Abstract
The assessment of response to therapy in glioblastoma remains a challenge, because the surrogate measures of survival are subject to radiographic misinterpretation. A solid and reliable definition of progression is needed for both clinical decision-making and for evaluating response within the clinical trials. Historically, assessment criteria have used radiologic and clinical features aimed to correctly classify patients into progressive or non-progressive disease. The widely used RANO criteria are a valuable tool in disease evaluation, both in the clinical setting and in the clinical trials. However, assessment criteria have certain limitations that emerging image techniques have tried to overcome. Differentiating true progression from treatment-related changes (like pseudoprogression or pseudoresponse) is crucial in order not to prematurely discontinue adjuvant chemotherapy or redirect the patient to second-line options. This fact underscores the need for advanced radiologic techniques, like specific diffusion and perfusion MRI sequences, MR spectroscopy and PET, which seem to play a role in distinguishing these phenomena.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor found in adults and is widely known for its poor prognosis [1]. Median survival for newly diagnosed GBM ranges from 12 to 18 months when treated with maximum safe resection plus adjuvant chemoradiotherapy [2]. In 2005, Stupp et al. [3] established the benefit of adding concurrent and adjuvant temozolomide (TMZ) to radiotherapy in terms of survival. Although this trial changed the treatment paradigm of GBM, progression almost invariably occurs despite continuation of TMZ cycles or initiation of second-line therapies and fewer than 10% of patients survive 5 years after the diagnosis [2]. At the time of recurrence, there is not yet any consensus as to the standard of treatment and no significant survival gain is expected following these secondary measures [4].
Overall survival (OS) is generally considered the primary endpoint in clinical trials assessing the efficacy of therapeutic agents for GBM. However, OS may not directly measure the impact of a specific regimen due to the possible confounding effects related to the many variables affecting these patients [4, 5]. Surrogate measures like progression-free survival (PFS) and objective radiographic response (RR) have been reported to be also valuable endpoints that may help to address the effect of a specific therapy, acknowledging their potential limitations in terms of inter-observer variability [6, 7].
Clinical methodologies used to determine the tumor response and techniques aimed to assess the radiographic response are currently evolving. Aside from OS, PFS and RR, and other measures, like the neurologic status and the dependence on steroids, have been introduced in an effort to further refine the accuracy of assessment criteria [8]. However, variables like quality of life (QOL) and performance status remain to be included as primary endpoints in the majority of trials. Moreover, tumor genetic parameters, recently incorporated into the new World Health Organization (WHO) classification of brain tumors [9], are also unaccounted by the currently used assessment criteria.
It is not uncommon that a brain magnetic resonance (MR) scan performed early after completing the concurrent chemoradiotherapy exhibits contrast-enhancing lesions that may represent either real progression of the tumor or post-radiotherapy changes that tend to improve spontaneously over time, the so-called pseudoprogression [10,11,12]. Differentiating both the situations is crucial to avoid unnecessary reoperations, premature discontinuation of adjuvant TMZ or its substitution for second-line agents or other salvage therapies. It must be noted that, although pseudoprogression and radiation necrosis (radionecrosis) are different clinical entities within the same pathological spectrum, the terms have been occasionally interchanged.
Correlation of enhancing disease with tumor progression can also be challenging in GBM patients undergoing anti-angiogenic therapy like bevacizumab or cediranib. These agents can rapidly reduce enhancement, giving the erroneous impression of a remarkable RR, which does not ultimately translate into improved survival [13]. This phenomenon is termed as pseudoresponse and it is likely to be a specific effect on blood vessel permeability, not a true anti-tumor action [11, 12].
Histopathologic examination is the reference method in the differential diagnosis of progression, pseudoprogression and radionecrosis. However, distinguishing these conditions is still difficult in some patients, because pseudoprogression may contain some portions of viable tumor [14] and surgical specimens are not always available for pathological study in every case. This fact underscores the need for new and reliable non-invasive radiographic methods that may help in distinguishing true tumor progression from treatment-related radiologic and histopathologic changes [14, 15].
