers including colon cancer, ovarian carcinoma, head and neck squamous cell carcinoma and prostate cancer [6]. Also, CD133 is introduced as an important CSC marker in brain tumor, osteosarcoma, and cancer including prostate, colorectal; ovarian and lung. Also, markers including CD24, CD29, ESA, CD49f, P63, Sca1, Ly-6A/E, NCAM, CD34, Thy-1, c-Kit, Flt-3 were found as important issue for CSCs identifications and therapeutic aims in several cancers [7].
Different mechanisms have been proposed for CSCs radiation resistance, which have been well documented in previous studies. It demonstrated that CSCs activate the DNA repair pathways. Activation of DNA damage checkpoint kinases (Chk1 and Chk2) efficiently were observed in CSCs in contrast to other tumor cells. In addition, overexpression of reactive oxygen species (ROS) scavenger proteins was shown by CSCs that this mechanism limits radiation-induced DNA damage. Chemotherapy agents can inactive via express enzyme [for example aldehyde dehydrogenase (ALDH)] by CSCs. CSCs can reduce cytotoxic effects of chemotherapy agent by different mechanisms, including activating Notch and Wnt pathways and upregulating BCL2 family proteins. Some CSCs remain in a quiescent state, thus are resistant to radiation and chemotherapy. One of the main parts of CSC niche is hypoxic regions. Hypoxic areas have a poor vascularization that limits the penetration of chemotherapy agents. Hypoxia decreases oxidative DNA injury. Under hypoxic conditions, expression of hypoxia-inducible factor 1α (HIF1α) not only leads to survival and proliferation of CSCs but also increases self-renewal CSCs by modulation of the activity of Notch signaling. Other causes of therapeutic resistance of CSCs which are associated to CSC niche, including the cancer-associated fibroblasts, the extracellular matrix and immune cells [8,9,10]. The main mechanisms of CSCs radiation resistance are shown in Fig. 1.
In fractionated radiotherapy, the main aim is to deliver radiation in daily fractions to maximize tumor control and minimize normal tissue injuries based on 4 R’s including Repair of normal tissues sub-lethal damages, Reoxygenation of hypoxic tumor cells, Redistribution of tumor cells in radiosensitive phases of cell cycle and Repopulation of normal tissue cells. But based on CSCs hypothesis these Rs remain challenging and unsatisfactory to enhance radiotherapy outcome. The causes of the radioresistance of CSCs to fractionated radiotherapy within the framework of the 4R’s of radiobiology are shown in Fig. 2. In a review by Pajonk et al. they related recent findings on CSCs to these four phenomena [11].
Recently, multiple strategies have been used for the destruction of CSCs. Approaches including chemical, biological to physical methods have been addressed and proposed to remove CSCs. In the present study, we aimed to review current physical methods for CSCs therapy. Methods including nanomaterial-based heat treatment, particle therapy, alone or combined therapy have been discussed in the present work.
Particle therapy for cancer stem cells therapy
Low linear energy transfer (LET) radiations such as X-rays and γ-rays mostly induce cell death through generation of ROS (indirect effect) [12]. Because CSCs reduce ROS levels and increase protection from oxidative damage, therefore they are resistant to conventional radiotherapy [13]. Furthermore, fractionated radiotherapy regimen may lead to repopulation of CSCs [14]. However, failure to remove CSCs leads to tumor recurrence.
Particle therapy is a form of radiation therapy (RT) that uses neutral (such as neutron) and charged particles (such as protons, carbon ions and other charged particles) [15]. The most important clinical benefit of charged particles depends on their physics. Charged particles release little energy at the entrance to the matter (tissue or patients body), when their velocity is high, and deposit most of their energy at the end of their range (Bragg peak) [16]. Owing to Bragg peak and energy deposition in small region, integral dose to normal tissue is low with charged particles such as protons than with photon beams [17, 18]. With due attention to charged particles depth dose curve, there is a sharp dose fall off beyond the Bragg peak that decreases normal tissue’s absorbed dose compared with photon beams [19]. In addition, heavier ions such as carbon ions have additional advantages in dose distribution in contrast to protons such as lesser lateral scattering and a reduction in range straggling [20, 21]. Proton beams have sharper beam penumbra than photon beams that this property is important for treatment tumor dose escalation close to the critical structure [15].
Most important notions to understand the work on heavy charged particles are relative biological effectiveness (RBE), linear energy transfer (LET) and Bragg peak [22]. The LET relates to the velocity and charge of the ions. Therefore, heavy ions such as carbon ions have higher LET than light ions such as proton, they are more efficient for cell death [16]. The RBE depends on several parameters such as particle type, energy, dose, dose rate, LET, cell type, experimental endpoint, cell cycle phase, oxygenation status and culture conditions. Therefore, it is difficult to determine absolute value of RBE [23,24,25]. The RBE determines the photon-equivalent dose that is why the RBE is the most important quantity in biological treatment planning of heavy-ion therapy [19]. Furthermore, heavier ions have more biological effectiveness in tumor irradiation than protons because those have greater RBE, lesser oxygen enhancement ratio (OER) owing to higher LET in Bragg peak region (tumor location) that lead to a decrease in the dependence of heavier ions function on the oxygen levels, cell cycle, hypoxia, fractionation and an increased cell death [16, 24] also have been reported to suppress cell migration and angiogenesis. RBE value of proton beam nearly is the same photon beam. For proton beam, RBE is 1.1 in routine clinical practice [26].
Treatment of hypoxia tumor regions is one of the challenges in radiotherapy. In large tumors hypoxia region issue become more important due to not enough angiogenesis. These regions are radioresistance. OER quantifies oxygen effect. The difference between hypoxic and normoxic regions is decreased for high-LET particles when those used for tumor treatment. Along with reduction of OER the RBE for heavy ion such as carbon more increases in the spreadout Bragg peak (SOBP). Therefore, carbon ions are more efficient in treatment of tumor with hypoxia cells [19]. High-LET heavy ion radiation-induced cell death is independent of ROS. Those lead to complex DNA damage [16]. Two main groups of complex DNA damage are including double strand breaks (DSBs) and non-DSB oxidative clustered DNA lesions (OCDL). The repair of complex DNA damage is difficult than other types of damage. Therefore, heavy ion could be more effective for eradication of CSCs [22]. In summary carbon ion beams are less dependent to the cell cycle and the five R’s of radiobiology (repair, redistribution, reoxygenation, repopulations and radiosensitivity). radiobiological characteristics of SOBP region of depth dose curve of heavy ion beams are summarized in Fig. 3. Proton beam has low LET compared with carbon ion beam and causes DNA damage through indirect effect therefore they are dependent to oxygen level [23, 27]. It was demonstrated that increasing ionization densities arises generation of ROS. For instance, 250 meV protons at the end of their range produce more ROS [28].
Recently, many studies have reported that CSCs could be more effectively sensitized or eliminated by proton beams, carbon ion or neutron beams in contrast to photon beams or in combination with a chemotherapy agent in colon, breast, brain, lung; pancreas and skin cancers. Quan et al. compared the impact of 2 Gy proton and gamma radiation on the short-term apoptosis and long-term clonogenic survival of SW620 colon cancer cells. Significant difference between the long-term cologenic survival and short-term apoptosis ratio proton and gamma beams were not observed. In fact, proton and gamma radiation have the identical impact on tumor volume control. From these findings, it could be observed that CD133+ expression significantly was reduced by proton beams. Also, proton beam decreased mammosphere formation capacity. Therefore, proton beam could be more effective for CSCs depletion in compared to gamma radiation [29]. In another study, proton beam generated higher apoptosis ratio of CSCs from human MCF-7 cell line than γ-ray irradiation. In addition, larger Foci size was produced by proton beams. After proton irradiation, cell’s reparability was reduced owing to more complex DNA damage [30]. It has been reported that proton beams can active pro-apoptotic MAP kinases such as p38 and JNK. High expression level of p21 indicates higher cell cycle arrest after proton irradiation. Up-regulation of KLF2 and ATF3 (mRNA proteins) result in cell cycle arrest and induction of apoptosis and adjust of caspases levels, respectively. Down-regulation of transforming growth factor beta (TGFβ), Wnt and ErbB signaling were found in human lung carcinoma cancer after proton irradiation that led to suppression of epithelial-mesenchymal transition (EMT) and CSCs phenotypes [31].
