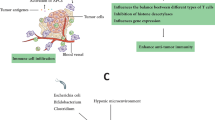
Abstract
Many combined therapies have been proposed to enhance radiotherapy outcome, but they have several limitations. As a new feasible strategy, combination of radiotherapy with bacteria showed a significant positive impact on the tumor treatment and metastasis inhibition. Although probiotic bacteria and radiotherapy alone can be effective in the treatment of different cancers, the combination of these two therapies seems to enhance therapeutic outcome and is cost-effective. Bacterial cells can act as therapeutic/gene/drug delivery vehicles as well as theranostic agents. In this communication, we reviewed current evidences, studies, suggestions, and future-based directions on combination of radiotherapy and bacteria. In another sections, an overview on tumor hypoxia, bacteria in cancer therapy, and combination of radiotherapy and bacteria is presented. A brief overview on trials and animal studies which used bacteria to protect normal tissues against radiotherapy-induced complications is also included.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of death throughout the world. According to global cancer statistics, 14.1 million new cancer cases were diagnosed in 2012 and 8.2 million people died from cancer [1]. In addition, by 2025, 19.3 million new cancer cases are expected to be diagnosed each year [2]. As a common feasible therapy approach, about 50–60% of cancer patients receive radiotherapy (RT) for their treatment courses [3]. However, despite of its efficiency, feasibility, availability, and specificity, RT was not proven to be very effective in different cancers. RT failure has a multifactorial etiology. These factors include large tumor and/or advanced tumor stage, relative radioresistance of tumors, failure to administer an optimal dose to the whole tumors, limitations in dose increasing due to complications of surrounding normal tissue, hypoxic and necrotic areas within the tumors and problems of tumor sites, and the ability of the surviving cells to repopulate within a treatment time of 6–7 weeks [4].
Advances in radiation dose delivery systems and machines, treatment planning systems, intensity and volumetric modulation, ion beams, and image guidance have been found much interest among clinicians as new RT approaches, but they have their own limitations and problems. Hyperthermia was shown to enhance RT and many research studies are in progress with this modality [5]. In the new era of nanomedicine and gene delivery, there is accumulating evidence which show that targeted therapy may become a future-based remedial way to enhance RT in cancer treatment [6, 7].
Different advanced strategies as combination therapy have been proposed to overcome failure and also enhance RT [8,9,10]. These strategies are based on the knowledge regarding the main mechanisms of tumor radiation resistance and also as a complementary mean to reduce radiation-induced normal tissue toxicities. Begg et al. [11] summarized best approaches to enhance RT. They concluded that modulating different biological mechanisms including DNA repair, cell-cycle checkpoints, signal transduction pathways, normal tissue damage, and modulating tumor microenvironment can enhance RT [11]. The two main approaches to enhance RT outcomes were suggested to be: “radiation sensitization” and “radiation protection”.
As a novel and future-based strategy, RT enhancement using bacteria is an active area of research. There are few interesting research directions which showed that bacteria can be used as an adjuvant modality to treat cancers combined with RT. To the best of our knowledge, there is no comprehensive review of all data in bacteria enhanced RT. In the other hand, normal tissue protection using bacteria has been investigated by many researchers and also as clinical trials.
In the present study, we aimed to review current evidences, studies, suggestions, and future-based directions on combination of RT and bacteria. We conducted an overview on tumor hypoxia, bacteria in cancer therapy, and combination of RT and bacteria. We also made a brief overview on trials and animal studies which used bacteria to protect normal tissues against RT-induced complications. At the end, we proposed conclusion remarks and future-based approaches.
Hypoxia and RT
Molecular oxygen (O2) plays a vital role in RT. It is an electron-affinic molecule which leads to DNA damage after the absorption of energy from ionizing radiation and must be present during irradiation. There are strong evidences which showed that well-oxygenated tumors are highly sensitive to radiation [12]. Tumors are formed as heterogeneous tissues and contain radio/chemoresistant hypoxic cells. Hypoxia has challenging definitions because of changing oxygen levels among different tissues. In tumors, hypoxia “develops as a result of an imbalance between supply of, and demand for, oxygen and, as such, depends both on the extent of blood perfusion and on the oxygen consumption of the cells in the tumor” [13]. Hypoxia may be caused by a number of factors and has a wide variety of concepts among different researchers. Speaking biologically, hypoxia has different impacts on tumors. It can enhance main mechanisms of tumor aggressiveness including angiogenesis, vasculogenesis, invasiveness, metastasis, epithelial-to-mesenchymal transition and suppressing immune reactivity [14]. In addition, hypoxia can change gene expressions that suppress apoptosis and receptor tyrosine kinase-mediated signaling [15]. Hypoxia also plays great roles in autophagy, cancer metabolism, tumor genotype, genomic instability, reactive oxygen species (ROS) production, and downregulation of DNA repair pathways [16]. At the clinic, the most significant feature of hypoxia is cancer therapy resistance, particularly RT. Hypoxia-induced RT resistance can be explained as the following: in normoxic conditions, molecular oxygen fixes ionizing radiation produced DNA radicals and causes DNA damages and then cell death, but in hypoxic conditions, DNA damage is reduced and leads to cell survival. At molecular level, hypoxia-inducible factor (HIF) and vascular endothelial growth factor A (VEGF A) have great impacts on radiation resistance. Under hypoxic conditions, the stabilization of hypoxia-inducible factor 1α (HIF1α) leads to upregulation of genes involved in cell survival. As RT induces ROS, it contributes to HIF1α stabilization and so cell survival. In addition, hypoxia increases VEGFA production that causes formation of abnormal vessels which contributes to tumor hypoxia [17]. HIF-1 is a transcription factor which regulates hypoxic response. Studies have shown irradiated tumors that express more HIF-1 which can lead to up regulation of different genes and signaling pathways which control cell proliferation, survival, metabolism, angiogenesis, and many other cellular functions that contribute to radiation resistance. In a recent study, Feng et al. [18] showed that hypoxia induces radiation resistance through activated autophagy to accelerate the clearance of cellular ROS products.
