Abstract
At least 50% of surgically resected non-functioning pituitary adenomas (NFPA) recur. Either early or late adjuvant radiotherapy is highly efficacious in controlling recurrent NFPA but associates potentially burdensome complications like hypopituitarism, vascular complications or secondary neoplasm. Reoperation is indicated in bulky tumor rests compressing the optic pathway. To date, no standardized medical therapy is available for recurrent NFPA although cabergoline and temozolomide show promising results. Guidelines on the management of recurrent NFPAs are now available. The new 2017 WHO pituitary tumor classification, based on immunohistochemistry and transcription factor assessment, identifies a group of aggressive NFPA variants that may benefit from earlier adjuvant therapy. Nevertheless, NFPA patients exhibit a reduced overall life expectancy largely due to hypopituitarism and treatment-related morbidity. The management of recurrent NFPA benefits from a multidisciplinary teamwork of surgeons, endocrinologists, radiation oncologists, ophthalmologists, pathologists and neuro-radiologists in order to provide individualized therapy and anticipate deterioration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas account for 10–20% of all primary brain tumors [1]. They are considered non-malignant lesions that arise from diverse cell lineages of the anterior pituitary gland. These tumors tend to grow primarily within the sella turcica although they may extend to the clivus or the supra and parasellar regions. The incidence of clinically relevant pituitary adenoma is 1 case per 100,000 annually and the prevalence is 80–100 cases per 100,000 population [2, 3]. However, autopsy and radiologic studies have shown that pituitary adenomas are indeed much more common, with an overall prevalence of 17% of the population (1 in 6 people), ranging between 14 and 23%, many of which are incidentally found [4, 5].
Clinically non-functioning pituitary adenomas (NFPAs) are hormonally inactive lesions that account for 15–30% of all pituitary adenomas [5, 6]. At least 60% of them are macroadenomas (> 10 mm in diameter) and usually present as headache, hormonal impairment, visual disturbance, cranial nerve palsy or combinations of them [7]. Presentation as apoplexy is also possible but less common. According to the immunomarkers, most NFPA are tumors derived from the gonadotroph lineage [8].
Although the primary treatment of symptomatic NFPAs is surgery [9], complete resection is achieved only in 40–50% of the cases [10], and at least 10–20% of completely resected tumors recur after 5–10 years [1, 11]. Moreover, when residual tumor is left after surgery, recurrence rate reaches 40 and 50% at 5 and 10 years, respectively [1, 12]. As a consequence, quality of life is likely to be impaired, largely due to hypopituitarism or treatment-related morbidity, and the standardized mortality rate in these patients is definitely increased [13]. Therefore, addressing NFPA recurrence is a relevant issue when confronting pituitary tumors.
Currently, we lack sound evidence supporting a specific treatment algorithm regarding recurrent NFPAs. Data from observational and other low quality studies suggest that certain patients seem to benefit from postoperative adjuvant therapy [1, 5,6,7]. Radiation therapy [14], some medical treatments [13] and temozolomide [15, 16] are known to enhance tumor control in recurrent or aggressive disease. However, many questions remain regarding the natural history of NFPAs, the optimal timing for adjuvant therapy and its long-term toxicity, what subset of NFPA patients are more prone to recur, or what is the residual tumor threshold for indicating reoperation or adjuvant therapy.
In this paper we review the implications of the new 2017 World Health Organization (WHO) classification of pituitary tumors [8] on the current practice, highlight the main issues provided by the latest clinical guidelines, comment on the importance of peri-operative ophthalmological evaluation, describe the pros and cons of the various treatment options and discuss some controversial issues regarding recurrent NFPAs.
Discussion
Clinical implications of the new WHO classification of pituitary tumors
The 4th edition of the WHO classification of pituitary tumors [8] has abandoned the concept of hormone-producing pituitary adenoma and adopted an adenohypophyseal cell lineage designation with subsequent categorization of histological variants according to hormone content and specific histological and immunohistochemical features [17, 18] (see Table 1). The new classification is largely based on hormone immunohystochemistry and pituitary-specific transcription factors. In order to identify clinically aggressive adenomas, evaluation of tumor proliferation (by mitotic count and Ki-67 index) and tumor invasiveness (by imaging features) for each particular patient is also underscored [17]. Additionally, the classification provides clinical information on prognosis by differentiating variants of tumors with an increased risk of recurrence.
In addition to morphological features and immunomarkers, transcription factors can be used to classify adenomas into three groups: acidophilic lineage, which are positive to PIT-1, leading to somatotroph, lactotroph and tyrotroph tumors; adenomas positive to SF-1, leading to gonadotroph tumors; and adenomas positive to T-PIT, leading to corticotroph tumors. The positivity to cytokeratin immunostaining differentiates sparsely (worse prognosis) from densely granulated somatotroph tumors [8]. Immunohystochemistry, hormone and transcription factors determination leave ultrastructural imaging rarely necessary [17]. Under this new classification, null cell adenomas are defined as hormone immune-negative lesions, also negative for transcription factors, meaning they lack any defined cell lineage [19]. However, it has been reported that 95% of hormone-immunonegative adenomas express some lineage-specific transcription factor: about 67% show gonadotroph differentiation (SF-1 and/or Estrogen Receptor-alfa positivity), 27% expresses T-PIT suggesting corticotroph lineage, and only 2% expresses PIT-1 as in the acidophilic lineage [20]. Therefore, very few hormone-negative tumors are actually null cell adenomas.
The so-called silent or non-functional pituitary adenoma present without clinical or laboratory repercussion although they usually express lineage-specific immuno-staining. This group comprises more than one-third of all neuroendocrine pituitary tumors and the majority derives from the gonadotroph lineage [21]. Likewise, the majority of gonadotroph adenomas are hormonally silent and they are usually discovered as macroadenomas presenting with mass effect. They can be distinguished from null cell adenomas by their positivity to SF-1. The next most frequent silent adenoma is the corticotroph tumor, with 20% of them not inducing Cushing’s syndrome [21]. Lactotroph and somatotroph tumors are rarely silent.