In treatment of glioma stem cells (GSCs), reduction of CD133+ surviving fraction confirmed that proton therapy induces higher cytotoxicity in GSCs than conventional radiotherapy. Chk1/2 has a determinant role in repair of DNA in GSCs. G2 arrest recovery reduced after proton irradiation because it increases Chk2 phosphorylation. Thus, induces greater DNA damage and cell apoptosis. Another mechanism that is associated with proton therapy cytotoxicity is the raise of ROS production. Regenerative capacity of GSCs is changed by ROS. Cell cycle redistribution and apoptosis that were produced by proton beam are ROS–dependent [32]. The results of Zhang et al. study on human non–small-cell lung cancer (NSCLC) cell lines A549, H460 and normal human bronchial epithelial (NHBE) cells were in line with above findings. CSC-like cell lines can be more sensitive to the proton beams than photon beams at the identical RBE owing to the greater ROS produced by proton irradiation [33].
Takahashi and co-authors compared the effect of X-ray and carbon ion beam on radiosensitivity sphere-type A172 human glioblastoma cells. In comparison with X-ray, Carbon ion beams have relatively a more effect on killing of the sphere-type cells. Thus, heavy ion particles may overcome on the tumor resistance due to cell stemness [34]. One of the mechanisms of CSCs radioresistance is activation anti-apoptotic pathway [10]. One of the signaling pathways of this mechanism is through AKT survival signaling. Study has been shown that one of the causes of head and neck (HN) CSCs radioresistance can be attributed to upregulation of the AKT signaling [35]. High LET radiation such as carbon ion beams is capable to depress AKT-related survival signaling. Also, it can increase apoptosis [36]. Carbon ion therapy can efficiently induce autophagy by inhibition AKT-mTOR through unfolded protein response (UPR) [37]. According to this, Takahashi et al. compared the impact of carbon ion therapy and X-rays radiation on human SAS HN squamous carcinoma cells (HNSCC). From the results of this study, it can be seen that pyknosis increased in cells by carbon ion irradiation. Also, AKT survival signaling was suppressed by carbon ion beams. These findings are in agreement with previous reports [34, 37]. They suggested that carbon ion therapy may increase apoptosis and autophagy through the activation of cell death signaling and target CSCs [38].
Lin28B (an oncogene) suppresses mature let-7 miRNA (tumor suppressor) production. Thus, it has a main role in melanoma CSC regulation. Recently, a work by Park et al. examined the impact of carbon ion and X-ray beam on killing of melanoma stem-like cells. Overexpression of Lin28B was observed by G361, A375s2 and SK-MEL5 cell lines that it increases sphere-forming capability. Moreover, mature let-7 miRNA expression was repressed by up-regulation of Lin28B, and it depends on cell type. Carbon ion irradiation completely abrogated X-ray irradiation–induced radioresistance. Also, the Lin28B-induced X-ray resistance is blocked through modulation of the DNA damage response after carbon ion irradiation [39].
The population of CD133+, CD44+ and epithelial specific antigen positive (ESA+) cells in human colon cancer cells increases after X-rays irradiation. In in vitro, RBE values were calculated 1.63–1.74, while RBE values were 2.05–2.28 for carbon ion beam relative to X-rays. In examination of xenograft tumor model after 4 weeks, 15–30 Gy of carbon ion irradiation induced severe damages such as tumor cell cavitation and fibrosis relative to X-ray irradiation. Thus, it can target putative colon CSCs [40]. The greater RBE value of carbon ion beams in contrast to X-rays, complex DSBs and reducing CSCs repair capacity owing to higher G2/M cell cycle arrest after carbon ion irradiation have shown that carbon ion irradiation can effectively eliminate putative pancreatic CSCs [41]. In a study, Sun et al. compared the effect carbon ion beam and X-ray on glioma CSCs. The results of this study were in agreement with previous reports. The results of in vitro experiment showed the repair rate of DNA damage was reduced by carbon ion beam. In in vivo experiment, Carbon ion beam significantly decreased the percentage of CD133+ glioma CSCs. Their findings showed that the initial yield of DNA damages is high after photon irradiation in compared with carbon ion beam. But, carbon ion beams induce more complex DNA damages [42]. Carbon ion therapy is effective in elimination of glioma patient-derived stem and non-stem cells than proton and photon beams. Clonogenic survival shows average RBE carbon ion approximately is threefold of proton beam that this can be attributed to reducing the DNA repair capacity of GSC. Also, more than 70.2% of residual gamma H2AX-positive cells were observed after carbon irradiation [43].
The improvement new treatment such as combination carbon ion therapy and chemotherapy may cause an increase local control and progression-free and overall survival of patients. HNSCC-CSCs represent higher invasiveness and migration in comparison to non-CSCs. Resistance to cetuximab is seen in HNSCC-CSCs with low epidermal growth factor receptor (EGFR) expression. The combination of carbon ion beam and cetuximab can inhibit invasion in HNSCC-CSCs [44]. In a work, Bertrand et al. to overcome HNCSCs resistance to photon and carbon ion radiation used of following two pharmacological approaches. (1) UCN-01, a cyclin-dependent kinase (CDK) modulator, induced the relapse of G2/M arrest and radiosensitization of SQ20B-CSCs. (2) All-trans retinoic acid (ATRA) resulted in an inhibition of ALDH activity, and induction of the differentiation and radiosensitization of SQ20B/SP/CD44+/ALDH high cells. The combination of two pharmacological treatments with radiation significantly reduced the surviving fraction at 2 Gy of SQ20B-CSCs. The utilization of ATRA and inhibition of Chk1/2 increased radiation response. Therefore, these pharmacological strategies can sensitize CSCs to photon and carbon ion radiation [45].
Carbon ion beam combined with a chemotherapy agent can effectively kill CSCs. Large number of gamma H2AX-foci and significant reduction of clonoy and spheroid formation in pancreatic CSCs and triple negative BCSCs after carbon ion irradiation combined with gemcitabine and cisplatin, respectively. The inhibition of cell cycle progression and elimination of CSCs markers expression were seen after carbon ion irradiation plus a chemotherapy agent. The combination of carbon ion therapy and chemotherapy lead to more DSBs and higher complexity of cluster DSBs [46, 47].
An alternative treatment for cancer is boron neutron capture therapy (BNCT). In BNCT boron-10 nucleus reacts with thermal neutron (neutron capture). This reaction results in production of boron-11 nucleus. Boron-11 nucleus decomposes automatically into two high LET particles, i.e. α-particle and Li-7 nucleus (fission reaction).
These particles have high RBE due to having high LET [48,49,50]. Therefore, BNCT can effectively kill anoxic and quiescent cells [51]. BNCT can be optimized by two independent parameters could be attributed to boron concentration in tumor and normal cells, another parameter is thermal neutron fluence rate in tumor and healthy tissue. In fact, these two parameters determine BNCT gain and it is necessary that cancerous cells contain a high concentration of boron-10. Furthermore, tumor tissue should irradiate with an enough amount of thermal neutrons [52]. These high energy particles have a limited range (5–9 μm in tissue) therefore their destructive effects are limited to boron-containing cells [49, 52]. On the other hand, BNCT is a treatment modality that selectively target tumor tissue. According to these properties, Sun et al. showed that BNCT can lead to glioma stem/progenitor cells killing in vitro. The results of this study showed that the expression of G2/M phase-associated proteins (cyclin B1 and CDK1) reduced after BNCT, and the cell cycle arrest in the G2/M phase leads to increase in radiosensitivity of cells. Also, apoptosis markers such as cytochrome c and caspases-9 increased after BNCT that induces cell apoptosis via the mitochondrial pathway [53].