During last decades, several approaches have been proposed to overcome hypoxia and also enhance tumor oxygenation [19, 20]. Remedial methods including hyperbaric oxygen [21], combination of carbogen (95% oxygen-5% carbon dioxide) and nicotinamide [22], RSR-13 [23], erythropoietin [24], blood transfusions [25], local oxygenation, nitroimidazoles [26], bioreductive drugs (mitomycin C and tirapazamine) [27], and various hypoxic radiosensitizers have been used. Hypoxic radiosensitizers mimic oxygen effect and thereby enhance RT-induced DNA damages. On the other hand, bioreductive drugs undergo intracellular reduction to form active cytotoxic species under low oxygen tension.
Radiotherapy-induced normal tissue damages
Unwanted exposure to therapeutic beams during the course of RT can result in a wide range of normal tissues complications including acute toxicities, mild chronic symptoms, or severe organ dysfunction [28]. Based on clinical literatures, the volume of tissue irradiated, radiation dose, fractionation scheme, the delivery of radiation modifiers, intrinsic radiation sensitivity, tissue architecture, and different biological pathways determine the severity of such complications [29]. The pathobiological mechanisms of RT-induced normal tissue effects are complex and different signaling pathways were explained and advanced researches are in progress. According to time of manifestation, processes of such damages begin immediately after radiation exposure, but the clinical effects may appear weeks, months, or even years after treatment. According to this time, RT injuries can be classified as acute, consequential, or late effects. Acute effects are observed during the course or within a few weeks after treatment. Consequential effects may be caused by acute damage and appear later than acute effects and late effects emerge months to years after RT. To appear an RT-induced normal tissue injury, a communication system of cells within the tissue, DNA damage repair system, tissue vasculature, and its coagulation system, bone marrow-derived inflammatory and immune systems has the greatest role. The main mechanism of RT-induced injuries points to DNA damage. There are two types of DNA damage: direct and indirect. In indirect defect, high-energy radiation ionizes atoms and molecules (mostly water), which results in highly reactive ions as ROS. The most important free radicals are hydroxyl ROS which are highly reactive with DNA, proteins, and lipids. Radiation is also capable of directly damaging DNA and other critical molecules. By splitting biological bonds, radiation creates single-strand breaks (SSBs) and double-strand breaks (DSBs) within DNA molecule. DSB are among the most serious types of DNA damage and their signaling and repair is critical for all cells and organisms. SSBs may lead to point mutations [30].
Bacteria in cancer therapy
The use of bacteria for cancer therapy has been studied for more than one century; however, live bacteria were used for first time [31]. Bacteria are used for cancer therapy with different approaches. They have been used as immunotherapeutic agents, vectors to carry tumoricidal agents, and bacterial enzyme, also their toxins and spores serve as anticancer drugs [32]. Bacteria, such Salmonella typhimurium, Streptococcus pyogenes, Bifidobacterium longum, Escherichia coli, and Clostridium spices (C. sporogenes, C. oncolyticum, C. novyi, C. butyricum, C. beijerinckii, C. acetobutyricum, and C. novyi-NT) were used for cancer therapy [33]. Most of these bacteria can grow in hypoxic regions of tumor selectively and destroy cancer cells by producing enzymes including proteases, lipases, and hydrolytic enzymes [34]. They also employ inflammatory cells and, therefore, activate anti-tumor immune responses to remove malignant cells. The most bacteria used for cancer therapy is clostridium. The genus Clostridium includes sporogenic, anaerobic, Gram-positive rods, which are a phylogenetically heterogeneous group of obligatory anaerobic cells [35]. Clostridia are a group of anaerobes that can successfully colonize necrotic tissue. There is a wealth of papers in this subject, but we reviewed main concepts of bacterial-assisted cancer therapy.
Bacterial toxin-assisted cancer therapy
As an important issue with regard to bacterial-assisted cancer therapy, bacterial toxins are of particular interest. These agents act as proliferation, differentiation, and apoptosis regulator materials, and thereafter control the carcinogenesis processes [36]. They are also able to kill cancer cells directly. In regards to cell-cycle control, bacterial toxins have different actions and they may act as cell-cycle inhibitors or cell-cycle stimulators [32]. Cytolethal distending toxins (CDTs) and cycle inhibiting factor (Cif) are two main toxins as cell-cycle inhibitors which block mitosis and compromise the immune system by inhibiting lymphocytes proliferation [37]. These toxins can be released by some Gram-negative bacteria such as Campylobacter jejuni, S. typhi (release CDTs), and enteropathogenic and enterohaemorrhagic E. coli (release Cif) [37]. Cytotoxic necrotizing factor (CNF) is a cell-cycle stimulator toxin which can be released by E. coli. This toxin acts on cell proliferation and differentiation and plays key roles in carcinogenesis [38]. In addition, there are specific bacteria which release toxins that bind to antigens present on tumor surface. For example, diphtheria toxin (DT), pseudomonas exotoxin, Clostridium perfringens enterotoxin (CPE), botulinum neurotoxin (BoNT), alfa-toxin from Staphylococcus aureus, AC-toxin from Bordetella pertussis, shiga like toxins, and cholera toxin bind to the surface of cells and have remarkable roles in cancer therapy [32]. On the other hand, cancer therapy can be performed by conjugating toxins to cell-binding proteins such as monoclonal antibodies or growth factors [39]. This approach can be accomplished by targeting protein toxins such as Pseudomonas exotoxin, diphtheria toxin, and ricin [40].
Bacterial spore-assisted cancer therapy
Spores are protected structure which are formed by bacteria and allow them to survive in highly toxic conditions. In suitable conditions, the spores germinate and the bacteria thrive, thereby making them ideal to target cancers. Several animal studies have shown that administration (e.g., intratumoral and intravenous injection) of bacterial spores such as C. novyi-NT, C. histolyticum, and C. sporogenes is feasible approach to treat cancers with lowest side effects [41,42,43]. Therefore, bacterial spores have been employed as targeted delivery agents to carry therapeutic and cytotoxic materials into tumor cells as well as vectors for gene therapy [44].