In this review, we focus on the controversies relating to response assessment criteria in GBM patients undergoing surgical and chemoradiation therapy. The concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis are also discussed.
Discussion
The current standard of care in newly diagnosed high-grade glioma is largely based on the results from the EORTC 26981-22981 trial conducted by Stupp et al. [2], published in 2009. This protocol recommends maximal resection or biopsy followed by concomitant TMZ and radiation (60 Gy in 30 fractions) followed by six cycles of adjuvant TMZ. This regimen has demonstrated a significant impact on survival compared to postoperative radiation only: a gain of at least 2 months in median overall survival and a fivefold increase in survival at 5 years [2].
However, at the time of recurrence or progression, a standardized therapy protocol is yet to be established. Currently, continuation of anti-angiogenic therapy or other chemotherapy agents, the use of alternating electric fields applied to the scalp, participation in clinical trials or a combination of these is generally offered [16]. The results from a series of landmark trials [2, 17,18,19,20,21,22,23] published from 2004 to 2015 have set the indication and effectiveness of concomitant TMZ-radiation and adjuvant TMZ in newly diagnosed GBM, demonstrated a modest impact of the therapies in prolonging PFS (but not OS), highlighted the prognostic importance of age and O6-methylguanine-DNA-methyltransferase (MGMT) promoted methylation, and showed the positive impact of adjuvant TMZ and bevacizumab on QOL and performance status.
Response assessment criteria
High-grade glioma patients commonly undergo several treatment modalities throughout the course of their disease. Evaluation of treatment response in clinical trials by OS alone is subject to some limitations, including the use of consecutive therapies, the cross-over effect, in which the true efficacy of the original treatment may be masked by subsequent therapies. PFS, either as a mean duration or defined at a specific time point (like PFS at 3 or 6 months) may be a good substitute for OS, although it requires a solid definition of progression [4, 10]. Surrogate measures of tumor burden, like PFS and RR, also have limitations, which include clinical variability and inter-observer discordance in radiograph interpretation [5, 24]. Table 1 summarizes the definitions of progression according to the various assessment criteria historically and currently proposed in malignant glial tumors.
Neuro-oncologists are familiar with the concept of response assessment criteria as a measure of disease status [5]. Before 1990, response to therapy was estimated using the Levin criteria, which involved qualitatively imaging evaluation acknowledging a number of factors including contrast enhancement, mass effect and edema [25]. The WHO established response criteria was introduced some years later and used contrast-enhanced CT images to estimate the affected area by multiplying the maximal cross-sectional enhancing diameters [26]. Limitations of these criteria included marked inter-observer variability and the fact that contrast enhancement can be affected by several factors unrelated to the tumor [5, 27].
In 1990, the Macdonald criteria were introduced and for 20 years, they were considered the standard in assessment of response and progression in high-grade glial tumors [28]. They were based upon changes in contrast-enhanced CT/MRI two-dimensional tumor areas. According to these criteria, progression was defined as a greater than or equal to 25% increase in the size of the enhancing tumor, the appearance of any new tumor on CT or MRI or the occurrence of clinical deterioration. However, no time lapse was specified as to which refers to these changes. The Macdonald criteria classified responses into four categories: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), a well-known terminology used before in solid tumor oncology [5].