Recently, a BNCT agent was developed to target CSCs selectively and effectively. For raising boron-10 uptake in GSCs, a bioconjugate nanoparticles (NPs) were designed. The lethal effects of neutron radiation significantly increased in combination with bioconjugate NPs owing to the particular uptake of the bioconjugate NPs in CD133+ GSCs and CD133-independent cellular targeting. Also, the combination of the biconjugate NPs and mercaptoundecahydrododecaborate (BSH) in comparison to BSH alone led to significantly expended survival in in vivo [54].
High LET radiation may overcome radioresistance of GSCs against low LET radiation. The human glioblastoma multiforme (GBM) cell line A172 was exposed to Co-60 gamma rays and reactor neutron beams. The radiosensitivity of cells significantly increased with neutron beams in contrast to gamma irradiation. Furthermore, neutron beams induced more gamma-H2AX foci. From these results, it is concluded that neutron beams are more efficient to produce unrepairable DNA DSBs in GSCs. However, further study is required to examine the efficacy of neutron beams for GSCs control [55].
Tables 1 and 2 ssummarize in vitro or in vitro and in vivo studies on the effect of particles therapy on CSCs and exhibit inhibition mechanisms of CSCs radioresistance.
The role of nanoparticles in radiosensitization of cancer stem cells
The aim of RT is to deliver a curative dose of radiation within tumor tissue while sparing the surrounding healthy tissue. This goal is limited by tolerance dose of normal tissue although imaging modalities and radiation source have progressed. Tolerance dose of organ at risks is a dose limit factor in dose escalation. Thus, it is necessary to utilize a strategy for enhancement sensitivity of tumor tissue to radiation compared to normal tissue. With this strategy can use a low dose of radiation. One of the strategies is application of NPs as a radiosensitizer.
Cui et al. showed that miR-200c NPs can use as radiosensitizer in gastric cancer cells. miR-200c NPs delay the repair of radiation-induced DNA DSBs. Moreover, combination of miR-200c NPs and radiation increase ROS level. In fact this strategy suppresses possible mechanisms of CSCs radioresistance. Furthermore, miR-200c under Gelatinase-stimuli PEG-Pep-PCL NP drug delivery system can cause CSCs depletion by regulation of self-renewal, invasiveness and differentiation [56]. The use of ultrasmall gadolinium-based NPs (GBNs) on several radio-resistant human HNSCC showed that the combination of GBNs and 250 keV photon irradiation overcame the radiation resistance in SQ20B stem-like cells through non-reparable DNA DSBs, reduce in tumor proliferation, production of ROS, cell arrest in G2/M phase [57]. In a work, Hu et al. investigated the role of glucose coated (Glu)-Au NPs for targeted treatment of cancer metastasis and CSCs in human monocytic cell line. Results of this work showed that Glu-Au NPs can increase the irradiation effect owing to the accumulation of cells in G2/M phase after Glu-Au NPs followed by X-ray irradiation. Radiosensitization mechanism of Au NPs can be associated to absorbing radiation and generation of ROS therefore can increase radiotherapy efficacy in term of tumor cell killing [58].
Recently, Castro Nava and co-authors demonstrated that catechin-loaded and gelatin-conjugated CNTs (Gel_CT_CNTs) in combination with X-ray irradiation can potentially eliminate prostate CSCs. This strategy significantly inhibited sphere formation ability of DU145 cells and increaseed the cancer cell radiosensitivity. Increase in radiosensitivity can be attributed to inhibition of WNT/b-catenin pathway, enhanced apoptosis and impaired DNA [59]. You et al. developed cyclopamine-loaded lipid NPs for radiosensitization in 4T1 murine breast cancer and Miapaca-2 human pancreatic carcinoma models. Inhibition of sonic hedgehog signaling by cyclopamine increases tumor radiation-response. For combination strategy, they used (177)Lu-conjugated polymeric micelles radiation source intratumorally that provides localized radiotherapy. CPA-LLP increased response of tumor cells to CCPM-177Lu. The combination therapy showed slow tumor growth [60].
In summary, radiosensitization mechanisms of NPs can be associated with disruption of DNA damage repair, redistribution of cell cycles to radiosensitive G2/M phases, generation of ROS, dose enhancement using high atomic number (Z) materials and targeted delivery of radiosensitizer agent.
The potential of targeted alpha-particle therapy for cancer stem cells
Radioimmunotherapy (RIT) is one of the therapeutic modalities for cancer. RIT is an elective internal RT that uses of radioactive isotope conjugated to tumor-directed monoclonal antibodies. The most common characteristics of radionuclide are including high energy and short-path-length. Radionuclide emits α-particles, β-particles or Auger [61,62,63]. The results of RIT in treatment of cancer were clear and repeatable on many tumor xenograft mouse models. Preclinical data showed that RIT has been successful in the treatment of hematological malignancies. Many studies have showed that RIT can eliminate CSCs by antigen-based approaches and inhibition of self-renewal pathways. In study by Jandal and co-authors, mice bearing A2058 melanoma xenograft was treated by (188)Re-labeled anti-melanin monoclonal antibody (mAb) 6D2. The results indicated that RIT destroys melanoma stem cells at the same rate of bulk cells [64].
It has demonstrated that treating to (177)Lu labeled anti-EGFR mAb alone significantly decreases the percentage of CD44+/CD24−/EpCAM+ breast CSCs (BCSCs), furthermore, in combination with chemotherapy and a poly ADP(adenosine diphosphate ribose) polymerase inhibitor cured tumor-bearing mice. These findings can be attributed to apoptosis and elimination of putative CSCs. The data suggested that RIT has a potential benefit for targeting CSCs [65]. For direct targeting leukemia stem cells, Leyton and co-workers used a radiolabeled antibody. Viability of leukemia stem cell and self-renewal were reduced by high dose RIT [66]. Recently, Weng et al. targeted CD133+ colonic CSCs with RIT. 131I-AC133.1 mAb caused a reduction of protein level of CD133, ALDH1, Lgr5, Vimentin, Snail1and proliferative rate and an increased tumor necrosis. Authors suggested that 131I-AC133.1 mAb can eradicate CSCs and inhibit tumor [67].
As mentioned in the studies above, RIT is performed with β-emitter radionuclides that can have some disadvantages because β-particles are low LET and, therefore, their energy deposition in tumor relatively is low. Furthermore, β-particles have long range (2–10 mm) that increases their cross fire effect. As a result, non-targeted cells are exposed to radiation [63]. Targeted alpha therapy (TAT) is an in-development method of RIT, where an alpha-emitting radionuclide is bound to a carrier (molecular carrier targeting a specific tumor antigen) [68]. In alpha particle decay, two protons and two neutrons are released by atomic nucleus. Some of the main characteristics of alpha particles include short penetration depth and high-LET. Short range of alpha particles limits damage to normal tissue [68,69,70].
High-LET radiations are more effective than low-LET radiations for cellular damage due to following reasons: (1) high-LET radiation generally causes more irreparable clustered and double-stranded DNA breaks and results in more severe chromosomal damage, including shattered chromosomes at mitosis and complex chromosomal rearrangements. (2) high-LET radiations causes more pronounced G2-phase delays [71]. The LET of alpha-particles ranges from 25 to 230 keV/μm [67] and DSBs are mainly produced in LET 100–200 keV/μm [71]. Therefore, alpha-particles are highly cytotoxic and can directly act on the specific tumor cell antigens. Meanwhile, it has super chance of substantially adding to hitherto failing curative adjuvant treatments for different types of cancer, e.g., prostate, breast, colon, and ovarian cancer. Also it can be addressed as systemic conformal radiotherapy at the cellular level [68, 72].