Bacteria-assisted immunomodulation cancer therapy
A number of studies have shown bacteria which can be recruited as immunotherapeutic agents to enhance the antigenicity of tumor cells [31]. In vitro and in vivo investigations have reported that attenuated but still invasive strains of bacteria such as S. typhimurium can trigger the immune response and thereby destroy cancer cells [45, 46]. Avogadri et al. [47] vaccinated tumor bearing mice with S. typhimurium and then injected Salmonella into tumors. They concluded that invasive S. typhimurium can infect malignant cells both in vitro and in vivo. Several studies have shown that C. novyi-induced massive leukocytosis and inflammation have anti-tumor effects. Spore of this bacterium can activate inflammatory pathways using production of different cytokines including G-CSF, KC, IL-6, TIMP-1, and MIP-2, and also kill cancer cells directly [48, 49]. In addition, they confirmed that inflammatory reactions which are contribute to bacterial infections destroy cancer cells via production of degradative enzymes such as proteases, production of ROSs and stimulation of cellular immune response [50]. More animal studies and clinical trials are in progress with genetically engineering bacteria as immunomodulatory agents to treat cancer.
Bacteria as tumoricidal agents
Several studies have revealed that different live, non-pathogenic (or pathogenic), attenuated, or genetically modified bacteria serve as anti-tumor agents [51]. The previous experimental studies have not dealt with pathogenic species due to toxicity, illness, and death. A considerable amount of literature has been published on potential roles of anaerobic bacteria against cancer [52]. Because of their specific characteristics, these strains colonize anaerobic parts of tumors following intravenous administration and destroy cancer cells via destruction of cell membrane and microtubule destabilization. Different live probiotic bacteria were used for their anticancer effects [53]. Experimental demonstration of this effect was carried out on colorectal, breast, and bladder cancer. Lactic acid bacteria include Lactobacillus spp., Bifidobacterium spp., and Streptococcus spp. Lactobacillus spp. were applied on colon cancer [54].
In recent years, numerous animal studies carried out on genetically engineered bacteria. The main aim for genetically modification of bacteria is to reduce their pathogenicity to the host and increase their stability and effectiveness on tumors [31]. Salmonella, a Gram-negative non-spore forming facultative anaerobic bacterium is one of the most common bacteria which have been used in the field of genetic engineering as a vehicle for drug/gene delivery and also as a therapeutic vaccine [34]. There are pre/clinical trials which showed that genetically attenuated serovar Typhimurium VNP20009 has low immune and toxic side effects on experimental tumors [31].
Combination of radiotherapy with bacteria
Bacterial-assisted RT is the newest approach to deal with hypoxic tumors. The combination of radiotherapy with bacteria is a new active area of research. Although there are few studies that applied bacteria to enhance radiotherapy, but this field would be optimized as a new feasible strategy in clinical radiation oncology. Bettegowda et al. [55] used combination of spores of C. novyi-NT with different radiation therapy approaches to treat transplanted tumors in mice. They tested external beam radiation derived from a Cs-137 source, systemic radioimmunotherapy with an I-131-conjugated monoclonal antibody, and brachytherapy using plaques loaded with I-125 seeds on several mouse models. Their results showed that C. novyi-NT spores have little therapeutic effect and the combination resulted in long-term remissions in a significant fraction of animal. In this study, complete and partial responses were found in combination of a single dose of C. novyi-NT with brachytherapy and external beam therapy, respectively. They suggested that this combined therapy with the conventional doses of radiation is toxic for sites such as liver. In addition, they also reported that combination of C. novyi-NT with radioactive iodine could allow patients to be treated with lower doses of radiolabeled antibodies, thereby minimizing toxicity to normal tissues such as the bone marrow. Nuyts et al. [56] conducted a genetic radiotherapy research and applied radiation as a potential gene delivery agent. In this study, they isolated recA and recN genes, two radiation-inducible genes of the SOS repair system of Clostridium acetobutylicum DSM792, and confirmed radiation activation of these genes at a dose of 2 Gy. These results indicated that fractionated radiotherapy could lead to repeated gene induction resulting in prolonged and enhanced protein expression. Thus, gene targeting by ionizing radiation could provide a new means of increasing the therapeutic ratio in cancer treatment. Spatial and temporal expression of therapeutic genes such as tumor necrosis factor and cytosine deaminase could be induced [56]. They suggested that radio-responsive recA promoter significantly increases TNFα production in recombinant clostridia after 2 Gy irradiation [57]. Jiang et al. [58] evaluated the tumor suppression effects of combining RT with bacteria. In this study, E. coli carrying pAClyA was injected to CT26-bearing BALB/c mice and then they were irradiated to various doses of radiation (0, 8, 15, 21 Gy). The best outcome occurred was obtained in combination of the bacterium with 21 Gy of radiation for the highest tumor shrinkage and the complete eradication of the CT26 tumors. At 8 or 15 Gy, significant tumor shrinkage was observed, but tumor regrowth started about 15 days after bacterial injection. On the second trial, they treated tumor bearing mice with 21 Gy radiation and various doses of engineered E. coli (0, 5 × 106, 1 × 107, and 5 × 107 CFU). In this phase, complete response was obtained at 21 Gy combined with 5 × 107 CFU E. coli and also survival was significantly prolonged. At lower doses, results were not significant. This study revealed that engineered bacteria such as Escherichia coli strain K-12 can produce cytolysin A (ClyA) to enhance the therapeutic effects of radiation. In addition, the results confirmed that bacteriolytic therapy and radiotherapy could exert a striking inhibitory effect on the development of tumor metastasis. Platt et al. [59] investigated anti-tumoral effect of lipid mutants Salmonella strains (Salmonella YS1456 and YS1646) in combination with X-rays against tumor bearing mice. They conducted their experiment as follows: C57B6 or DBA/2J female mice were injected with B16F10 or Cloudman S91 melanoma cancer cells and then were further inoculated intraperitoneally or intravenously with Salmonella 2 × 105 CFU/mouse. After that, tumors were irradiated with 0–15 Gy X-rays act 1.109 Gy/min dose rate (using 250 kV 15 mA X-ray machine with 2 mm aluminum equivalent filtration). Results showed that combination of Salmonella and X-rays has supra-additive anti-tumor effects, with a greater slope of the dose–response curve. They also suggested that at higher doses (25–50 Gy), the supra-additive effect was not distinct, because this would have required full fractional dose–response. Liu et al. [60] studied combination of radiotherapy with engineered Salmonella typhimurium ΔppGpp (S.t ΔppGpp). This research was conducted to study an engineered theranostic bacterium to carry imaging probes and therapeutic molecules for tumor imaging and therapy, respectively. Imaging probe was bacterial luciferase, Lux, and Cytolysin A has been selected as therapeutic agent. Radiotherapy contributed to Salmonella. Typhimurium colonization in a colon tumor (CT26) model of BALB/c mice. RT was delivered in 21 Gy at three fractions. Bioluminescence imaging also was done and data were analyzed. Results indicated that combination of bacterial therapy and radiotherapy treatments reduced tumor growth compared with sole bacteria therapy.