The advent of new MRI technical advances and the use of novel anti-angiogenic agents have highlighted the limitations of the Macdonald criteria. First, it is firmly recognized that there is an increased enhancement of the walls of the surgical cavity, even in completely resected tumors, beginning 24–48 h postoperatively. It is related to blood–brain-barrier permeability impairment caused by direct surgical trauma, not necessarily a true residual tumor enhancement [29]. Therefore, an early (within 48 h) baseline postoperative MRI is mandatory to rule out tumor rests. Second, about 25% of patients undergoing surgical resection and chemoradiation exhibit abnormal contrast enhancement soon after completing adjuvant therapy that resolves within the following weeks (pseudoprogression) and does not imply a worsened prognosis [11, 30]. Third, anti-angiogenic agents affect and may transiently normalize tumor vasculature, resulting in a prompt and markedly reduced contrast enhancement. This equivocal improvement in contrast enhancement (pseudoresponse) is attributed to a diminished vascular permeability induced by these agents, meaning not a true anti-tumor action [11, 12]. Additionally, a percentage of patients progressing on anti-angiogenics harbors non-enhancing lesions, an event also unaccounted by the Macdonald criteria. Finally, high-grade tumors commonly exhibit irregular shapes, multiplicity and postoperative or postradiation cyst walls enhancement, none of which were measured by the Macdonald criteria.
In 2006, the BRAIN study was initiated, a phase II trial aimed to evaluate the effectiveness and safety of bevacizumab with or without irinotecan in relapsed GBM [31]. It used WHO general criteria for response assessment, but introduced clinical progression as an indicator of PD and any new area of non-enhancing T2 or FLAIR signal consistent with tumor was considered PD as well. A confirmatory MRI performed 4 weeks after response was also needed. In this study, RR achieved in the combined therapy arm occurred in 35 and 71% of patients, according to the modified Macdonald and Levin criteria, respectively. They concluded that the Levin criteria was more sensible to early decreases in the enhancement, edema and mass effect compared to the reductions in the diameter of the enhancing tumor proposed by Macdonald. Moreover, this early decrease in enhancement correlated with PFS better than the response evaluated by the Macdonald criteria [32].
In 2009, the Response Evaluation Criteria in Solid Tumors (RECIST) group updated the guidelines previously published in 2000. It used unidimensional measurements of tumor diameters, not taking into consideration the clinical findings or steroid usage [33, 34]. Interestingly, strong concordance between the different methods of assessment (Macdonald, RECIST and RANO) was found both in the patients treated with anti-angiogenic agents [35] and in those not treated with them [36]. Criteria integrating FLAIR hyperintensity tended, however, to underestimate RR and PFS compared with the criteria considering only contrast enhancement [35].
The RANO criteria
In 2010, the Response Assessment in Neuro-Oncology (RANO) Working Group presented the RANO criteria [8] which were published concurrently and just after the initiation of the AVAglio trial [22]. Both of them considered non-enhancing lesions in the evaluation of disease status and they also contained specific guidelines to differentiate pseudoprogression from PD. However, the RANO criteria required stability or improvement of clinical symptoms to qualify for CR, PR or SD, although they did not specify according to what methodology it should be measured (KPS, ECOG status, WHO performance score). In contrast, the AVAglio protocol specified that SD must be confirmed with stable or improved neurologic symptoms and patients needed to be regularly examined by Mini-Mental State Examination as well as with radiographic evaluation.
The definition of PD according to the RANO criteria was a ≥ 25% increase of enhancing lesions on stable or increasing doses of corticosteroids, or a significant increase in non-enhancing (T2/FLAIR) lesions, or the appearance of any new lesion, or a clear clinical deterioration, non-attributable to other causes from the tumor or changes in corticosteroid dose, or clear progression of the non-measurable disease (see Table 1).
It is important to notice that, according to the RANO criteria, within the first 12 weeks after completing chemoradiation, clinical deterioration alone is insufficient to establish the diagnosis of PD. Besides, PD requires enhancement outside the radiation field to rule out pseudoprogression. The presence of viable tumor in biopsy samples also helps in differentiating PD from treatment-related changes. After those 12 weeks, a new contrast enhancement outside the radiation field or any increase in size is necessary for diagnosing progression. Any increase in the size of non-enhancing tumor is also suggestive of progression in patients receiving anti-angiogenics. Another difference with the Macdonald criteria is the use of T2/FLAIR images for non-enhancing lesions evaluation to define CR, PR and SD [8].