Corticotroph adenomas present specific histological and immunohistochemical features, with immune-stain for ACTH and expression of T-PIT. Three morphological variants are defined: densely granulated adenoma (the most common), sparsely granulated adenoma and Crooke’s cell adenoma [8]. Silent corticotroph adenomas may exhibit densely granulated basophilic features with strong reactivity to ACTH (previously known as silent corticotroph subtype 1), but also sparsely granulated chromophobic features with focal ACTH immunoreactivity (formerly silent corticotroph subtype 2). As in hormonally active tumors, silent corticotroph adenomas show T-PIT expression. Silent corticotroph tumors are commonly found as macroadenomas with frequent cavernous sinus, clivus or sphenoid sinus invasion. They are prone to intra-tumor infarction or hemorrhage. Thus, identification of this NFPA subtype is clinically important because of their aggressive biologic behavior, tendency to apoplexy and increased recurrence rate.
Importantly, the new 2017 WHO classification of pituitary tumors recognizes a series of high-risk adenoma variants with increased propensity to recurrence [8]. They are listed in Table 2. Definition of some tumor variants, like null cell adenoma or plurihormonal PIT-1 positive adenoma (former silent adenoma subtype 3), needs specific transcription factor expression assessment [8, 17]. However, not all laboratories are currently able to perform transcription factors methodology and commercial antibodies for T-PIT (corticotroph lineage) are not yet available. Therefore, immunohistochemical staining remains crucial for pituitary adenoma identification and classification. As an example, Fig. 1 shows the morphological features of a previously silent densely granulated corticotroph adenoma with strong positivity to ACTH that ultimately recurred and produced Cushing’s syndrome.
a Left: pathology specimen of a silent corticotroph tumor showing a basophilic and densely granulated adenoma, formerly known as silent corticotroph subtype 1 (Hematoxylin–Eosin 40×). Right: strong ACTH immunostaining of the same specimen. b Left: microphotograph of the previous tumor at the time of recurrence exhibiting similar characteristics. At this point, the patient however, presented with Cushing’s syndrome (Hematoxylin–Eosin 40×). Right: ACTH immunostaining of the same case
Natural history of recurrent NFPA
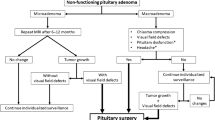
The postoperative follow-up of NFPAs remains a challenge in the daily practice. The usual scenario is that of an incompletely resected tumor in which standardized treatment strategies are yet to be defined. For an adequate decision, we should ideally take into consideration the growth rate of the residual tumor, the immune-phenotypic tumor subtype, the treatment-related morbidity and the preference of the patient in terms of therapy aggressiveness.
According to several studies, the presence of a postoperative residue greatly conditions the natural history of the disease [1, 12, 13, 22]. The study by O’Sullivan et al. [22] analyzed the course of 159 NFPA patients undergoing surgery and concluded that the risk of recurrence was 3.7 times higher in the subgroup of patients with extrasellar remnants compared to those with just intrasellar rests. Moreover, only 20.1% of the completely resected cohort did not recur over time. In patients with either intra or extrasellar rests, the recurrence rate was higher at 10 years compared to 5 years, leading to the conclusion that follow-up should be kept for an indefinite period and the approach to patients with extrasellar rests should be more aggressive. A meta-analysis [1] published in 2012 over 971 NFPA patients surgically treated confirmed these findings and noticed that within the cohort without residue (N = 300), recurrence also occurred (12 versus 46%) even after 10 years of follow-up.
According to some retrospective studies [23, 24], it appears that certain immune-histological subtypes of NFPAs tend to recur more often [25]. In a large cohort of 814 NFPA patients operated on, including gonadotroph (18.9%), silent corticotroph (4.7%) and silent somatotroph (2.1%) tumors, the rate of recurrence was significantly higher among corticotroph and somatotroph tumors, which additionally ended up in radiation therapy more frequently. Interestingly, 5.1% of silent corticotroph adenomas evolved to hypersecreting tumors presenting with symptomatic hypercortisolism (Fig. 1), an aggressive behavior previously reported by others [26, 27].
The term aggressive NFPA generally refers to lesions with higher probability of recurrence and bad response to conventional therapy (Table 2). Histopathologically, pituitary carcinomas cannot be distinguished from adenomas except for their ability to develop metastases [28]. In 2014, Raverot et al. [25] developed a novel histological and clinical classification of NFPAs aiming to predict their biological behavior. They proposed a score that included size, radiological invasiveness and proliferation index (Ki67≥3%, p53 positive, and more than two mitosis per 10 high power fields). Tumors were classified into five grades, from grade 1a (non-invasive and non-proliferative) to grade 3 (metastatic). According to this grading system, a study on 213 pituitary adenoma patients [29] followed an average of 3.5 years found that tumors in grade 2b (invasive and proliferative) presented a risk of recurrence and progression 3.7 times higher compared to those in grade 1a.