Bacteria as radioprotector
Different approaches were suggested and applied to reduce radiotherapy-induced normal tissue toxicity [61, 62]. As clinically important agents, radiation modifiers and protectors can alter the response of normal tissues to RT. The application of bacteria to reduce normal tissue damages during or after RT has been suggested and implemented by different researchers. Different types of bacteria were suggested to reduce RT side effects. Based on their special property, gas producing bacteria have many beneficial effects [63,64,65,66].
As probiotics, bacteria have many health benefits. The beneficial effects of bacteria include modulation of antioxidant status, apoptosis, DNA damage, and stimulation of immune system according to World Health Organization; probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [67]. The use of probiotics to preserve normal tissue during radiotherapy has been shown in preclinical and clinical studies. These studies showed that probiotics have a role in the prevention of radiation-induced injuries. Ciorba et al. [68] tested probiotic bacteria (Lactobacillus rhamnosus GG) as potential radioprotective agents in C57BL/6 wild-type mice intestine before 12 Gy whole body radiation. The bacterium reduced radiation-induced epithelial injury and improved crypt survival. A TLR-2/MyD88 signaling mechanism can lead to repositioning of constitutive COX-2-expressing mesenchymal stem cells of the lamina propria from the villi to the crypt region.
In an animal study by Demirer et al. [69], supplementation of Lactobacillus delbrueckii, subspec. Bulgaricus B3 strain as a probiotic agent led to intestinal and gastrointestinal toxicity prevention in irradiated male Wistar rats. In another animal study, a probiotic compound containing Lactobacillus acidophilus, Lactobacillus helveticus, and Bifidobacterium spp was administrated to male Wistar rats and acute radiation enteritis with particular interest in endotoxinemia and bacterial translocation was studied [70]. They conduced that probiotics may have worthwhile therapeutic and preventive effects for radiation-induced enteritis. There are several, randomized clinical trials and a meta-analysis which studied therapeutic and preventive effects of probiotics in RT patients [71]. Most of these trials are related to reduce RT-induced diarrhea and also other parts of lower gastrointestinal tract. In a large double-blind, randomized, placebo-controlled study trial (n = 482), patients receiving a probiotic mixture containing viable lyophilized bacteria from several different strains of lactobacilli showed significantly lower incidence severity of RT diarrhea compared to placebo [72]. In another study, the preventive effect of Lactobacillus brevis CD2 lozenges against oral mucositis in head and neck cancer patients receiving chemoradiotherapy was studied [73]. In a randomized double-blind control trial, Demers et al. [74] administrated L. acidophilus-361 and B. longum probiotics as prophylaxis to 229 pelvic RT patients. Results showed that severe diarrhea was significantly lower at day 60 in the standard-dose, but not high dose group compared to placebo. In a Swedish randomized trial, 24 pelvic RT patients received a probiotic compound containing live L. acidophilus bacteria in a daily regimen as 5 days prior to RT and to 10 days after completing RT. Results showed that the bacterium probiotic significantly reduces the incidence of diarrhea [75]. In a Spanish prospective randomized placebo-controlled trial, 85 pelvic RT patients received liquid yogurt containing L. casei or placebo three times per day starting 1 week prior to RT. They observed no significant reduction in diarrhea [76]. In a large Italian double-blinded, placebo-controlled randomized trial, 490 patients undergoing postoperative RT for colorectal or cervical cancer were called to take viable lyophilized probiotic bacteria including strains of Lactobacilli, Bifidobacteria, and Streptococcus. Result of this study showed that diarrhea significantly was reduced [77]. Therefore, probiotics clearly have a beneficial role in prevention of radiation-induced toxicity. However, a summary of studies on probiotics used as radioprotective agents is listed in Table 1.
Theranostic approaches
Theranostic, as a new personalized medicine subject, can be defined as a material that combines therapeutic and diagnostic. It aims to deliver therapeutic drugs and diagnostic imaging agents at the same time within the same dose [78]. The main aim of theranostics is combination of therapy and diagnosis into one package to image and monitor the diseased tissue, delivery kinetics, and drug efficacy with the long-term hope of gaining the ability to tune the therapy and dose [79].
In recent years, there has been a body of research evidence in the engineering of different types of theranostic nanoparticles for cancer imaging and therapy [79]. The main aim of these studies was to find the most efficient, targeted, biocompatible, and biodegradable theranostic nanoparticles to obtain highest imaging and therapy outcome. Ideal theranostic nanoparticles have to pass different criteria including safety, high clearance from the body, rapid, and selective accumulation in target tissue, report biological characteristics of target, and efficiently deliver sufficient therapeutic agents to target tissue with the lowest damage to surrounding healthy tissues. Chen et al. [80] have discussed on the ways by which theranostic nanoparticles can be engineered. These were: (a) loading or conjugating therapeutic agents to existing imaging nanoparticles (e.g., quantum dots, iron oxide nanoparticles, and gold nanocages); (b) tagging of imaging contrast agents (e.g., fluorescent dyes, optical or magnetic nanoparticles, and various radioisotopes) to the existing therapeutic nanoparticles; (c) encapsulating both imaging and therapeutic agents together in biocompatible nanoplatforms (e.g., polymeric nanoparticles, ferritin nanocages, and porous silica nanoparticles); (d) engineering of unique nanoparticles (e.g., porphysomes, 64Cu-CuS, and gold nanoshells or cages) with intrinsic imaging.