However, and despite their widespread use, validation of the RANO guidelines is still lacking, since they were developed for clinical trials. Although these criteria also have limitations, especially referring to the evaluation of non-enhancing tumor burden, they are currently considered as a useful guide for disease assessment in clinical practice. In 2015, the RANO criteria were applied to patients undergoing immunotherapy [37]. The recently published 2016 WHO classification of brain tumors incorporated genetic parameters in addition to the classical histopathological findings [9]. These genetic alterations have prognostic implications in terms of survival and response to therapies. Although some of these parameters (e.g., IDH/MGMT methylation status) are currently acknowledged in the decision-making process, no formal guidelines are yet available as to recommend the stratification of patients according to such parameters when assessing response. However, it is likely that future criteria may take into consideration some of these genetic markers.
Currently, there are no consensus guidelines defining the optimal frequency for the MRI follow-up after treatment. A proposed protocol from the National Comprehensive Cancer Network (NCCN) [38] suggests repeating scans 4 weeks after completion of radiation therapy and then every 2–4 months during the next 2–3 years and less frequently thereafter. Thus, the usual schedule of neuroimaging in high-grade glioma would ideally include: a preoperative MRI (set for navigation, 5-aminolevulinic acid-guided resection and including functional sequences for tumors within eloquent areas), intraoperative MRI for optimal tumor resection (where available), early postoperative MRI (before 48–72 h, to rule out tumor rests and suitable for radiation planning), post-irradiation MRI (4 weeks after completion of radiotherapy), and then repeat it every 2–4 months according to disease status and clinical course. Patients surviving more than 2 years need repeating MRI according to the effectiveness of second-line therapies. The International Standardized Brain Tumor Imaging Protocol [39] has established the minimum image acquisition requirements for 1.5T and 3T MR scans: sagittal/axial T1, axial FLAIR and axial DWI prior to contrast administration (0.1 mmol/Kg dose injection with a gadolinium-chelated contrast agent at 3–5 cc/s) and axial T2 and sagittal/axial T1 after contrast infusion.
Although the RANO criteria introduced the evaluation of non-enhancing lesions, variations in T2 or FLAIR images have proven difficult to be quantitatively measured and no standardized threshold for determination of PD has been established [5]. Regarding contrast-enhancing lesions, angiogenesis inhibitors and many tumor-extrinsic factors also affect vascular permeability and the leakage of gadolinium into the brain, which may lead to either pseudoprogression or pseudoresponse [30].
The AVAglio study
The AVAglio trial was a randomized, placebo-controlled, phase III trial [22] that began recruiting patients in 2009. The trial tested the effectiveness and safety of radiotherapy and TMZ with (458 patients) or without (463 patients) bevacizumab following surgical resection or biopsy in newly diagnosed GBM. The primary endpoints were OS and investigator-assessed PFS. PFS resulted significantly longer in the bevacizumab arm (10.6 vs. 6.2 months), but OS did not improve. Moreover, QOL and performance status was maintained for a longer period and the requirements for steroids were lower in the bevacizumab group at the expense, however, of an increased rate of grade three adverse events.
The definition of PD in the AVAglio trial included several new terms: ≥ 25% increase in index lesions or unequivocal progression of non-index lesions, or any new lesion or worsened neurologic symptoms, only if corticosteroid dose was stable or increased. Index lesions were defined as all measurable lesions (contrast-enhancing lesions with clear borders with both diameters ≥ 10 mm) identified at baseline. Non-index lesions included contrast-enhancing lesions too small or irregular in shape to be considered measurable and any non-enhancing lesion compatible with tumor. The latter were evaluated qualitatively as present, absent or not assessable. The designation unable to assess was: both index and non-index lesions that could not be reliably measured due to technical reasons, but not due to doubtful interpretation. Overall, the AVAglio trial tried to dichotomize disease assessment into PD or non-PD. Non-PD was all the other scenarios not accounted in PD or pseudoprogression and in patients with gross total resection with neither measurable nor non-measurable disease, for which there was no change in the radiologic assessment.