Using to mathematical modeling Sgouros and Song investigated the possibility of use of the Alpha-Particle Emitter, Bismuth-213, for CSC targeting. Their investigation showed that specific activity, antigen site density, and number of target cells are important parameters for effective cell death. If antigen site density increases by a factor of 2, tumor control probability will highly disappropriate increase [73]. Dekempeneer et al. reviewed importance of TAT and advantage of use of nanobodies as vehicles for TAT. They suggested that nanobodies have several advantages including following factors: (1) targeted tracers, (2) high affinity and specificity for their cognate antigen and facile production and (3) efficient radiotracers directed against a variety of membrane-bound biomarkers in various applications. In addition to, the nanobodies have more advantages than conventional antibodies such as lower molecular weight, low immunogenicity, sufficiently penetration of nanobodies in tumor tissue and link tumor antigens rapidly and specifically in vivo. Meanwhile, they expressed the nanobodies’ pharmacokinetic properties match perfectly with the interesting decay properties of the short-lived alpha-particle emitting radionuclides [74].
Elgqvist et al. discussed about obstacles and potential TAT. This review showed that TAT is engaging treatment strategy to treat cancer. The most important difference between RIT with β-particle emitters and α-particle emitters this is that RIT with α-particle emitters labeled to a targeting vector can directly kill single cancer cells (by self-irradiation). Clinical trial performed or in progress using of TAT have been shown the possibility of TAT for treating disseminated and/or micro-metastatic. Radium-233-dichloride is food and drug administration-approved for bone metastases in castration-resistant prostate cancer (CRPC). The main problem about TAT in clinic is availability with optimum cost. TAT now is a potentially profitable treatment strategy. Thus, it is required to conduct randomized and controlled clinical studies [72]. Recently, Ceder and Elgqvist presented possibility targeting prostate CSCs with alpha-particle therapy. They suggested that TAT can potentially use for treatment of metastatic cancerous diseases. CRPC is now an irremediable disease that TAT can increase the probability of prolonged survival in mentioned disease. The suitable alpha-particle emitters for TAT are including Astatine-211, Bismuth-212, Bismuth-213, Radium-223, Actinium-225 [68].
Hyperthermia
Cancer hyperthermia (HT) refers to the elevation of tumor temperature in the range of 40–46 °C that is usually applied in order to enhance the effectiveness of other therapeutic modalities such as radiotherapy and chemotherapy [75]. Various sources of HT are currently employed to heat up the tumor including microwaves, radiofrequency waves, infrared laser and high intensity focused ultrasound [76, 77]. However, conventional HT has two major disadvantages: (1) inability to access the deep-seated tumor sites, and (2) non-selectivity of tumor heating which is associated with undesired thermal damages to the surrounding normal tissues [78]. Recently, nanotechnology has shown great promise to promote the effectiveness of the conventional HT. A variety of NPs can absorb the energy of the external HT sources and then generate a localized heat within the tumor, thereby realizing a tumor-specific HT strategy without unwanted heating of collateral healthy tissues. Moreover, NPs have the potential to combine HT with other therapeutic modalities such as RT and chemotherapy in order to simultaneously deliver multiple treatments to the tumor. The most common thermo-responsive NPs for HT include gold NPs (mostly in the form of nanoshells and nanorods), superparamagnetic iron oxide NPs (SPIONs) and carbon based nanomaterials [79, 80].
HT may either induce direct cell killing if the temperature is high enough (> 47 °C) or sensitize tumor cells toward chemotherapy and radiotherapy via various mechanisms (indirect effects). Temperature and duration of HT are two main determinant factors of the type and extent of cellular injury [8]. The direct and indirect effects of HT on tumor cells are summarized in Table 3. Several studies have indicated that HT can eradicate CSCs via different mechanisms [9]. As a result, HT can enhance the sensitivity of the tumor to RT. Meanwhile, HT is independent of cell cycle state and it can destroy quiescent CSCs. HT targets immune response and leads to heat shock protein (HSP)-induced cell killing. Moreover, HT may inhibit the cellular repair pathway of DNA damages such as DNA DSBs, thus altering the sublethal and potentially lethal radiation induced damages into lethal events. HT can target cells in hypoxic regions and increases the blood flow and improves tumor oxygenation. HT can change microenvironment by re-oxygenation [81, 82]. Furthermore, HT inhibits endothelial cell adhesion and cell proliferation and increases apoptosis.
It has been demonstrated that HT may cause cell death via apoptotic route [83, 84]. HT may induce heat stress to cells and then results in mitochondrial protein denaturation [85]. HT can also increase the level of ROS production by mitochondria that promote oxidative stress. Taken together, heat stress and oxidative stress induced by HT can activate the intrinsic pathway of apoptosis, resulting in cell death and phagocytosis [76, 86, 87]
Magnetic hyperthermia for cancer stem cells therapy
Among other types of NPs, iron oxide NPs (IONPs) have been widely utilized in biomedical applications because of their ease of preparation and cost-effectiveness [88]. The superparamagnetic property of IONPs renders them an ideal candidate for magnetic HT (MHT), where magnetic NPs (MNPs) generate heat under an alternating magnetic field (AMF) [87]. MHT has provided remarkable advantages over conventional HT techniques as listed below:
-
The heat generation effect of MNPs under AMF excitation along with their contrast enhancement capability in magnetic resonance imaging (MRI) may enable simultaneous diagnosis and therapy [89].
-
MNPs are able to realize a combinatorial cancer treatment strategy through the concurrent delivery of HT in conjugation with other cancer treatment modalities such as radiotherapy and chemotherapy [89].
-
MHT is able to selectively and uniformly deliver heat to the tumor due to the ability of MNPs to distribute into small regions [88].
-
MHT has access to the deep-seated area of body without penetration depth limitation [88].
-
MHT can particularly target the tumor vasculature or hypoxic tumor cells (effective targeting of CSCs) [90].
Magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the most common types of IONPs in MHT applications owing to their good biocompatibility and biodegradability [91, 92]. Several reports have shown that magnetic IONPs can increase generation of ROS [93,94,95]. The primary sources of oxidative stress in response to IONPs could be attributed to direct production of ROS from the NPs surface, generation of ROS via leaching of iron molecules, altering mitochondrial and other organelle functions and induction of cell signaling pathways [96]. SPION can induce (geno) toxicity by different mechanism. For examples, production of ROS and high level of Fe ions in tissue can lead to DNA damage and cytotoxicity [97].
Recent investigations have shown that nanoparticle-mediated MHT can effectively kill CSCs and cause tumor growth suppression. Sadhukha et al. utilized SPIONs to induce MHT for the elimination of CSCs in A549 and MDA-MB-231 cancer cell lines. The results indicated that MHT for 30 min resulted in about 90% cell death in both cell lines, whereas conventional HT for the same exposure time showed much lower cell killing potency. Further experiments demonstrated that MHT could effectively reduce CSCs population mainly via two pathways: (1) the short-term effect that is associated with acute necrotic cell death immediately after MHT, and (2) the long-term effect which is related to the increased level of ROS generation and eventually leads to apoptosis after some latency [98].
In another report, Kwon et al. investigated the effect of particle size and found that among magnetic nanocluster (MNC) of various sizes within 20–140 nm range, the 60 nm MNC exhibited the highest value of specific absorption rate (SAR) and determined as the optimum particle size. Compared to other particle sizes, the 60 nm MNC demonstrated the highest temperature rise under AMF and thus dramatically reduced cell viability. Further mechanistic study proved that the expression levels of HSP 70 and 90 that protect cells against heat stress, were downregulated following MHT, thereby promoting the apoptotic process. The expression of BCSCs markers (ALDH1 and CD44+/CD24−) was reduced by MHT [99].
Nano-photo thermal therapy for CSCs therapy
Nano-photo thermal therapy (NPTT) is a minimally invasive cancer treatment modality that exploits nanophotosensitizer in order to convert laser light into heat in the site of interest, thereby selective targeting tumor cells [100]. In NPTT, plasmonic NPs are designed to absorb non-toxic light in the near-infrared range (NIR; 650–1064 nm) because this wavelength range is less absorbed by tissue components (e.g., water and hemoglobin) relative to visible light wavelength and therefore has more penetration depth into biological tissues [101]. When plasmonic NPs are irradiated by electromagnetic radiation (i.e. NIR), strong electric fields are induced owing to the synchronized excitation of the conduction band electrons on the surface of NPs (that is called surface plasmon resonance; SPR). In conclusion, a localized heat is generated by the rapid relaxation of these oscillated free electrons and causes irreversible damages [102, 103]. NPs have two main roles in NPTT including selective delivery of photon energy into tumor tissue and light-to-heat conversion [104, 105]. The performance of NPTT or tumor temperature rise depends on several parameters such as the concentration of NPs in the tumor tissue, photothermal (PT) conversion efficiency of NPs, exposure time and the power density of laser [100, 101].