The enhancement of RT effects using nanoparticles is one of the most rising landscapes in personalized oncology era. Radiosensitizing effects of nanoparticles enhance the biological effects of RT within the target and spares healthy organs [81]. As the physical base, the main interaction between RT and high atomic number nanoparticles is photoelectric effect which leads to diffused photons, photoelectrons, Auger electrons, and ROS that increase biological damages [82]. The main nanoparticles are gold (Au), gadolinium (Gd), iron (Fe), bismote (Bi), titanium (Ti), and hafnium (Hf). As discussed for theranostic approach, gadolinium is the most well-known nanoparticle which can be applied as contrast agent for MRI to obtain high spatial resolution and excellent soft-tissue image. It is also an effective radiosensitizer, since it has a high capture cross section due to its high atomic number (Z = 64) and can interact with high energetic radiations [83].
Application of bacteria as theranostics agents is of particular interest. Because bacteria have special biological characteristics, they can be used theranostically; for example, they are able to transfer genes into cancer cells and can be used for tumor diagnosis. In an interesting study, Park et al. [84] constructed bacteria-fluorescent bead conjugates for tumors imaging in living animals. Genetic engineered interventions led bacteria to express specific proteins which are of importance in cancer cell death pathways such as TNF-related apoptosis-inducing ligand (TRAIL), Fas ligand, and convertor enzymes [85]. More recently, studies have noticed interesting findings about quorum sensing (QS) system in engineered bacteria. QS has been reported as a switching mechanism among bacteria to distinguish between cancer and normal tissues. This approach was reported in QS-sensing-engineered E. coli and Salmonella. In another bacteria-assisted theranostics issue, because of difference in immune system strength between normal and cancer tissues, some (engineered) bacteria can be cleared by immune system in normal tissue but not in tumors [86]. Quispe-Tintaya et al. [87] benefited from this method and developed radioactive Listeria (conjugation of radioactive Rhenium using Listeria-binding antibodies) as a theranostic approach to image and treat pancreatic cancer recurrence and metastases.
In a recent paper, Luo et al. [88] introduced the bacteria-mediated targeting hypoxia as a theranostic agent. In this work, two delivery methods including a cargo-carrying and an antibody-directed method were recruited for delivering upconversion and gold nanorods for imaging and photo-thermal therapy, respectively. In this subject, two forms of the anaerobic Bifidobacterium breve and Clostridium difficile were used as mediating agents for nanorods delivery which had been exited using near-infrared light.
Theranostic nanoparticle producing bacteria
There are different microorganisms which biosynthesize intracellular and extracellular nanoparticles. They grab metal ions from the environment and turn them into metal element using enzymes derived by cell activities. Theses nanoparticles have a wide variety of applications including biosensors, drug and gene delivery, cancer imaging and treatment, antibacterial agents, and in many different sciences [88]. These biosynthesized nanoparticles can be categorized to metal, oxide, sulfide, and other nanoparticles.
As discussed earlier, different nanoparticle can be used theranostically. There are no reports of using nanoparticle producing bacteria as theranostic agents, but they were used as contrast media to image tissues. Magnetotactic bacteria (MTB) are well-identified bacteria in this field as magnetic resonance imaging (MRI) contrast agents [89]. These bacteria have specific intracellular structures, called magnetosomes, which synthesize nanometer-sized minerals magnetite (Fe3O4) or greigite (Fe3S4). For example, Magnetospirillum magneticum strain AMB-1 (AMB-1) coordinates over 100 genes to synthesize magnetic nanoparticles which are highly effective MRI contrast agents [90].
Theranostic gas producing bacteria
A considerable amount of literature has been published on gas producing microorganisms particularly bacteria. They produce various gases as a result of intestinal microbial colonization, metabolism, and subsequent fermentation. Studies show that these gases have several effects on gastrointestinal system and can be used as diagnostic markers. These gases include hydrogen (H2), carbon dioxide (CO2), hydrogen sulfide (H2S), and methane (CH4). There are indications that these gases are produced as gas vesicles [91]. For example, there are some few species of Haloarchaea (e.g. Halobacterium salinarum and Haloferax mediterranei) which produce filled gas proteinaceous nanocompartment vesicles [92]. These vesicles are gas permeable protein-shelled compartments with typical widths of 45–250 nm and lengths of 100–600 nm which enable cells to migrate to regions with optimal conditions. In an interesting study, Shapiro et al. [93] employed these vesicles as contrast agents for ultrasound imaging. They imaged intravenously injected gas vesicles (from Anabaena flos-aquae and Halobacterium NRC-1) mice using a scanning single-element ultrasound imaging system operating at 18 MHz. Their result showed that gas vesicles are promising new molecular reporters for ultrasonography with a feasible image quality. The main mechanism of these agents as contrast molecules is cavitation. Gas bubbles can act as nuclei for cavitation in an ultrasonic filed. In addition, these microbubbles can be used as therapeutic agents and also as vehicles for drugs or genes delivery [94]. Scientific evidence suggested that destruction of microbubbles with ultrasound is an ongoing mechanism to treat cancer and many other diseases. Several theranostic microbubble approaches are tested in animal trial studies and many others are currently being undertaken [95]. For example, Wang et al. [96] created and tested a theranostic microbubble containing a recombinant fibrinolytic drug, an echo-enhancing microbubble, and a recombinant thrombus-targeting device in form of an activated-platelet-specific single-chain antibody for ultrasound imaging and thrombolytic therapy in a mouse model. Ultrasound-guided RT using such contrast agents provides better image quality and survey motions during treatment. In this era, bacterial-assisted image guidance RT may be suggested for future-based personalized medicine.
Discussion
Radiotherapy remains the most feasible modality to treat many cancers. Different randomized trials have shown innovations in radiotherapy technology. They yielded better patient care over time; however, these technologies have several limitations including cost, availability, maintenance, complexity, and many others. Various problems such as tumor hypoxia, radiation resistance, and normal tissue damages have unsuitable impacts on RT.