Noticeably, NFPA patients seem to have an increased overall mortality rate compared to the normal population. A retrospective Swedish study on 2795 NFPA patients (53% operated and 54% presenting with hypopituitarism) found an increased mortality rate only in women, mainly due to sepsis and cardiovascular events [30]. The authors also reported higher rates of diabetes, myocardial infarction, brain infarct and fractures in women compared to men and the general population [31]. The same authors have recently hypothesized that this excess mortality among women tended to normalize after the year 2007, coincidentally with an improvement of surgical techniques and in the management of postoperative hypopituitarism [32]. A British study [33] on 546 NFPA patients (all with pathological confirmation and followed within a structured protocol) showed a standardized mortality rate 3.6 times higher in comparison to the general population, attributable to cardiovascular events, infections and neoplasm. The only predictive factor was age over 50 years at the time of diagnosis. However, sex, presentation as apoplexy, degree of tumor extension, use of radiotherapy, recurrence or hormone deficit did not correlate with increased mortality [33]. Data from the Swedish study cohort presenting with hypocortisolemia (N = 392) confirmed that replacement doses of hydrocortisone ≥ 20 mg per day or ≥ 0,30 mg/kg/day also correlated with higher mortality [34], an important finding with practical clinical implications.
Ophthalmological evaluation of NFPA patients
Only 10% of NFPAs patients are diagnosed because of neuro-ophthalmological abnormalities, although more than 50–60% present with some kind of visual disturbance [7]. Ophthalmological affectation is attributed to the anatomical proximity between the pituitary gland and the optic pathway, especially the chiasm [35], where the fibers originating from the nasal hemi-retinas of both eyes decussate.
The review by Ortiz-Pérez et al. [35] describes several clinical syndromes linked to pituitary tumors compressing the optic pathway. The Central Chiasm Syndrome is the most common and occurs in patients with a typical centrally located chiasm (about 79% of the cases). Decussated nasal fibers are affected leading to bitemporal hemianopsia with a variable degree of central vision impairment. Neuro-ophthalmological examination of pituitary tumor patients should include visual acuity, intrinsic and extrinsic ocular motility, visual field, and fundus examination under dilated pupil [7, 35]. Optic discs are usually normal or may present with mild diffuse atrophy, or the so-called bow-tie atrophy, due to retinal fibers loss nasal to the fovea.
Currently, optical coherence tomography (OCT) is able to determine the integrity of the afferent visual pathway. This technique can quantify optic atrophy reproducibly, and is of prognostic value for postoperative visual recovery [36]. OCT provides a noninvasive means of capturing manifestations of axonal and neuronal loss and represents a surrogate marker of structural integrity of the central nervous system [37]. According to the study by Altun et al. [38] on 36 patients with micro or macroadenomas, there were significant differences in all OCT parameters between tumor patients and healthy controls: retinal nerve fiber layer (RNFL), ganglion cell layer, inner plexiform layer thickness, and choroid thickness. However, there were no significant differences in RNFL and ganglion cell layer measurements between microadenoma and macroadenoma. These authors conclude that neurodegeneration occurs along the course of pituitary tumors, present with decreased ganglion cell layer thickness at early stages and, as the disease progresses, other layers of the ganglion cell complex may be involved like RNFL and the inner plexiform layer (Fig. 2).
Optic coherence tomography (OCT) of a NFPA patient with visual affectation. There is a decreased thickness of the nasal retinal nerve fiber layer (RNFL) as they enter the optic nerve, leading to typical bow-tie atrophy (black dotted arrow). These are the fibers decussating at the chiasm that are compressed by pituitary adenomas. OCT allows visualization and quantification of the degree of thickness reduction in ganglion cell layer (CGL++) and internal plexiform layer (CGL+) belonging to the nasal hemi-retinas (black solid arrow)
The study by Danesh-Meyer et al. [39] on 107 patients undergoing pituitary decompression confirmed that long-term visual recovery after surgical decompression of pituitary lesions is predicted by pre-operative OCT RNFL, and patients with normal RNFL thickness show an increased propensity for visual recovery. This effect continues after long-term follow-up, although most visual recovery occurs within the first 6–10 weeks.
Both optic nerve, usually the temporal fibers, and the ganglion cell layer of the retina, usually the central and macular area and the nasal hemi-retina are affected in NFPA patients [35, 40]. Some OCT and other visual parameters seem to be predictive of reversibility after treatment: integrity of the optic nerve fibers and the ganglion cell layer of the retina, visual acuity over 0.2, absence of optic nerve atrophy, and tumor size [7, 35]. Older patients and patients with longer duration (> 4 months) of vision loss should be warned about the reduced chance of postoperative vision improvement [41].
Following treatment of the tumor, 80% of NFPA patients experience some visual improvement, with complete recovery in only 25% of the cases [7, 35]. However, improvement can be achieved up to 36 months after treatment. Timing for ophthalmological evaluation varies according to clinical guidelines [5,6,7]. A reasonable schedule would include assessment prior to surgery, 1 week afterwards, at 6 months and yearly thereafter in order to anticipate recurrences [7].
Overview of the current guidelines for the management of NFPAs
Recently, some consensus clinical guidelines on the management of NFPAs have been published [5,6,7]. Table 3 enumerates the main conclusions for each topic addressed by the recently released Congress of Neurological Surgeons (CNS) guidelines along with their level of evidence. Unfortunately, we lack level I evidence supporting any of the statements from these consensus guidelines and none of the topics addressed include information as to provide a standard of management. The CNS evidence-based guidelines included information collected from 300 studies and produced a total of 6 level II and 24 level III recommendations (Table 3).
These guidelines recommend routine preoperative endocrine, radiographic and ophthalmologic evaluations, including all pituitary axes and the degree of pretreatment visual impairment. Preoperative hormonal replacement may be needed in cases associating hypocortisolemia and/or hypothyroidism. Treatment options for symptomatic NFPAs include surgery and radiation, although small asymptomatic or incidental tumors may be followed. Surgery is undeniably the primary treatment in the majority of NFPA patients. Regarding the surgical technique, the endoscopic approach provides better visualization of the intrasellar cavity during resection compared to the classical transsphenoidal microsurgical approach. The sublabial-transsphenoidal approach provides a wider surgical corridor (up to 2.8 cm) compared to the transnasal-transsphenoidal approach (about 1.3 cm), and may be preferred for large sellar masses with significant lateral extension. The trans-nasal technique is the least invasive technique and requires minimum or no nasal packing. It appears, however, that individual surgeon experience greatly conditions the choice of approach.