A wide range of light-responsive nanomaterials have been employed for NPTT. For example, gold nanomaterials with different structures [106], copper-based nanocrystals [107, 108], and carbon-based nanomaterials such as graphene [109], single-walled or multi-walled carbon nanotubes [110,111,112] and fullerene [113] have shown promising potential in NPTT. Owing to their unique physicochemical properties such as biocompatibility, non-toxicity and bioinertness, facile preparation, easy surface functionalization and high light-to-heat conversion efficiency, gold nanomaterials have been presented as the main mediators of NPTT [114,115,116,117]
Necrosis and apoptosis are two primary mechanisms of cell death triggered by NPTT [101]. Induction of apoptosis and necrosis following NPTT depends on the localization of NPs inside cells and laser irradiation parameters [118, 119]. Recent reports have demonstrated that the application of NPTT with lower laser power and shorter exposure time can skew the response toward apoptotic cell death rather than necrotic death [120]. Moreover, the accumulation of NPs in cell nucleus or cytoplasm may affect cell death mechanisms in response to NPTT [119].
A recent effort in the area of CSCs therapy tried to compare the effectiveness of traditional HT and NPTT. Traditional HT was shown to be ineffective in eradicating BCSCs due to the overexpression of HSP 90 which is known as a determinant factor of thermoresistance. In sharp contrast, NPTT exhibited the potential to overcome the inherent resistance of BCSCs to thermal therapy. The treatment of cells with multi-walled carbon nanotubes (MWCNTs) and subsequent laser irradiation resulted in significant reduction in cell viability through membrane permeabilization and necrosis. The in vivo antitumor study further proved the high efficiency of this modality, where MWCNTs mediated NPTT yielded complete tumor regression and significantly prolonged survival of mice bearing breast tumors [112].
It has been found that CSCs are more resistant to ionizing radiation than the bulk of tumor cells and they result in metastasis or tumor relapse after successful radiotherapy treatment. A report by Atkinson et al. revealed that NPTT can be implemented in combination with radiotherapy in order to increase the sensitivity of CSCs to ionizing radiation. To assess this effect, mice bearing breast tumors were exposed to a single dose of 6 Gy followed by 20 min NIR laser irradiation at 42 °C using intravenously administrated gold nanoshells. The analysis of cells derived from tumors indicated that the combination of radiotherapy and NPTT caused a greater reduction in the proportion of CSCs than radiotherapy alone. Moreover, while NPTT alone had no inhibitory effect on tumor growth and radiotherapy alone inhibited the tumor growth to some extent, the combination of radiotherapy plus NPTT resulted in significantly higher tumor growth inhibition. These results corroborate the positive role of gold nanoshells mediated NPTT for thermal enhancement of radiotherapy through effective eradication of radioresistant CSCs. Irradiation plus HT inhibited HSP90 and AKT pathway. In addition, NPTT can has an effect on the tumor microenvironment and destroy CSC niche [121].
Based on the facts that CSCs are also resistant to chemotherapy and the combination of heat and drug results in synergistic therapeutic effects, a new combinatorial strategy can be provided for inhibiting CSCs by utilizing thermo-responsive nanomaterials that carry anticancer agents. To exploit this potential, Xu et al. loaded salinomycin (SA) as a CSCs inhibitor in polydiallyldimethylammonium chloride (PDC) conjugated gold nanorods (Au/SA@PDC) for targeted delivery of NPTT and chemotherapy in the presence of NIR laser irradiation. The results demonstrated that while Au/SA@PDC induced slight toxicity (cell viability: ~ 85%) in MCF-7 breast cancer cells due to the slow release rate of SA from the nanocarrier, the combined action of Au/SA@PDC plus NIR laser dramatically reduced cell viability to 18%. Moreover, the combination of Au/SA@PDC plus NIR laser showed great potential in inhibiting CSCs and reduced the percentage of CSCs in MCF-7 cells to zero. This synergistic interaction between NPTT and chemotherapy was attributed to the accelerated release of SA from Au/SA@PDC due to NIR irradiation. AuNRs-mediated PTT resulted in reduction of ALDH+ cell subpopulation, gene expression of stem cell markers (ALDH1 and KLF4), and mammosphere forming capability [122].
Photodynamic therapy for cancer stem cells therapy
Photodynamic therapy (PDT) is another light-driven cancer treatment modality with promising potential for CSCs therapy [123]. PDT uses photosensitizers that are activated by visible light at the appropriate wavelength to produce an excited state which may generate cytotoxic species through two following reactions: (1) generation of free radicals through electron or hydrogen transfer from a substrate molecule (type Ι mechanism), and (2) generation of singlet oxygen as the main cytotoxic species in PDT resulting from energy transfer to oxygen molecules (type Π mechanism) [124, 125].
Type Π mechanism dominantly occurred by most photosensitizers and it depends on the oxygen concentration of the tumor microenvironment. Therefore, the efficacy of PDT may be greatly limited at hypoxic condition which is often found in tumor microenvironment. However, type Ι mechanism is less sensitive to oxygen concentration, and therefore it can address the limitation of PDT. Usacheva et al. reported that incorporation of methylene blue (MB) into alginate-Aerosol OT NPs can increase the level of ROS production under hypoxic condition via type Ι mechanism. This is attributed to the increased interaction of MB with solvent molecules mediated by alginate polymer. As a consequence, PDT with MB NPs more effectively eradicated BCSCs and reduced mammosphere formation ability under either normoxia or hypoxia compared to that with free MB [126]. Table 4 summarizes the recent studies regarding the use of NPs for elimination of CSCs.
Future prospective and conclusion
In the present work, we reviewed the current studies on new physical approaches for cancer stem cell therapy. Although we had a wide overview on this topic, but there are several issues that remain unstudied. Also, in order to provide newer ideas for CSCs treatment, we have several points as future directions and prospective.
First, combination of two more physical therapy approaches. In this regard, each approach has its beneficial effects and their combination could provide better therapy outcomes. It also should be reminded that, side effects are the main limiting factors. Further clinical and experimental studies are needed to find the optimum dose, mechanisms of action and unwanted side effects. On the other hand, because CSCs is a new topic in cancer therapy, there is no well documented study on the late effects of CSCs therapy approaches. There are clinical needs to survey the CSCs treatment outcomes in long time follow-ups to find best solutions.
Second, testing combination of new physical/biological and chemical agents. Biological therapy is a new area of research which is found as a feasible therapy method for removing cancer cells. Studies have identified that immunotherapy would be an effective tool for CSCs eradication. Immunological experiments have shown that antigen-specific targeting using T and natural killer cells had promising results on CSCs treatment [138]. Also several immunological mechanistic issues have been proposed to solve the CSCs therapeutic resistances. Combination of immunological therapies with radiation, although is tested for CSCs, but is new yet and could be updated by particle beams, nanoparticles, hyperthermia and other physical approaches. Several studies have shown that combination of hyperthermia and immunological cells including natural killer cells could improve the final outcomes of cancer therapy. This combination therapy may be tested for cancer stem cells.
Third, radiogenomic assays. Ongoing studies on personalized radiotherapy have revealed that genomic assays could predict patient’s radiation response including tumor and normal tissues response to radiotherapy [139, 140]. In this light, “physico-genomics”, association between genes and physical modalities response, could modify the use of physical therapy approaches in cancer therapy clinics. We could offer to use of genes for finding physical sensitivity for cancer patients. For example hypersensitive patients to nanoparticles, hyperthermia, and particle beams may have different response and different therapy outcomes. In this era, because CSCs resistance to current therapies has a genetically basis, physico-genomic assays will results in more personalized therapy.