The definition of pseudoprogression in AVAglio was applicable to the period prior to the maintenance phase, usually 4 weeks after radiotherapy: a ≥ 25% increase in the sum of the longest perpendicular diameters of all the enhancing lesions compared with baseline or unequivocal progression of non-enhancing lesions and no new lesions outside the radiation field and no significant clinical neurologic worsening. Concomitant decrease in steroid dose within the 2 months after radiation rules out the designation of PD at this point. According to AVAglio, pseudoprogression needs to be confirmed at the next disease assessment, 2 months later, and it will designate if the patient should continue treatment or not. If the scan performed after 12 weeks of chemoradiation is still compatible with progression, treatment is discontinued. Conversely, if the evaluation is PR or SD, then the designation is pseudoprogression and the patient is continued on treatment. In these cases, the MRI performed right after radiotherapy is now considered the new baseline image (the so-called re-baselining effect), although these patients were excluded from the response analysis population of this trial.
When comparing RANO versus AVAglio, both the criteria consider non-enhancing lesions (not clearly measured, however) and provide specific guidelines to distinguish pseudoprogression from PD. In RANO, patients must be clinically stabilized or improved to qualify for CP, PR or SD. However, it is not specified how to measure clinical deterioration. AVAglio specifies that neurologic examination by MMSE needs to be regularly scheduled along with radiologic assessment. PD in RANO criteria can only be determined if, within the first 12 weeks after completing radiotherapy, the majority of the new enhancement is outside of the radiation field (for instance, beyond the 80% isodose line) [8]. In AVAglio, a scheduled scan 12 weeks after radiotherapy is needed to rule out pseudoprogression, which is integrated into the follow-up algorithm. A recent update of the RANO criteria applicable to surgically delivered therapies [40] (wafers, brachytherapy) and immunotherapy [37] is also available for better assessment.
The RTOG 0825 trial [41] was conducted in the United States largely in parallel with the AVAglio trial, conducted mostly in Europe. The RTOG 0825 was also a randomized trial aiming to assess the efficacy of bevacizumab versus placebo as concomitant and adjuvant to radiotherapy and TMZ in newly diagnosed GBM. The results on 637 randomized patients showed that OS was not improved, although PFS was longer in the bevacizumab group (10.7 vs. 7.3 months) at the expense of an increased symptom burden, a worse QOL and impairment of cognitive function. The long-term results from both the studies, reported in 2013 and 2014, respectively, showed similar perspectives: PFS was significantly prolonged, overall survival was not improved, safety and tolerability were acceptable in both the trials and QOL was preserved in the AVAglio trial, but not in the RTOG 0825 [42].
Modified RANO criteria for clinical trials
Ellingson et al. [4] have recently suggested a series of modifications to the current RANO criteria. First, they proposed a volumetric measurement of the lesion, in which a confirmed change in volume of the enhancing mass (either just the enhancing volume or the total enhancing volume including intra-tumor necrosis and cysts) may be predictive of survival [43]. Second, the use of contrast-enhanced T1 subtraction maps a voxel-to-voxel image subtraction of pre-contrast from post-contrast T1 weighted images, for better delineation of the tumor. Third, as recommended by the AVAglio trial, they consider post-radiotherapy MR image as the baseline for future response assessment in newly diagnosed GBM, instead of the usual postoperative images. The presence of blood products within the surgical cavity, the performance of the scan not following image protocols recommended for clinical trials, the variability in steroid dosage and timing of the MRI and, of course, the unpredictable occurrence of the pseudoprogression and the pseudoresponse phenomena are some of the reasons adduced. Fourth, they suggest discarding qualitative non-enhancing tumor evaluation requirements and consider only objective and measurable enhancing disease for better inter-observer concordance. And finally, they encourage the use of a systematic protocol for identifying pseudoprogression, pseudoresponse and, what has been termed as confirmed durable response in both the newly diagnosed and recurrent GBM.