Since early postoperative radiographic changes are difficult to interpret, MR imaging should not be performed before 3 months postoperatively [5, 6]. Biomarkers differentiate aggressive NFPAs, which are more prone to recur or progress in order to implement earlier intervention (re-operation, radiation or chemotherapy). However, the timing for post-surgery adjuvant therapy is not standardized. High-risk for recurrence or incompletely resected tumors should ideally benefit from radiation therapy acknowledging the possibility of mid and long-term complications of irradiation, mainly hypopituitarism but also vascular complications and secondary neoplasm. Length of post-treatment follow-up is not standardized but patients with visual or endocrine dysfunction probably need long-term or indefinite duration assessment.
The French Endocrinology Society set up in 2012 a multidisciplinary working group that elaborated guidelines [6] on clinically NFPAs. These guidelines provide information and recommendations about relapsing NFPA and two special situations: pituitary incidentaloma and apoplexy. According to these guidelines, when there is no residue after surgery, annual surveillance is recommended for 5 years and then at 7, 10 and 15 years. If there is residue or doubtful MR imaging, annual MRI is recommended for an indefinite period. Re-intervention is indicated for symptomatic optic pathway compression or in case of post-radiation progression. Radiotherapy is usually postponed until the remnant growth is confirmed as efficacy of early and late radiotherapy seems to be comparable, and there is a high risk of radiation-induced hypopituitarism, whatever the technique [40]. Figure 3 shows a radiographic example of long-term stability after surgical resection.
a A radiographic example of non-functioning macroadenoma with upward displacement of the optic pathway in a 67-year-old woman who presented with a 2-month history of headache and asthenia. She was operated on via transsphenoidal approach experiencing immediate postoperative symptom relief. b Four months later, brain MRI showed adequate optic pathway decompression and a minimum intrasellar residue. The pathological study revealed a gonadotroph adenoma. c Five years later, the patient remains asymptomatic and no signs of progression or recurrence are present
Pituitary incidentaloma is defined as a pituitary lesion found fortuitously on brain imaging performed by some other reason [6]. The French guidelines distinguish between small (< 5 mm) pituitary micro-incidentalomas, in which patient reassurance is the attitude recommended without radiologic nor hormonal surveillance, and large (> 5 mm) micro-incidentalomas, in which radiological surveillance is recommended at 6 months, 1 and 2 years. If the lesion is stable, surveillance can be stopped; if it progresses, annual MRI should guide management: surgery or surveillance. Macro-incidentalomas (> 10 mm) away from the optic chiasm (at least 5 mm apart) need close radiologic follow-up, periodic ophthalmological evaluation and assessment for hypopituitarism. If the lesion is stable MRI can be repeated every 2 years. However, macroincidentalomas close to the chiasm need hormonal, visual and radiologic evaluation every 6 months. At this point preventive surgery may be discussed with the patient, taking into consideration the natural history of NFPA, the morbidity associated to surgery, future plans for pregnancy and the patient’s compliance with surveillance.
Apoplexy is a severe condition characterized by acute headache and visual disorder due to chiasmal compression or oculomotor involvement [6]. It usually presents over non-functioning macroadenomas and two-thirds of the cases associate corticotroph deficiency [43]. Altered consciousness, recent or worsening visual defect, and maybe isolated oculomotor palsy are indications for surgery. Tumor decompression should be performed as soon as possible, preferably by an experienced surgeon. Conservative management is also possible as long as the patient is under high-dose corticoid therapy, no visual worsening occurs, adequate consciousness is maintained, and symptoms tend to improve over the following days. Patients with pituitary macroadenomas close to the chiasm should be warned of the risk of apoplexy.
Treatment options for recurrent NFPA
Surgery
Surgical revision is eventually needed in 30–50% of NFPA patients previously operated on [22, 44]. However, a second surgery does not seem to improve tumor control, and persistent residue can be found in 72% of reoperations [42], with a clinical benefit comparable if not inferior to radiotherapy. Visual improvement is also less frequent and intraoperative complications are slightly more common [42, 45]. Therefore, indications for revision surgery include progressive residue accessible for complete resection (without cavernous sinus invasion), persistence of optic pathway compression, to obtain a safe margin of at least 3–5 mm between the optic pathway and the tumor adequate for radiation planning, and in cases of progression after radiotherapy. Sometimes, primary surgery needs to be planned in two steps: first the intrasellar adenoma is resected and a second surgery is performed once the suprasellar rests descend. Neuronavigation can be useful in revision surgery since important anatomic landmarks like the sphenoid rostrum and sphenoid sinus septum are often distorted or removed during the first intervention [46, 47]. Additionally, the use of intraoperative ultrasound appears to be a safe and effective technologic adjunct to transsphenoidal surgery [48].
Surgical resection of NFPA with extrasellar extension is challenging and parasellar invasion classifications (Hardy [49], Knosp [50]) are helpful to predict the chances of gross total removal and endocrine remission. The interesting study by Micko et al. [51] confirmed that with increasing grades, the likelihood of surgically observed invasion rises and the chance of total removal and endocrine remission decreases. Moreover, direct endoscopic view confirmed that Knosp grades 1, 2 and 3 showed significantly lower rates of invasion than those previously believed, and in cases in which the intracavernous internal carotid artery was encased (Grade 4), all adenomas were invasive. The authors suggest subdividing grade 3 to distinguish the strikingly different outcomes of adenomas invading the superior cavernous sinus compartments (grade 3A) from those invading the inferior compartments (grade 3B), the latter presenting with higher rate of invasion.