Fourth, the oxygen effect. Although some studies have applied hyperbaric oxygen therapy to treat cancer stem cells, but there are several limitations in these studies which should be updated based on more mechanistic issues. This therapy will be more effective if mechanistically combined with other approaches. In this regard, several further studies are needed to find best remedial issues.
In conclusion, this paper reviews several physical therapy methods for CSCs eradication. To our knowledge there is no such study, and it also could be improved by new other studies. By advances in biology and techniques, CSCs could be investigated in more details in terms of their targeting with new therapy modalities. Physical therapy, although seems feasible, but they have several limitations including their high cost (e.g. particle beams), unavailability in any clinic, challenges in their use as an approved agents (particularly nanoparticles) and fully known mechanisms. But they are future based approaches and many researches are in progress to enhance these methods.
References
Abdollahi H, Shiri I, Atashzar M, Sarebani M, Moloudi K, Samadian H. Radiation protection and secondary cancer prevention using biological radioprotectors in radiotherapy. Int J Cancer Ther Oncol. 2015;3(33):335. https://doi.org/10.14319/ijcto.33.5
Khademi S, Abdollahi H. Application of hydrogen producing microorganisms in radiotherapy: an idea. Iran J Pub Helath. 2014;43:1018–9.
Newhauser WD, Berrington de Gonzalez A, Schulte R, Lee CA. Review of radiotherapy-induced late effects research after advanced technology treatments. Front Oncol. 2016;6:13.
Kouhsari E, Ghadimi-Daresajini A, Abdollahi H, Amirmozafari N, Mahdavi SR, Abbasian S, et al. The potential roles of bacteria to improve radiation treatment outcome. Clin Transl Oncol. 2018;20:127–39.
Dou J, Gu N. Biomarkers of cancer stem cells. Adv Cancer Stem Cell Biol: Springer; 2012. p. 45–67.
D’Andrea V, Panarese A, Tonda M, Biffoni M, Monti M. Cancer stem cells as functional biomarkers. Cancer Biomark. 2017;20:231–4.
Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, et al. Cancer stem cell markers in common cancers–therapeutic implications. Trends Mol Med. 2008;14:450–60.
Huang H, Yu K, Mohammadi A, Karanthanasis E, Godley A, Yu JS. It’s getting hot in here: targeting cancer stem-like cells with hyperthermia. J Stem Trans Bio. 2017;2:113.
Oei AL, Vriend LE, Krawczyk PM, Horsman MR, Franken NA, Crezee J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;2:1–12. https://doi.org/10.1080/02656736.2017.1279757
Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73.
Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells (Dayton, Ohio). 2010;28:639–48.
Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment—tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–75.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–25.
Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol. 2014;32:2855–63.
Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43.
Loeffler JS, Durante M. Charged particle therapy–optimization, challenges and future directions. Nat Rev Clin Oncol. 2013;10:411–24.
Mendenhall NP, Malyapa RS, Su Z, Yeung D, Mendenhall WM, Li Z. Proton therapy for head and neck cancer: rationale, potential indications, practical considerations, and current clinical evidence. Acta Oncol. 2011;50:763–71.
Schardt D, Elsässer T, Schulz-Ertner D. Heavy-ion tumor therapy: physical and radiobiological benefits. Rev Mod Phys. 2010;82:383–425.
Tobias CA, Lyman JT, Chatterjee A, Howard J, Maccabee HD, Raju MR, et al. Radiological physics characteristics of the extracted heavy ion beams of the bevatron. Science. 1971;174:1131–4.
Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22.
Okayasu R. Repair of DNA damage induced by accelerated heavy ions–a mini review. Int J Cancer. 2012;130:991–1000.
Blakely EA, Chang PY. Biology of charged particles. Cancer J. 2009;15:271–84.
Durante M. New challenges in high-energy particle radiobiology. Br J Radiol. 2014;87:20130626.
Held KD, Kawamura H, Kaminuma T, Paz AE, Yoshida Y, Liu Q, et al. Effects of charged particles on human tumor cells. Front Oncol. 2016;6:23.
Liu H, Chang JY. Proton therapy in clinical practice. Chin J Cancer. 2011;30:315–26.
Tobias CA, Blakely EA, Alpen EL, Castro JR, Ainsworth EJ, Curtis SB, et al. Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys. 1982;8:2109–20.
Tommasino F, Durante M. Proton radiobiology. Cancers (Basel). 2015;7:353–81.
Quan Y, Wang W, Fu Q, Mei T, Wu J, Li J, et al. Accumulation efficiency of cancer stem-like cells post γ-ray and proton irradiation. Nucl Instrum Methods Phys Res, Sect B. 2012;286:341–5.
Fu Q, Quan Y, Wang W, Mei T, Wu J, Li J, et al. Response of cancer stem-like cells and non-stem cancer cells to proton and γ-ray irradiation. Nucl Instrum Methods Phys Res Sect B. 2012;286:346–50.
Narang H, Kumar A, Bhat N, Pandey BN, Ghosh A. Effect of proton and gamma irradiation on human lung carcinoma cells: gene expression, cell cycle, cell death, epithelial-mesenchymal transition and cancer-stem cell trait as biological end points. Mutat Res. 2015;780:35–46.
Alan Mitteer R, Wang Y, Shah J, Gordon S, Fager M, Butter PP, et al. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep. 2015;5:13961.
Zhang X, Lin SH, Fang B, Gillin M, Mohan R, Chang JY. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. J Thorac Oncol. 2013;8:1484–91.
Takahashi M, Hirakawa H, Yajima H, Izumi-Nakajima N, Okayasu R, Fujimori A. Carbon ion beam is more effective to induce cell death in sphere-type A172 human glioblastoma cells compared with X-rays. Int J Radiat Biol. 2014;90:1125–32.
Ettl T, Viale-Bouroncle S, Hautmann MG, Gosau M, Kolbl O, Reichert TE, et al. AKT and MET signalling mediates antiapoptotic radioresistance in head neck cancer cell lines. Oral Oncol. 2015;51:158–63.
Nakagawa Y, Takahashi A, Kajihara A, Yamakawa N, Imai Y, Ota I, et al. Depression of p53-independent Akt survival signals in human oral cancer cells bearing mutated p53 gene after exposure to high-LET radiation. Biochem Biophys Res Commun. 2012;423:654–60.
Jin X, Li F, Zheng X, Liu Y, Hirayama R, Liu X, et al. Carbon ions induce autophagy effectively through stimulating the unfolded protein response and subsequent inhibiting Akt phosphorylation in tumor cells. Sci Rep. 2015;5:13815.
Takahashi A, Ma H, Nakagawa A, Yoshida Y, Kanai T, Ohno T, et al. Carbon-ion beams efficiently induce cell killing in x-ray resistant human squamous tongue cancer cells. Int J Med Phys Clin Eng Radiat Oncol. 2014;03:133–42.
Park SJ, Heo K, Choi C, Yang K, Adachi A, Okada H, et al. Carbon ion irradiation abrogates Lin28B-induced X-ray resistance in melanoma cells. J Radiat Res. 2017;58(6):765–71. https://doi.org/10.1093/jrr/rrx022
Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011;71:3676–87.
Oonishi K, Cui X, Hirakawa H, Fujimori A, Kamijo T, Yamada S, et al. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother Oncol. 2012;105:258–65.
Sun F, Zhang X, Zhou X, Hua J, Zhang Y, Wang B, et al. Comparisons between bio-radiation effects of X-rays and carbon-ion irradiation on glioma stem cells. Int J Clin Exp Med. 2017;10:4639–48.
Chiblak S, Tang Z, Campos B, Gal Z, Unterberg A, Debus J, et al. Radiosensitivity of Patient-Derived Glioma Stem Cell 3-Dimensional Cultures to Photon, Proton, and Carbon Irradiation. Int J Radiat Oncol Biol Phys. 2016;95:112–9.