In recent years, several studies indicated that bacteria have potential roles to enhance RT effectiveness as radiation sensitizer/protectors. Several mechanisms underlying bacteria-induced radiation sensitization were proposed. Bettegowda et al. [55] discussed the mechanism through which C. novyi-NT enhances the RT effect. They concluded that C. novyi-NT sensitizes hypoxic radioresistant parts of tumors and thus, exacerbating bacteriolysis of tumor endothelial cells which have undergone RT-induced microvascular damages. Such microvascular damages would increase the niche for C. novyi-NT growth by creating more hypoxic areas within tumor. It should be reminded that this bacterium is not a predefined or classical radiosensitizer, and may be called a radioenhancer, due to its different mechanism of action. Platt et al. [59] suggested that Salmonella secrete molecules which inhibit repair of radiation damage and thereby increase cellular radiosensitivity. In addition, radiation can change bacterial microenvironment and rendering it more accessible to induce infection. In the other hand, radiation make tumor cells more vulnerable to Salmonella infection/toxin and then to immune attacks. They also reported that radiation can increase the number of Salmonella. The fact that bacteria colonize within hypoxia area in tumors can make them suitable for cancer treatment. Bacteria also can directly target tumors and are able to have anticancer effects. There is a body of research evidence which has proposed tumor vascular abnormalities as a feasible reason for bacterial-assisted cancer therapy. Existing evidence shows that when a tumor is developing, new blood vessel formation has a disorganized manner with incomplete endothelial linings/blind ends which make them leaky and thereby permeable to bacteria. Because such disorders are contributing to inefficient transfer of oxygen and nutrients in tumors, it can provided favorable conditions for anaerobic bacteria colonization. Jibu et al. [97] showed that intravenously injection of E. coli as well as E. cloacae in forms of live as well as inactive cells reduce the number of metastatic lung colonies after abdominal irradiation. They concluded that bacterial lipopolysaccharide (LPS) is an effective component for this inhibition. LPS can activate several immunological effectors such as macrophages, NK cells and thereby induction of TNF, interferon, vascular endothelial damage, and augmentation of the adhesion of granulocytes and lymphocyte.
The combined therapy using radiation and bacteria has several benefits. Because bacterial cell production is simple and cost-effective, this therapy provides an alternative treatment for advanced cancers and metastases. In addition, genetic manipulation of bacteria makes them more targeting agents with lowest side effects. Specific biological property of bacteria including enhanced permeability and retention (EPR) effect causes bacteria to preferentially accumulate in tumors after injection with highest values and clear rapidly from normal tissues [98].
There are interesting studies which have used combination of bacteria and hyperthermia to treat cancer. Dietzel et al. [99] tested a combined modality of C. oncolyticum and radio-frequency hyperthermia on mice-bearing neck tumors. They revealed that warming of the tumor up to of 42–44 °C using local RF hyperthermia significantly enhanced tumor oncolysis by C. oncolyticum. In similar work, this effect was tested on slowly and rapidly growing tumors based on the thermic dose. They concluded that oncolysis intensification in tumors is mostly marked 12 h after hyperthermia and rapidly growing tumors regenerate more quickly than the slowly growing. In addition, a period of 12 h between hyperthermia and injection of clostridia represents a favorable interval for the timing of slowly as well as of rapidly growing tumors [100].
It also should be reminded that almost all bacteria are radioresistant and fractionated radiotherapy in the conventional doses or also in single doses had very little effect on the survival of bacteria. For example, Platt al. showed that exposure of the Salmonella to 20 Gy X-rays resulted in >90% survival of the bacteria. However, radiosensitivity is dependent to type of strain [59].
Based on several evidences mentioned above, in combination with radiation, bacteria act as a double edge sword. On the other hand, microbiota plays a vital role in response to radiation. As consisted of 10–100 trillion bacteria, human body (adults) is full of natural radiosensitizer/protectors. The role of gut microbiota in regulation of intestinal radiosensitivity was studied by Crawford and Gordon [101]. They reported that Fiaf, a microbiota-regulated, epithelial-derived, secreted protein, has a major role in radioresistance. In addition, it has been suggested that intestinal microflora enhance the mitotic rate of epithelial cells and act as radiosensitizer. Therefore, intestinal microflora play a radioprotective role against radiation-induced damages by activating Toll-like receptors (TLRs) present in intestinal tissues [102]. Different components of these bacteria including peptidoglycan, teichoic acid, lipopolysaccharides, ‘O’ side chain, flagellins, and lipopeptides are potent activators of TLRs. Activation of TLRs regulates genes associated with immune system stimulation, DNA repair enhancement, apoptosis reduction, and G2 phase cell accumulation.
There are several limitations and problems associated with using bacteria-assisted cancer therapy, including bacteria-induced toxicity, systemic infection, DNA damage, and also selection of feasible bacterial strain and convenient dose. However, these limitations can be removed using genetic manipulations.
In future, engineered bacteria in the forms of theranostic and therapeutic may have more feasible applications to enhance radiotherapy outcomes. Developing such agents needs further research studies and also clinical trials.
Conclusion
Here, we have reviewed the principles and applications of bacteria alone or in combination with radiotherapy to enhance cancer treatment. Application of bacteria as hypoxic radiosensitizer and also as normal tissue protector is an interesting remedial approach to enhance radiotherapy outcome. The potential role of bacteria as theranostic agents is an active area of research and many research studies are under investigation. In the future, bacteria as therapeutic or theranostic agents would maximize the likelihood of radiotherapy efficacy and minimize the risk of normal tissue toxicity.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Bentzen SM, Heeren G, Cottier B, Slotman B, Glimelius B, Lievens Y, et al. Towards evidence-based guidelines for radiotherapy infrastructure and staffing needs in Europe: the ESTRO QUARTS project. Radiother Oncol. 2005;75(3):355–65.
Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Tansl Med. 2013;5(173):1732sr2-sr2.
Datta N, Ordóñez SG, Gaipl U, Paulides M, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–53.
Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309.
Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296(5577):2404–7.
Higgins GS, O’Cathail SM, Muschel RJ, McKenna WG. Drug radiotherapy combinations: review of previous failures and reasons for future optimism. Cancer Treat Rev. 2015;41(2):105–13.
Winczura P, Jassem J. Combined treatment with cytoprotective agents and radiotherapy. Cancer Treat Rev. 2010;36(3):268–75.
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65.
Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–53.
Richardson RB, Harper M-E. Mitochondrial stress controls the radiosensitivity of the oxygen effect: implications for radiotherapy. Oncotarget. 2016;7(16):21469.
Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and predicting radiation response. Semin Radiat Oncol. 2015;25(4):260–72.
Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21.
Wang W-M, Zhao Z-L, Ma S-R, Yu G-T, Liu B, Zhang L, et al. Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1α and notch1 in head neck squamous cell carcinoma. PLoS One. 2015;10(2):e0119723.
Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307.
Feng H, Wang J, Chen W, Shan B, Guo Y, Xu J, et al. Hypoxia-induced autophagy as an additional mechanism in human osteosarcoma radioresistance. J Bone Oncol. 2016;5(2):67–73.
Corry J, Rischin D. Strategies to overcome accelerated repopulation and hypoxia—What have we learned from clinical trials? Semin Oncol. 2004;31(6):802–8.
Peters LJ. Targeting hypoxia in head and neck cancer. Act Oncol. 2001;40(8):937–40.
Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26(2):241–8.
Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6(1):10–21.
Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9(4):442–58.
Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys. 2007;69(4):1008–17.
Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63(1):25–36.
Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol. 2007;19(6):397–417.
Denny WA, Wilson WR. Tirapazamine: a bioreductive anticancer drug that exploits tumour hypoxia. Exp Opin Invest Drugs. 2000;9(12):2889–901.
Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9(2):134–42.
Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17(2):81–8.
Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–54.
Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–94.
Patyar S, Joshi R, Byrav DP, Prakash A, Medhi B, Das B. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):21.
Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in cancer therapy: renaissance of an old concept. Int J Microbiol. 2016;8(45):17–28.
Nallar SC, Xu D-Q, Kalvakolanu DV. Bacteria and genetically modified bacteria as cancer therapeutics: current advances and challenges. Cytokine. 2017;89:160–72.
Minton NP. Clostridia in cancer therapy. Nat Rev Microbiol. 2003;1(3):237–42.
Carswell E, Old LJ, Kassel R, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Nat Acad Sci. 1975;72(9):3666–70.
Nougayrède J-P, Taieb F, De Rycke J, Oswald E. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trend Microbiol. 2005;13(3):103–10.
Oswald E, Sugai M, Labigne A, Wu HC, Fiorentini C, Boquet P, et al. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Nat Acad Sci. 1994;91(9):3814–8.
Pastan I, FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991;254(5035):1173.
Antignani A, FitzGerald D. Immunotoxins: the role of the toxin. Toxins. 2013;5(8):1486–502.
Wei MQ, Ellem KA, Dunn P, West MJ, Bai CX, Vogelstein B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur J Cancer. 2007;43(3):490–6.
Ryan RM, Green J, Lewis CE. Use of bacteria in anti-cancer therapies. BioEssays. 2006;28(1):84–94.
Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti-tumour agents. Trend Microbiol. 2006;14(4):190–6.
Zhou S. Synthetic biology: bacteria synchronized for drug delivery. Nature. 2016;536(7614):33–4.
Fensterle J, Bergmann B, Yone C, Hotz C, Meyer S, Spreng S, et al. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 2008;15(2):85–93.
Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66(15):7647–52.
Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65(9):3920–7.
Frankel AE, Rossi P, Kuzel TM, Foss F. Diphtheria fusion protein therapy of chemoresistant malignancies. Curr Cancer Drug Targets. 2002;2(1):19–36.
Hagihara N, Walbridge S, Olson AW, Oldfield EH, Youle RJ. Vascular protection by chloroquine during brain tumor therapy with Tf-CRM107. Cancer Res. 2000;60(2):230–4.
Xu J, Liu XS, Zhou S-F, Wei MQ. Combination of immunotherapy with anaerobic bacteria for immunogene therapy of solid tumours. Gene Ther Mol Biol. 2009;13:36–52.
Sznol M, Lin SL, Bermudes D, Zheng L-M, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105(8):1027–30.
Jain RK, Forbes NS. Can engineered bacteria help control cancer? Proc Nat Acad Sci. 2001;98(26):14748–50.
Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100(6):1171–85.
Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73(2):451s–5s.
Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Nat Acad Sci. 2003;100(25):15083–8.
Nuyts S, Van Mellaert L, Theys J, Landuyt W, Lambin P, Anné J. The use of radiation-induced bacterial promoters in anaerobic conditions: a means to control gene expression in clostridium-mediated therapy for cancer. Radiat Res. 2001;155(5):716–23.
Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anné J, et al. Radio-responsive recA promoter significantly increases TNF [alpha] production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001;8(15):1197.
Jiang S-N, Phan TX, Nam T-K, Nguyen VH, Kim H-S, Bom H-S, et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli–mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18(3):635–42.
Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low K, et al. Antitumour effects of genetically engineered Salmonella in combination with radiation. Euro J Cancer. 2000;36(18):2397–402.
Liu X, Jiang S, Piao L, Yuan F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim. 2016;65(4):413–8.
Abdollahi H. Beneficial effects of cellular autofluorescence following ionization radiation: hypothetical approaches for radiation protection and enhancing radiotherapy effectiveness. Med Hypotheses. 2015;84(3):194–8.
Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–45.
Abdollahi H. Probiotic-based protection of normal tissues during radiotherapy. Nutrition. 2014;30(4):495.
Khademi S, Abdollahi H. Application of hydrogen producing microorganisms in radiotherapy: an idea. Iran J Public Health. 2014;43:1018–9.
Abdollahi H, Shiri I, Atashzar M, Sarebani M, Moloudi K, Samadian H. Radiation protection and secondary cancer prevention using biological radioprotectors in radiotherapy. Int J Cancer Ther Oncol. 2015;3(3):1–9.
Abdollahi H, Atashzar M, Amini M. The potential use of biogas producing microorganisms in radiation protection. J Med Hypotheses Ideas. 2015;9(2):67–71.
Group JFWW, Group JFWW. Guidelines for the evaluation of probiotics in food. London: World Health Organization, ON, Canada: Food and Agriculture Organization. 2002.
Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61(6):829–38.
Demirer S, Aydıntug S, Aslım B, Kepenekci I, Sengül N, Evirgen O, et al. Effects of probiotics on radiation-induced intestinal injury in rats. Nutrition. 2006;22(2):179–86.
Seal M, Naito Y, Barreto R, Lorenzetti A, Safran P, Marotta F. Experimental radiotherapy-induced enteritis: a probiotic interventional study. J Dig Dis. 2007;8(3):143–7.
Mego M, Holec V, Drgona L, Hainova K, Ciernikova S, Zajac V. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement Ther Med. 2013;21(6):712–23.
Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13(6):912.
Sharma A, Rath G, Chaudhary S, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation-and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer. 2012;48(6):875–81.
Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33(5):761–7.
Österlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97(8):1028–34.
Giralt J, Regadera JP, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys. 2008;71(4):1213–9.
Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, et al. Prevention of radiation-induced diarrhea with the use of VSL# 3, a new high-potency probiotic preparation. Am J Gastroenterol. 2002;97(8):2150.
Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62(11):1064–79.
Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62(11):1052–63.
Chen F, Ehlerding EB, Cai W. Theranostic nanoparticles. J Nucl Med. 2014;55(12):1919–22.
Miladi I, Alric C, Dufort S, Mowat P, Dutour A, Mandon C, et al. The in vivo radiosensitizing effect of gold nanoparticles based MRI contrast agents. Small. 2014;10(6):1116–24.
Detappe A, Lux F, Tillement O. Pushing radiation therapy limitations with theranostic nanoparticles. Nanomed. 2016;11(9):997–9.
Sancey L, Lux F, Kotb S, Roux S, Dufort S, Bianchi A, et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br J Radiol. 1041;2014(87):20140134.
Park SJ, Park S-H, Cho S, Kim D-M, Lee Y, Ko SY, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394.
Kojima R, Aubel D, Fussenegger M. Toward a world of theranostic medication: programming biological sentinel systems for therapeutic intervention. Adv Drug Deliv Rev. 2016;105:66–76.
Wu HC, Tsao CY, Quan DN, Cheng Y, Servinsky MD, Carter KK, et al. Autonomous bacterial localization and gene expression based on nearby cell receptor density. Mol Syst Biol. 2013;9(1):636.
Quispe-Tintaya W, Chandra D, Jahangir A, Harris M, Casadevall A, Dadachova E, et al. Nontoxic radioactive Listeriaat is a highly effective therapy against metastatic pancreatic cancer. Proc Nat Acad Sci. 2013;110(21):8668–73.
Luo C-H, Huang C-T, Su C-H, Yeh C-S. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–9.
Martel S, Mohammadi M, Felfoul O, Lu Z, Pouponneau P. Flagellated magnetotactic bacteria as controlled MRI-trackable propulsion and steering systems for medical nanorobots operating in the human microvasculature. Int J Robot Res. 2009;28(4):571–82.
Zurkiya O, Chan AW, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Mag Resonan Med. 2008;59(6):1225–31.
Pfeifer F. Distribution, formation and regulation of gas vesicles. Nat Rev Microbiol. 2012;10(10):705–15.
Oren A. The function of gas vesicles in halophilic archaea and bacteria: theories and experimental evidence. Life. 2012;3(1):1–20.
Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat Nanotechnol. 2014;9(4):311–6.
Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Dev Ther. 2013;7:375–88.
Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J Nucl Med. 2012;53(3):345–8.
Wang X, Gkanatsas Y, Palasubramaniam J, Hohmann JD, Chen YC, Lim B, et al. Thrombus-targeted theranostic microbubbles: a new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics. 2016;6(5):726.
Jibu T, Ando K, Matsumoto T, Koike S, Kobori O, Morioka Y, et al. Active components of intestinal bacteria for abdominal irradiation-induced inhibition of lung metastases. Clin Exp Metastasis. 1991;9(6):529–40.
Fang J, Liao L, Yin H, Nakamura H, Shin T, Maeda H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J Pharm Sci. 2014;103(10):3235–43.
Dietzel F, Gericke D, König W. Tumor hyperthermia using high frequency for increase of oncolysis by Clostridium butyricum (M 55). Strahlentherapie. 1976;152(6):537–41.
Dietzel F, Gericke D. Intensification of the oncolysis by clostridia by means of radio-frequency hyperthermy in experiments on animals–dependence on dosage and on intervals (author’s transl). Strahlentherapie. 1977;153(4):263–6.
Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Nat Acad Sci USA. 2005;102(37):13254–9.
Kainthola A, Gupta N, Agrawala PK. Gastrointestinal microflora in radiation injury and countermeasure. Ann Res Rev Biol. 2016;10(1):1–22.
Liu Q, Nobaek S, Adawi D, Mao Y, Wang M, Molin G, et al. Administration of Lactobacillus plantarum 299v reduces side‐effects of external radiation on colon anastomotic healing in an experimental model. Colorectal Dis. 2001;3(4):245–52.
Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010;5(1):31.
Tanaka I, Tanaka M, Satoh A, Kurematsu A, Ishiwata A, Suzuki K, et al. Alteration of radioprotective effects of heat-killed Lactobacillus casei in X-irradiated C3H/He mouse related to blood level of proinflammatory cytokines by corticoids. J Radiat Res. 2010;51(1):81–6.
García-Peris P, Gimeno CV, Lozano M, Moreno Y, Paron L, de la Cuerda Compés C, et al. Effect of a mixture of inulin and fructo-oligosaccharide on lactobacillus and bifidobacterium intestinal microbiota of patients receiving radiotherapy; a randomised, double-blind, placebo-controlled trial. Nutr Hosp. 2012;27(6):1908–15.
Ki Y, Kim W, Cho H, Ahn K, Choi Y, Kim D. The effect of probiotics for preventing radiation-induced morphological changes in intestinal mucosa of rats. J Korean Med Sci. 2014;29(10):1372–8.
Nomoto K, Yokokura T, Tsuneoka K, Shikita M. Radioprotection of mice by a single subcutaneous injection of heat-killed Lactobacillus casei after irradiation. Radiation Res. 1991;125(3):293–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Ebrahim Kouhsari declares that he has no conflict of interest. Ali Ghadimi-Daresajini declares that he has no conflict of interest. Noor Amirmozafari declares that he has no conflict of interest. Seied Rabi Mahdavi declares that he has no conflict of interest. Sara Abbasian declares that she has no conflict of interest. Seyed Hamzeh Mousavi declares that he has no conflict of interest. Hashem Fakhre Yaseri declares that he has no conflict of interest. Masoud Moghaderi declares that he has no conflict of interest. Hamid Abdollahi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kouhsari, E., Ghadimi-Daresajini, A., Abdollahi, H. et al. The potential roles of bacteria to improve radiation treatment outcome. Clin Transl Oncol 20, 127–139 (2018). https://doi.org/10.1007/s12094-017-1701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1701-7