Radiation therapy
Conventional radiotherapy has been indicated in recurrences and as adjuvant therapy in partially resected NFPAs. According to the meta-analysis by Murad et al. [52] the relative risk of recurrence of operated NFPAs without radiotherapy is 1.97 as compared to the irradiated. A 10-year progression-free survival rate greater than 90% is expected after radiotherapy compared to 50–60% of those not irradiated [11, 53]. Traditionally, radiation has been reserved for clinically aggressive tumors, large extrasellar residual lesions or confirmed recurrences.
The dose is usually 45–50 Gy in 25–30 fractions (1.8–2 Gy per fraction) both for conventional conformal radiotherapy or fractionated stereotactic radiotherapy. The usual marginal dose in radiosurgery is 13–16 Gy, allowing enough distance from the optic pathway to ensure < 8 Gy irradiation of the chiasm and the optic nerves [44]. Overall, radiotherapy offers a threefold decrease in global relapse rate, especially in patients with postoperative residue [11, 54, 55]. When total or subtotal surgical resection is achieved, radiotherapy can be deferred to the time of recurrence since late RT seems to be as effective as immediate postoperative RT in terms of tumor control [56].
Post-radiation hypopituitarism is a common complication occurring in 5–40% of the cases and up to 80% over a 10-year period [42]. Radiation induces pituitary but also hypothalamic deficiency (frequently associating hyperprolactinemia) which may ensue over the years. Keeping total dose < 50 Gy, fractionating at 1.8 Gy per day, and performing 3D dosimetry have virtually eliminated radionecrosis and visual complications [57]. However, radiation-induced neoplasm is a rare but well-established complication. These are mainly glioma, glioblastoma, sarcoma or meningioma, occurring even 20 years after irradiation [58]. The risk estimation is 2% at 10 years, 2.4% at 20 years and up to 8.5% at 30 years, according to the study by Minninti et al. [59] over 426 patients irradiated and followed for 5749 person-years. This incidence is 10 times more often as compared to the general population. Vascular complications like stroke [60] have been reported with conventional external RT but not with fractionated stereotactic radiotherapy [61]. Ionizing radiation induces a pro-thrombotic state and a vascular inflammatory reaction that aggravates atherosclerosis [62].
Radiosurgery (12–18 Gy in a single session) achieves 83–100% tumor control and induces hypopituitarism in 0–39% of patients harboring tumors under 3 cm in diameter and at least 3 mm apart from the optic pathways [63]. Fractionated stereotactic radiotherapy (48–54 Gy with 2 Gy per fraction) was developed to minimize the rate of complications and improve precision with respect to conventional radiotherapy (1–2 mm instead of 3–5 mm). Hypofractionated schemes (25 Gy in 5 fractions or 21 Gy in 3 fractions) are also effective [42].
There is no consensus about the optimal timing for adjuvant radiotherapy. According to the results of the recent study by Sadik et al. [12] adjuvant radiosurgical treatment (before 6 months postoperatively) yields the same long-term tumor control as delayed radiosurgery (at the time of progression) with effectiveness rates of 92 and 96%, respectively. Moreover, neither adjuvant nor delayed treatment induced additional neurologic complications. However, there seemed to be a trend that adjuvant treatment induced less additional endocrine deficits compared with delayed therapy. Another multicenter study [64] over 222 patients followed a median of 68 months, concluded that early gamma knife radiosurgery was associated with a lower risk of radiological progression in subtotally resected NFPAs compared with expectant management followed by late radiosurgery. The authors also stated that delaying radiosurgery might increase patient risk for long-term progression but the timing of radiosurgery did not appear to significantly affect the rate of delayed endocrinopathy. Figure 4 shows an example of clinical and radiologic stability after radiotherapy.
a A non-functioning very large macroadenoma presented as visual acuity impairment, bitemporal hemianopsia, severe headache and malaise in a 39-year-old male. The lesion was promptly operated via transsphenoidal approach. Headache and visual affectation improved immediately after surgery. At that time, hypopituitarism was diagnosed and hormone replacement was prescribed. b Five months later, brain MRI showed adequate tumor debulking and absence of optic pathway compression. Pathological study confirmed the gonadotroph lineage of the tumor. However, visual acuity progressively worsened over the following 6 months and adjuvant radiotherapy was recommended. c Sixteen months after radiotherapy the lesion remains stable. The patient needs corticoid and thyroid replacement and visual acuity remains affected
In summary, the efficacy of postoperative radiotherapy is doubtless, regardless of the technique, and late radiotherapy seems as effective as early treatment in terms of local control. The current trend favors deferring radiotherapy to the stage of confirmed recurrence in order to minimize the probability of radiation-induced hypopituitarism or secondary brain tumor.
Medical treatment
Currently, we lack specific medical therapies against recurrent NFPA. However, some medications (cabergoline and temozolomide) have shown promising results in their capacity to avoid or delay the need for radiotherapy or reoperation [65]. The study by Greenman et al. [66] on 79 NFPA patients with postoperative tumor rests and followed > 8 years on average, showed that patients treated with a median weekly dose of 1.5 ± 0.7 mg of cabergoline achieved tumor control in 87.3% of the cases versus 46.7% of untreated controls. Moreover, only 12.7% of those treated with cabergoline eventually needed a new operation or radiotherapy, as compared to 41.7% of controls. Interestingly, a correlation between the degree of expression of somatostatin receptors and response to cabergoline could not be confirmed. The results from a recently finished Brazilian trial on 140 NFPA patients operated and randomized to either cabergoline or placebo (Clinical Trials.Gov identifier: NCT03271918) will likely provide valuable clinical information.