Moncharmont C, Guy JB, Wozny AS, Gilormini M, Battiston-Montagne P, Ardail D, et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in head and neck squamous cell carcinoma (HNSCC). Oncotarget. 2016;7:47738–49.
Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, et al. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014;10:114–26.
Sai S, Wakai T, Vares G, Yamada S, Kamijo T, Kamada T, et al. Combination of carbon ion beam and gemcitabine causes irreparable DNA damage and death of radioresistant pancreatic cancer stem-like cells in vitro and in vivo. Oncotarget. 2015;6:5517–35.
Sai S, Vares G, Kim EH, Karasawa K, Wang B, Nenoi M, et al. Carbon ion beam combined with cisplatin effectively disrupts triple negative breast cancer stem-like cells in vitro. Mol Cancer. 2015;14:166.
Gabel D, Foster S, Fairchild RG. The Monte Carlo simulation of the biological effect of the 10B(n, alpha)7Li reaction in cells and tissue and its implication for boron neutron capture therapy. Radiat Res. 1987;111:14–25.
Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002.
Yura Y, Fujita Y. Boron neutron capture therapy as a novel modality of radiotherapy for oral cancer: principle and antitumor effect. Oral Sci Int. 2013;10:9–14.
Utsumi H, Ichihashi M, Kobayashi T, Elkind MM. Sublethal and potentially lethal damage repair on thermal neutron capture therapy. Pigment Cell Res. 1989;2:337–42.
Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151:1–18.
Sun T, Zhang Z, Li B, Chen G, Xie X, Wei Y, et al. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat Oncol. 2013;8:195.
Sun T, Li Y, Huang Y, Zhang Z, Yang W, Du Z, et al. Targeting glioma stem cells enhances anti-tumor effect of boron neutron capture therapy. Oncotarget. 2016;7:43095–108.
Hirota Y, Masunaga S, Kondo N, Kawabata S, Hirakawa H, Yajima H, et al. High linear-energy-transfer radiation can overcome radioresistance of glioma stem-like cells to low linear-energy-transfer radiation. J Radiat Res. 2014;55:75–83.
Cui FB, Liu Q, Li RT, Shen J, Wu PY, Yu LX, et al. Enhancement of radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer cells. Int J Nanomed. 2014;9:2345–58.
Miladi I, Aloy MT, Armandy E, Mowat P, Kryza D, Magne N, et al. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomedicine. 2015;11:247–57.
Hu C, Niestroj M, Yuan D, Chang S, Chen J. Treating cancer stem cells and cancer metastasis using glucose-coated gold nanoparticles. Int J Nanomed. 2015;10:2065–77.
Castro Nava A, Cojoc M, Peitzsch C, Cirillo G, Kurth I, Fuessel S, et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int J Cancer. 2015;137:2492–503.
You J, Zhao J, Wen X, Wu C, Huang Q, Guan F, et al. Chemoradiation therapy using cyclopamine-loaded liquid-lipid nanoparticles and lutetium-177-labeled core-crosslinked polymeric micelles. J Control Releas. 2015;202:40–8.
Kawashima H. Radioimmunotherapy: a specific treatment protocol for cancer by cytotoxic radioisotopes conjugated to antibodies. SciWorldJ. 2014;2014:492061.
Pohlman B, Sweetenham J, Macklis RM. Review of clinical radioimmunotherapy. Expert Rev Anticancer Ther. 2006;6:445–61.
Aghevlian S, Boyle AJ, Reilly RM. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting alpha-particles or Auger electrons. Adv Drug Deliv Rev. 2017;109:102–18.
Jandl T, Revskaya E, Jiang Z, Harris M, Dorokhova O, Tsukrov D, et al. Melanoma stem cells in experimental melanoma are killed by radioimmunotherapy. Nucl Med Biol. 2013;40:177–81.
Al-Ejeh F, Shi W, Miranda M, Simpson PT, Vargas AC, Song S, et al. Treatment of triple-negative breast cancer using anti-EGFR-directed radioimmunotherapy combined with radiosensitizing chemotherapy and PARP inhibitor. J Nucl Med. 2013;54:913–21.
Leyton JV, Gao C, Williams B, Keating A, Minden M, Reilly RM. A radiolabeled antibody targeting CD123(+) leukemia stem cells—initial radioimmunotherapy studies in NOD/SCID mice engrafted with primary human AML. Leuk Res Rep. 2015;4:55–9.
Weng D, Jin X, Qin S, Lan X, Chen C, Sun X, et al. Radioimmunotherapy for CD133(+) colonic cancer stem cells inhibits tumor development in nude mice. Oncotarget. 2017;8:44004–14.
Ceder J, Elgqvist J. Targeting prostate cancer stem cells with alpha-particle therapy. Front Oncol. 2016;6:273.
Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60:1402–6.
Lassmann M, Nosske D, Reiners C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: dosimetry and risk considerations. Radiat Environ Biophys. 2002;41:173–8.
Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted α-particle therapy. J Nucl Med. 2005;46:199S–204S.
Elgqvist J, Frost S, Pouget JP, Albertsson P. The potential and hurdles of targeted alpha therapy—clinical trials and beyond. Front Oncol. 2014;3:324.
Sgouros G, Song H. Cancer stem cell targeting using the alpha-particle emitter, 213Bi: mathematical modeling and feasibility analysis. Cancer Biother Radiopharm. 2008;23:74–81.
Dekempeneer Y, Keyaerts M, Krasniqi A, Puttemans J, Muyldermans S, Lahoutte T, et al. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther. 2016;16:1035–47.
Hegyi G, Szigeti GP, Szasz A. Hyperthermia versus oncothermia: cellular effects in complementary cancer therapy. Evid Based Complement Alternat Med. 2013;2013:672873.
Tran N, Webster TJ. Magnetic nanoparticles: biomedical applications and challenges. J Mater Chem. 2010;20:8760.
Cheung AY, Neyzari A. Deep local hyperthermia for cancer therapy: external electromagnetic and ultrasound techniques. Cancer Res. 1984;44:4736s–44s.
Shetake NG, Kumar A, Gaikwad S, Ray P, Desai S, Ningthoujam RS, et al. Magnetic nanoparticle-mediated hyperthermia therapy induces tumour growth inhibition by apoptosis and Hsp90/AKT modulation. Int J Hyperthermia. 2015;31:909–19.
Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv. 2011;2:1001–14.
Beik J, Abed Z, Ghoreishi FS, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–21.
Van Oorschot B, Granata G, Di Franco S, Ten Cate R, Rodermond HM, Todaro M, et al. Targeting DNA double strand break repair with hyperthermia and DNA-PKcs inhibition to enhance the effect of radiation treatment. Oncotarget. 2016;7:65504–13.
Man J, Shoemake JD, Ma T, Rizzo AE, Godley AR, Wu Q, et al. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015;75:1760–9.
Gorman AM, Heavey B, Creagh E, Cotter TG, Samali A. Antioxidant-mediated inhibition of the heat shock response leads to apoptosis. FEBS Lett. 1999;445:98–102.
Takasu T, Lyons JC, Park HJ, Song CW. Apoptosis and perturbation of cell cycle progression in an acidic environment after hyperthermia. Cancer Res. 1998;58:2504–8.
Slimen BI, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513–23.
Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS One. 2013;8:e75044.
Saldivar-Ramirez MM, Sanchez-Torres CG, Cortes-Hernandez DA, Escobedo-Bocardo JC, Almanza-Robles JM, Larson A, et al. Study on the efficiency of nanosized magnetite and mixed ferrites in magnetic hyperthermia. J Mater Sci Mater Med. 2014;25:2229–36.
Shetake NG, Balla MM, Kumar A. PANDEY BN. Magnetic hyperthermia therapy: an emerging modality of cancer treatment in combination with radiotherapy. J Radiat Cancer Res. 2016;7:13–7.
Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2003;36:R167–81.
Kelland LR. Targeting established tumor vasculature: a novel approach to cancer treatment. Curr Cancer Ther Rev. 2005;1:1–9.
Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16:023501.