In summary, assessment criteria have allowed standardized comparison of clinical data from GBM trials. The development of MRI techniques has incorporated accurate measurement of contrast-enhancing lesions, but somehow imprecise information about non-contrast tumor burden. Criteria that include FLAIR affectation usually underestimates RR and PFS relative to criteria that only take into consideration contrast enhancement for diagnosing progression [4, 5, 35]. PFS should ideally be supported by other measures that may further refine the description of the true clinical state. These include performance status, neurocognitive status, the need for steroids and QOL measurement [44]. Both RANO and AVAglio criteria can be reliably used in clinical practice. The AVAglio criteria avoid some of the uncertainty of the RANO criteria, which makes it more applicable for clinical trials. However, cases of pseudoprogression confirmed by AVAglio criteria need to be excluded from trials because of the mentioned re-baselining effect. Figure 1 shows a radiographic example of a GBM patient with postoperative tumor rest that underwent early reoperation. The subsequent local true progression was resected again. Figure 2 shows a radiographic example of typical postoperative MRI changes appearing prior to radiation therapy, and mimicking progression that ultimately evolved as stable non-measurable disease.
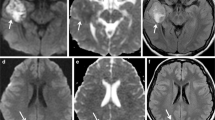
A radiographic example of postoperative tumor rest that underwent early reoperation and subsequent local true progression resected again. a Left parietal contrast-enhancing tumor consistent with glioblastoma on the histopathologic examination. b The patient is operated with 5-aminolevulinic acid guidance. Immediate postoperative MRI (after 48 h) shows an enhancing superficial tumor rest (white arrow). c The patient is re-operated (1 week after the first intervention) and the rest is resected. d 7 months afterwards, the tumor progresses in the nearby area. e The new enhancing lesion is again resected
A radiographic example of postoperative MRI changes (prior to radiation therapy) mimicking progression and presented as stable non-measurable disease. a Right temporal enhancing lesion diagnosed as glioblastoma upon histopathologic findings. b Immediate postoperative MRI showing complete resection. c New contrast enhancement (before radiation therapy was administered) around the surgical cavity (white arrow) considered to be non-measurable disease. d 2 months after completing radiation therapy, there is a marked reduction in the enhancement of the walls of the cavity. e 10 months afterwards, the lesion remains unchanged and the disease is stable
Treatment-related changes
Radiation necrosis (radionecrosis)
Radiation-related brain tissue injury can be acute (during irradiation), subacute (within 3 months after completing radiation) or late (months or years after radiation). Acute and subacute injuries seem to be caused by vasodilation, blood–brain-barrier impairment and changes in vascular permeability [11]. However, late injuries as in radionecrosis involve tissue necrosis linked to vessel damage and edema, secondary to capillary transudation [45].
The pathological features of radionecrosis include coagulation necrosis of the white matter, wall thickening and hyalinization of vessels and capillary obliteration with occasional reactive telangiectasia. These changes occur as a consequence of chronic inflammation and micro-vessel collapse around the tumor [46]. The typical appearance of radionecrosis in gadolinium-enhanced T1 MRI is the so-called Swiss cheese or soap bubble [46]. Radionecrosis usually appears months to years after irradiation and can be asymptomatic, although it commonly presents as a space occupying necrotic mass provoking neurological deficit.
Differentiating radionecrosis from PD or pseudoprogression is crucial for adequate treatment election. Some of the treatment modalities used in relapsing GBM like corticosteroids [47], bevacizumab [48,49,50] and surgery [51] are also applicable in radiation necrosis. Bevacizumab is currently considered the first choice, when steroids cannot reverse the symptoms [51]. Anticoagulation [52], hyperbaric oxygen therapy [53], laser interstitial thermal therapy [54] and oral vitamin E [55] are also available options that are associated with rather modest effectiveness.