The study by Losa et al. [67] on 31 aggressive pituitary tumors (10 aggressive NFPAs and 6 pituitary carcinomas) showed that temozolomide significantly improved survival at 2 and 4 years with minimum adverse effects. Very recently, the study by McCormmack et al. [68], promoted by the European Society of Endocrinology, has confirmed a survival gain with the use of temozolomide in aggressive tumors (40 carcinomas and 125 adenomas) with 6, 31, 33 and 30% rates of complete response, partial response, stability and progression, respectively. Interestingly, better response correlated with hormonally functioning tumors, lower expression of MGMT and concurrent use of radiotherapy [68]. In this study, the majority of patients in which the usual course of temozolomide was completed, eventually recurred, raising the question about the duration of treatment, and suggesting that temozolomide might be maintained especially in patients who do not exhibit significant adverse effects [68].
The recently published European Society of Endocrinology Guidelines [28] supports the use of temozolomide in monotherapy as first-line chemotherapy (maintaining treatment for at least 6 months in patients responding after the first three cycles) in aggressive pituitary tumors once tumor progression is confirmed.
Controversial issues and emerging therapies
Some unanswered questions remain regarding the management of recurrent NFPAs. Whether the election of a specific surgical approach conditions the chances of achieving gross total removal is still debatable. It is known that the endoscopic approach allows better visualization of the intra-tumor cavity and the lateral aspects of the sellar area. However, the widest corridor is obtained with the sublabial transsphenoidal approach. Although surgeon’s preference seems to greatly condition the choice of approach, it is our impression that senior experienced surgeons tend to favor the sublabial approach while younger surgeons may prefer the endoscopic approach.
We lack enough data regarding the optimal timing of adjuvant radiotherapy according to the various immune-histochemical NFPA variants. The current tendency is to offer early radiotherapy to aggressive lesions as defined by the new WHO classification. However, long-term follow-up is needed to ascertain the effectiveness and safety in each NFPA subtype.
There is a growing evidence [69] suggesting that cabergoline may be used on a regular basis as adjuvant therapy in partially resected NFPAs when radiotherapy is not immediately considered. This fact needs further research and confirmation in well-designed trials in the future.
Another controversial issue is whether volumetric measurement of the tumor is necessary to determine and quantify the degree of progression in NFPAs. Given the relatively small size of NFPAs, confirmation of progression can be challenging with the usual radiologic assessment criteria. Specification of volume change in mm3 would ideally yield earlier identification of progression and help in anticipating therapy. To date we do not know what is the minimum residue size or tumor growth rate threshold to indicate reoperation or adjuvant therapy.
Regarding emerging therapies, we still lack clinical in vivo trials that confirm the efficacy of any new medical therapy. Somatostatin analogs have shown to provide relief of symptoms in NFPA, maybe independently of an antitumor effect [42]. Pasireotide, a somatostatin analog with affinity to all somatostatin receptors except subtype 4 proved to be efficacious in vitro [70] but data in vivo are still unavailable, although a phase II trial is under way (ClinicalTrials.gov Identifier: NCT01283542). An association of somatostatin analogs plus dopaminergic agonists did not improve efficacy either [71]. Finally, mTOR (mammalian target of rapamycin) pathway inhibitor treatment concomitant with somatostatin analog octeotride [72] has shown promising results only in vitro.
Conclusions
Surgery is currently recognized as the primary treatment in NFPAs. However, at least half of the cases result in subtotal or partial resection leading to significant rates of recurrence. Adjuvant radiotherapy is efficacious in controlling tumor growth but associates potentially burdensome complications. Reoperation is indicated in bulky tumor rests compressing the optic pathway. To date, no standardized medical therapy is available although cabergoline and temozolomide show promising results.
The new WHO pituitary tumor classification is based on immune-histochemistry and transcription factor methodology and not only on the hormone produced. This classification identifies a group of aggressive NFPA variants that may benefit from earlier adjuvant therapy. Nevertheless, NFPA patients exhibit a reduced overall life expectancy largely due to hypopituitarism and treatment-related morbidity. Adequate hormone replacement, modern conformal radiation schemes and a thorough surgical resection should minimize mid and long-term complications. The management of recurrent NFPA patients ideally benefits from a coordinated work of surgeons, endocrinologists, radiation oncologists, ophthalmologists, pathologists and neuro-radiologists in a multidisciplinary team that should provide individualized therapy and anticipate deterioration.
References
Chen Y, Wang CD, Su ZP, Chen YX, Cai L, Zhuge QC, et al. Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology. 2012;96(4):333–42.
Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J Clin Endocrinol Metab. 2010;95(9):4268–75.
Day PF, Loto MG, Glerean M, Picasso MF, Lovazzano S, Giunta DH. Incidence and prevalence of clinically relevant pituitary adenomas: retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch Endocrinol Metab. 2016;60(6):554–61.
Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–9.
Aghi MK, Chen CC, Fleseriu M, Newman SA, Lucas JW, Kuo JS, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: executive summary. Neurosurgery. 2016;79(4):521–3.
Chanson P, Raverot G, Castinetti F, Cortet-Rudelli C, Galland F, Salenave S, et al. Management of clinically non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):239–47.
Cámara Gómez R. Non-functioning pituitary tumors: 2012 update. Endocrinol Nutr. 2014;61(3):160–70.
Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521–35.
Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z, et al. Congress of neurological surgeons systematic review and evidence-based guideline on primary management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E533–5.
Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15(1):71–83.
Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol. 2010;163(2):193–200.
Sadik ZHA, Voormolen EHJ, Depauw PRAM, Burhani B, Nieuwlaat WA, Verheul J, et al. Treatment of nonfunctional pituitary adenoma postoperative remnants: adjuvant or delayed gamma knife radiosurgery? World Neurosurg. 2017;100:361–8.