Salunkhe AB, Khot VM, Pawar SH. Magnetic hyperthermia with magnetic nanoparticles: a status review. Curr Top Med Chem. 2014;14:572–94.
Kim JE, Shin JY, Cho MH. Magnetic nanoparticles: an update of application for drug delivery and possible toxic effects. Arch Toxicol. 2012;86:685–700.
Shubayev VI, Pisanic TR 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–77.
Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–43.
Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9:1533–45.
Li L, Jiang L-L, Zeng Y, Liu G. Toxicity of superparamagnetic iron oxide nanoparticles: research strategies and implications for nanomedicine. Chin Phys B. 2013;22:127503.
Sadhukha T, Niu L, Wiedmann TS, Panyam J. Effective elimination of cancer stem cells by magnetic hyperthermia. Mol Pharm. 2013;10:1432–41.
Kwon Y-S, Sim K, Seo T, Lee J-K, Kwon Y, Yoon T-J. Optimization of magnetic hyperthermia effect for breast cancer stem cell therapy. RSC Adv. 2016;6:107298–304.
Chen F, Cai W. Nanomedicine for targeted photothermal cancer therapy: where are we now? Nanomedicine (Lond). 2015;10:1–3.
Melamed JR, Edelstein RS, Day ES. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano. 2015;9:6–11.
Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–83.
Huang X, El-Sayed MA. Plasmonic photo-thermal therapy (PPTT). Alex J Med. 2011;47:1–9.
Li J, Hu Y, Yang J, Wei P, Sun W, Shen M, et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21.
Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44:947–56.
Mirrahimi M, Hosseini V, Kamrava SK, Attaran N, Beik J, Kooranifar S, et al. Selective heat generation in cancer cells using a combination of 808 nm laser irradiation and the folate-conjugated Fe2O3 Au nanocomplex. Artif Cells Nanomed Biotechnol; 2018. https://doi.org/10.1080/21691401.2017.1420072
Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011;11:2560–6.
Zhou M, Zhao J, Tian M, Song S, Zhang R, Gupta S, et al. Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale. 2015;7:19438–47.
Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem Soc Rev. 2013;42:530–47.
Liang C, Diao S, Wang C, Gong H, Liu T, Hong G, et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv Mater. 2014;26:5646–52.
Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7:69–79.
Burke AR, Singh RN, Carroll DL, Wood JC, D’Agostino RB Jr, Ajayan PM, et al. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials. 2012;33:2961–70.
Chen Z, Ma L, Liu Y, Chen C. Applications of functionalized fullerenes in tumor theranostics. Theranostics. 2012;2:238–50.
Beik J, Khademi S, Attaran N, Sarkar S, Shakeri-Zadeh A, Ghaznavi H, et al. A nanotechnology-based strategy to increase the efficiency of cancer diagnosis and therapy: folate-conjugated gold nanoparticles. Curr Med Chem. 2017;24:4399–416.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Releas. 2000;65:271–84.
Liao HW, Hafner JH. Gold nanorod bioconjugates. Chem Mater. 2005;17:4636–41.
Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. https://doi.org/10.1002/wnan.1449
Pattani VP, Shah J, Atalis A, Sharma A, Tunnell JW. Role of apoptosis and necrosis in cell death induced by nanoparticle-mediated photothermal therapy. J Nanopart Res. 2015;17(1):20–31.
Huang X, Kang B, Qian W, Mackey MA, Chen PC, Oyelere AK, et al. Comparative study of photothermolysis of cancer cells with nuclear-targeted or cytoplasm-targeted gold nanospheres: continuous wave or pulsed lasers. J Biomed Opt. 2010;15:058002.
Brun E, Sanche L, Sicard-Roselli C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution. Colloids Surf B. 2009;72:128–34.
Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. 2010;2:55ra79.
Xu Y, Wang J, Li X, Liu Y, Dai L, Wu X, et al. Selective inhibition of breast cancer stem cells by gold nanorods mediated plasmonic hyperthermia. Biomaterials. 2014;35:4667–77.
Li W, Tan G, Cheng J, Zhao L, Wang Z, Jin Y. A novel photosensitizer 3(1),13(1)-phenylhydrazine -Mppa (BPHM) and its in vitro photodynamic therapy against HeLa cells. Molecules. 2016. https://doi.org/10.3390/molecules21050558
Konan YN, Gurny R, Allémann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B. 2002;66:89–106.
Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659.
Usacheva M, Swaminathan SK, Kirtane AR, Panyam J. Enhanced photodynamic therapy and effective elimination of cancer stem cells using surfactant-polymer nanoparticles. Mol Pharm. 2014;11:3186–95.
Galanzha EI, Kim JW, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in vivo detection and killing of circulating cancer stem cells. J Biophotonics. 2009;2:725–35.
Lee E, Hong Y, Choi J, Haam S, Suh JS, Huh YM, et al. Highly selective CD44-specific gold nanorods for photothermal ablation of tumorigenic subpopulations generated in MCF7 mammospheres. Nanotechnology. 2012;23:465101.
Wang J, Sefah K, Altman MB, Chen T, You M, Zhao Z, et al. Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem Asian J. 2013;8:2417–22.
Peng CA, Wang CH. Cancer stem-like cells photothermolysed by gold nanorod-mediated near-infrared laser irradiation. Int J Nanotechnol. 2014;11:1157–65.
Sun T, Wang Y, Wang Y, Xu J, Zhao X, Vangveravong S, et al. Using SV119-gold nanocage conjugates to eradicate cancer stem cells through a combination of photothermal and chemo therapies. Adv Healthc Mater. 2014;3:1283–91.
Thapa R, Galoforo S, Kandel SM, El-dakdouki MH, Wilson TG, Huang X, et al. Radiosensitizing and hyperthermic properties of hyaluronan conjugated, dextran-coated ferric oxide nanoparticles: implications for cancer stem cell therapy. J Nanomater. 2015;2015:1–11.
Yang R, Tang Q, Miao F, An Y, Li M, Han Y, et al. Inhibition of heat-shock protein 90 sensitizes liver cancer stem-like cells to magnetic hyperthermia and enhances anti-tumor effect on hepatocellular carcinoma-burdened nude mice. Int J Nanomedicine. 2015;10:7345–58.
Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C, et al. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics. 2015;5:970–84.
de Paula LB, Primo FL, Pinto MR, Morais PC, Tedesco AC. Combination of hyperthermia and photodynamic therapy on mesenchymal stem cell line treated with chloroaluminum phthalocyanine magnetic-nanoemulsion. J Magn Magn Mater. 2015;380:372–6.
Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X. Combined cancer therapy with hyaluronan-decorated fullerene-silica multifunctional nanoparticles to target cancer stem-like cells. Biomaterials. 2016;97:62–73.
Paholak HJ, Stevers NO, Chen H, Burnett JP, He M, Korkaya H, et al. Elimination of epithelial-like and mesenchymal-like breast cancer stem cells to inhibit metastasis following nanoparticle-mediated photothermal therapy. Biomaterials. 2016;104:145–57.
Kawakami Y, Matsushita M, Ueda R, Tsukamoto N, Ohta S. Immunotherapy targeting cancer stem cells. Nippon Rinsho Jpn J Clin Med. 2012;70:2142–6.
Kerns SL, Ostrer H, Rosenstein BS. Radiogenomics: using genetics to identify cancer patients at risk for development of adverse effects following radiotherapy. Cancer Discov. 2014;4:155–65.
Kouhsari E, Ghadimi-Daresajini A, Abdollahi H, Amirmozafari N, Mahdavi S, Abbasian S, et al. The potential roles of bacteria to improve radiation treatment outcome. Clin Trans Oncol. 2018;20(2):127–39. https://doi.org/10.1007/s12094-017-1701-7
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Consent is not required for this type of study.
Rights and permissions
About this article
Cite this article
Ghaffari, H., Beik, J., Talebi, A. et al. New physical approaches to treat cancer stem cells: a review. Clin Transl Oncol 20, 1502–1521 (2018). https://doi.org/10.1007/s12094-018-1896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1896-2