Pseudoprogression
Pseudoprogression can be considered a subacute radiation-related reaction with or without neurological deterioration [11]. It occurs in 10–30% of GBM patients undergoing the first MRI following radiotherapy and concurrent TMZ (within the first 12 weeks) [1, 8, 56,57,58] and in up to 30–48% of patients who exhibit image progression within 1 month of the end of radiotherapy [58, 59]. Pseudoprogression is considered part of the radiation-related spectrum, although its pathophysiology has not been clearly elucidated [56]. It has been attributed to early changes in the vascular endothelium and blood–brain-barrier and oligodendroglial injury leading to inflammation and increased permeability [56]. Treatment-related cellular hypoxia, which triggers the expression of hypoxia-regulated molecules from the tumor, seems to play a role in the appearance of the abnormal enhancement [60].
According to Brandes et al. [61], the incidence of pseudoprogression in MGMT methylated patients is more than double compared to the unmethylated (91% vs. 41%) with positive prognostic implications. It has also been reported, following interstitial chemotherapy [62]. In patients undergoing carmustine wafers implantation, there is a high risk of cyst development (up to 90%) in the surgical bed [62] and a transient increase in contrast enhancement and peripheral edema within the first 2 months of wafer placement [63].
Although pseudoprogression resolves spontaneously without modifying therapy, it complicates interpretation of results in clinical trials and confuses clinical decision-making. The consequences of not recognizing the pseudoprogression include premature discontinuation of adjuvant TMZ, performing unnecessary salvage surgery or inclusion of the patient in clinical trials, resulting in falsely improved RR and PFS [8, 13, 15]. This fact underscores the need for appropriate baseline images and clinical assessment and full acknowledgement of the pseudoprogression event. Analysis of data from the AVAglio trial demonstrated that pseudoprogression complicated progression assessment in a small but relevant number of patients (2.2% in the bevacizumab group and 9.3% in the placebo arm), although it seemed to have a negligible impact on PFS [64]. Figure 3 shows a radiographic example of pseudoprogression.
A radiographic example of pseudoprogression. a MR scan showing contrast-enhanced T1 weighted (upper image) and T2 weighted images (lower image) of a left temporal-occipital paraventricular glioblastoma. b Postoperative MRI (4 days after surgery) showing complete tumor resection and mild enhancement of the cavity walls denoting early surgical trauma-related changes. c 4 weeks after completing chemoradiation, a marked increase in enhancement is visible, even beyond the previous limits of the tumor (white arrow) but within the radiation field. d 2 months later, the new enhancement disappears and the lesion remains stable indicating pseudoprogression
Pseudoresponse
Although enhancing tumor burden usually correlates with GBM progression, patients undergoing anti-angiogenic therapy may exhibit a marked reduction in enhancement within 1–2 days after administration with a radiographic response in 25–60% of the cases [65]. This is a direct effect on blood vessel permeability, not a true anti-tumor effect that does not translate into improved survival [8, 66]. According to the RANO criteria, a RR needs to be present for at least 4 weeks before it is considered a true response. The modified 2017 RANO criteria [4] also take into consideration the concept of confirmed durable response both in newly diagnosed and recurrent GBM. However, anti-angiogenics tend to promote progression of non-enhancing disease by selecting tumor cells not relying on angiogenesis [4], which may explain why OS is not improved by these agents [11, 30]. Non-enhancing infiltrative disease is a common cause of clinical deterioration, not successfully accounted for by the RANO criteria.