Greenman Y, Cooper O, Yaish I, Robenshtok E, Sagiv N, Jonas-Kimchi T, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016;175(1):63–72.
Li X, Li Y, Cao Y, Li P, Liang B, Sun J, et al. Safety and efficacy of fractionated stereotactic radiotherapy and stereotactic radiosurgery for treatment of pituitary adenomas: a systematic review and meta-analysis. J Neurol Sci. 2017;15(372):110–6.
Losa M, Bogazzi F, Cannavo S, Ceccato F, Curtò L, De Marinis L, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126(3):519–25.
Bengtsson D, Schrøder HD, Andersen M, Maiter D, Berinder K, Feldt Rasmussen U, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–98.
Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28(3):228–43.
Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO classification of tumors of endocrine organs, vol. 10. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Lee JC, Pekmezci M, Lavezo JL, Vogel H, Katznelson L, Fraenkel M, et al. Utility of Pit-1 immunostaining in distinguishing pituitary adenomas of primitive differentiation from null cell adenomas. Endocr Pathol. 2017;28(4):287–92.
Nishioka H, Inoshita N, Mete O, Asa SL, Hayashi K, Takeshita A, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol. 2015;26(4):349–55.
Manojlovic-Gacic E, Engström BE, Casar-Borota O. Histopathological classification of non-functioning pituitary neuroendocrine tumors. Pituitary. 2018;21(2):119–29. https://doi.org/10.1007/s11102-017-0855-1.
O’Sullivan EP, Woods C, Glynn N, Behan LA, Crowley R, O’Kelly P, et al. The natural history of surgically treated but radiotherapy-naïve non-functioning pituitary adenomas. Clin Endocrinol. 2009;71:709–14.
Langlois F, Lim DST, Yedinak CG, Cetas I, McCartney S, Cetas J, et al. Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary. 2018;21(1):32–40.
Langlois F, Lim DST, Varlamov E, Yedinak CG, Cetas JS, McCartney S, et al. Clinical profile of silent growth hormone pituitary adenomas; higher recurrence rate compared to silent gonadotroph pituitary tumors, a large single center experience. Endocrine. 2017;58(3):528–34.
Raverot G, Jouanneau E, Trouillas J. Clinicopathological classification and molecular markers of pituitary tumours for personalized therapeutic strategies. Eur J Endocrinol. 2014;170:R121–32.
Fang H, Tian R, Wu H, Xu J, Fan H, Zhou J, Zhong J. Cushing disease after treatment of nonfunctional pituitary adenoma a case report and literature review. Med (Baltimore). 2015;94(51):e2134.
Zoli M, Faustini-Fustini M, Mazzatenta D, Marucci G, De Carlo E, Bacci A, et al. ACTH adenomas transforming their clinical expression: report of 5 cases. Neurosurg Focus. 2015;38(2):E15.
Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic E, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178:G1–24.
Raverot G, Dantony E, Beauvy J, Vasiljevic A, Mikolasek S, Borson-Chazot F, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab. 2017;102(2):3368–74.
Olsson DS, Nilsson AG, Bryngelsson IL, Trimpou P, Johannsson G, Andersson E. Excess mortality in women and young adults with nonfunctioning pituitary adenoma: a Swedish Nationwide Study. J Clin Endocrinol Metab. 2015;100(7):2651–8.
Olsson DS, Bryngelsson IL, Ragnarsson O. Higher incidence of morbidity in women than men with non-functioning pituitary adenoma: a Swedish nationwide study. Eur J Endocrinol. 2016;175:55–61.
Olsson DS, Bryngelsson IL, Ragnarsson O. Time trends of mortality in patients with non-functioning pituitary adenoma: a Swedish nationwide study. Pituitary. 2017;20:218–24.
Ntali G, Capatina C, Fazal-Sanderson V, Byrne JV, Cudlip S, Ashley B, et al. Mortality in patients with non-functioning pituitary adenoma is increased: systematic analysis of 546 cases with long follow-up. Eur J Endocrinol. 2016;174:137–45.
Hammarstrand C, Ragnarsson O, Hallén T, Andersson E, Skoglund T, Nilsson AG, Johannsson G, et al. Higher glucocorticoid replacement doses are associated with increased mortality in patients with pituitary adenoma. Eur J Endocrinol. 2017;177:251–6.
Ortiz-Pérez S, Sánchez Dalmau BF, Adan-Civera A. Manifestaciones Neuroftalmológicas de los adenomas de hipófisis, valor de la tomografía de Coherencia Óptica. Rev Neurol. 2009;18(2):85–90.
Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):210–9.
Costello F. Optical coherence tomography in neuro-ophthalmology. Neurol Clin. 2017;35(1):153–63.
Altun Y, Karadag AS, Yucetas SC, Saglam S, Tak AZA, Cag I, et al. Neuroretinal evaluation using optical coherence tomography in patients affected by pituitary tumors. Ann Ital Chir. 2017;88:7–14.
Danesh-Meyer HV, Wong A, Papchenko T, Matheos K, Stylli S, Nichols A, et al. Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci. 2015;22(7):1098–104.
Rebolleda G, Diez Alvarez L, Casado A, Sanchez C, Dompablo E, Gonzalez J, Muñoz-Negrete FJ. OCT new perspectives in neurophthalmology. Saudi J Ophthalmol. 2015;29(1):9–25.
Newman SA, Turbin RE, Bodach ME, Tumialan LM, Oyesiku NM, Litvack Z, et al. Congress of neurological surgeons systematic review and evidence-based guideline on pretreatment ophthalmology evaluation in patients with suspected nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E530–2.
Cortet-Rudelli C, Bonneville JF, Borson-Chazot F, Clavier L, Coche Dequéant B, Desailloud R, et al. Post-surgical management of non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):228–38.
Briet C, Salenave S, Chanson P. Pituitary apoplexy. Endocrinol Metab Clin N Am. 2015;44(1):199–209.
Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;108(3):525–32.
Brada M, Rajan B, Traish D, Ashley S, Holmes-Sellors PJ, Nussey S, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38(6):571–8.
Linsler S, Antes S, Senger S, Oertel J. The use of intraoperative computed tomography navigation in pituitary surgery promises a better intraoperative orientation in special cases. J Neurosci Rural. 2016;7(4):598–602.
Eboli P, Shafa B, Mayberg M. Intraoperative computed tomography registration and electromagnetic neuronavigation for transsphenoidal pituitary surgery: accuracy and time effectiveness. J Neurosurg. 2011;114(2):329–35.
Marcus HJ, Vercauteren T, Ourselin S, Dorward NL. Intraoperative ultrasound in patients undergoing transsphenoidal surgery for pituitary adenoma: systematic review [corrected]. World Neurosurg. 2017;106:680–5.
Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185–217.
Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610–7 (discussion 617–8).
Micko AS, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122(4):803–11.
Murad MH, Fernández-Balsells MM, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, et al. Outcomes of surgical treatment for nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2010;73(6):777–91.
Olsson DS, Buchfelder M, Schlaffer S, Bengtsson BA, Jakobsson KE, Johannsson G, et al. Comparing progression of non-functioning pituitary adenomas in hypopituitarism patients with and without long-term GH replacement therapy. Eur J Endocrinol. 2009;161(5):663–9.
Woollons AC, Hunn MK, Rajapakse YR, Toomath R, Hamilton DA, Conaglen JV, et al. Non-functioning pituitary adenomas: indications for postoperative radiotherapy. Clin Endocrinol (Oxf). 2000;53(6):713–7.
Ferrante E, Ferraroni M, Castrignanò T, Menicatti L, Anagni M, Reimondo G, et al. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol. 2006;155(6):823–9.
Park P, Chandler WF, Barkan AL, Orrego JJ, Cowan JA, Griffith KA, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55(1):100–6 (discussion 106–7).
Erridge SC, Conkey DS, Stockton D, Strachan MW, Statham PF, Whittle IR, et al. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009;93(3):597–601.
Chen Y, Li ZF, Zhang FX, Li JX, Cai L, Zhuge QC, et al. Gamma knife surgery for patients with volumetric classification of nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Eur J Endocrinol. 2013;169(4):487–95.
Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90(2):800–4 (Epub 2004 Nov 23).
Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Radiotherapy for pituitary adenomas: long-term outcome and complications. Radiat Oncol J. 2011;29(3):156–63.
Chand-Fouché ME, Colin P, Bondiau PY. Pituitary adenomas: multimodal management and modern irradiation techniques. Cancer Radiother. 2012;16(Suppl):S90–100. https://doi.org/10.1016/j.canrad.2012.01.004.
Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67(1):10–8.
Minniti G, Jaffrain-Rea ML, Osti M, Cantore G, Enrici RM. Radiotherapy for nonfunctioning pituitary adenomas: from conventional to modern stereotactic radiation techniques. Neurosurg Rev. 2007;30(3):167–75 (discussion 175–6. Epub 2007 May 5).
Pomeraniec IJ, Kano H, Xu Z, Nguyen B, Siddiqui ZA, Silva D, et al. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J Neurosurg. 2017;27:1–10 (Epub ahead of print).
Greenman Y. Management of endocrine disease: present and future perspectives for medical therapy of nonfunctioning pituitary adenomas. Eur J Endocrinol. 2017;177(3):R113–24.
Greenman Y, Cooper O, Yaish I, Robenshtok E, Sagiv N, Jonas-Kimchi T, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016;175:63–72.
Losa M, Bogazzi F, Cannavo S, Ceccato F, Curto L, De Marinis L, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126:519–25.
McCormack AI, Dekkers O, Petersenn S, Popovic V, Trouillas J, Raverot G, et al. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018. pii: EJE-17-0933. (Epub ahead of print).
Vieira Neto L, Wildemberg LE, Moraes AB, Colli LM, Kasuki L, Marques NV, et al. Dopamine receptor subtype 2 expression profile in nonfunctioning pituitary adenomas and in vivo response to cabergoline therapy. Clin Endocrinol (Oxf). 2015;82(5):739–46.
Andersen M, Bjerre P, Schrøder HD, Edal A, Høilund-Carlsen PF, Pedersen PH, et al. In vivo secretory potential and the effect of combination therapy with octreotide and cabergoline in patients with clinically non-functioning pituitary adenomas. Clin Endocrinol (Oxf). 2001;54(1):23–30.
Broson-Chazot F, Houzard C, Ajzenberg C, Nocaudie M, Duet M, Mundler O, et al. Somatostatin receptor imaging in somatotroph and non-functioning pituitary adenomas: correlation with hormonal and visual responses to octreotide. Clin Endocrinol (Oxf). 1997;47(5):589–98.
Cerovac V, Monteserin-Garcia J, Rubinfeld H, Buchfelder M, Losa M, Florio T, et al. The somatostatin analogue octreotide confers sensitivity to rapamycin treatment on pituitary tumor cells. Cancer Res. 2010;70(2):666–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest regarding the content of this manuscript.
Research involving human/animal participants
No animals participated in this study.
Informed consent
No informed consent was necessary for this review. No individual patient can be identified by the content of this paper.
Rights and permissions
About this article
Cite this article
Delgado-López, P.D., Pi-Barrio, J., Dueñas-Polo, M.T. et al. Recurrent non-functioning pituitary adenomas: a review on the new pathological classification, management guidelines and treatment options. Clin Transl Oncol 20, 1233–1245 (2018). https://doi.org/10.1007/s12094-018-1868-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1868-6