However, and according to some studies, the degree of subsequent enhancement decrease seems to correlate with survival [67] and normalization of permeability induced by bevacizumab or cediranib is associated with certain clinical benefits, whether an improbable true anti-tumor effect or just a pseudoresponse is observed, attributable to a reduction in vasogenic edema which improves neurological symptoms, QOL and reduces steroid dependence [30]. Thus, the presence of pseudoresponse, like pseudoprogression, may be considered a good prognostic marker [11, 12]. Figure 4 shows a radiographic example of pseudoresponse. Table 2 summarizes the main clinical, radiographic and management characteristics of these four phenomena.
A radiographic example of pseudoresponse. a A deep temporal contrast-enhanced lesion (upper: post-contrast T1 weighted images, lower: FLAIR images) with histopathologic confirmation of glioblastoma. b 1 month after surgery, enhancement appeared to be greatly increased and irradiation was dismissed at that point, so the patient continued under TMZ. c After two cycles of TMZ, the enhancing lesion further extends and second-line options are considered. At that moment, bevacizumab is initiated. d After 15 days (two doses of bevacizumab), there is a dramatic reduction in contrast enhancement within the lesion denoting pseudoresponse
A detailed description on the advanced MR techniques for differentiating pseudoprogression and radionecrosis from true progression is beyond the scope of this paper, but available elsewhere [15, 68,69,70]. Radiographic features reflecting hypercellularity [71,72,73,74] (like certain apparent diffusion coefficient histogram parameters from diffusion sequences) and neoangiogenesis/increased permeability [75,76,77,78,79,80] (from perfusion techniques), as well as amino acid PET and MR spectroscopy, are valuable tools [81,82,83], although the cut-off values need to be adjusted for specific clinical settings and image acquisition techniques [15].
Areas of controversy and uncertainty
It is not clearly established to what extent PFS is a good clinical endpoint in patients undergoing therapy for GBM. PFS may represent a statistical mixture of true anti-tumor effectiveness and also just the radiographic changes. In fact, we still do not know what the actual clinical meaning of pseudoresponse is or whether bevacizumab really does have a true anti-tumor effect [12]. Measures of the time from initial treatment to progression include the so-called time to progression (TTP). An important difference between TTP and PFS is that TTP refers exclusively to time to tumor progression, while PFS also includes time to death from any cause, which may be influenced by other factors [10]. However, we currently lack sound clinical evidence as to privilege the use of TTP over PFS as a surrogate measure of survival.
Another important issue is what follow-up image protocol should be routinely used for disease assessment in GBM. Although disease evaluation is largely based on MR imaging, other techniques like spectroscopy and PET are emerging tools that enhance accuracy and help in identifying PD. According to RANO criteria, measurable disease refers to bidimensional contrast-enhancing lesions with clearly defined margins and two perpendicular diameters of at least 10 mm that are visible on two or more axial slices no further than 5 mm apart without any interslice gaps [8]. However, the true radiological and clinical meaning of non-measurable disease is yet to be determined. To date, there is no minimum relevant tumor size threshold under which the presence of disease may be considered unimportant.
The coexistence of pseudoresponse and pseudoprogression in a particular patient is also an interesting issue. As pseudoprogression reflects increased permeability, administration of anti-angiogenics under the misdiagnosis of PD may show a falsely positive response. However, as we lack pathological confirmation for every suspected recurrence, counting these cases as a treatment effect may result in a falsely high response rate attributed to anti-angiogenics [12]. Thus, these two phenomena should not be considered separately, but within the same assessment protocol.
In a malignant disease such as GBM, it is paramount to maintain QOL as long as possible. Extensive surgical resections and aggressive chemotherapies may be a source of potential neurological and systemic morbidity affecting QOL throughout the course of the disease. Thus, when designing a treatment strategy for a particular patient, it is crucial to balance the maintenance of QOL, maybe at the expense of less therapeutic aggressiveness, against the possibility of a later rapid deterioration. Sacrificing longer survival for less morbidity is a matter of debate in GBM, in which second-line therapies are unlikely to provide real benefit in terms of survival. It seems reasonable that, before any therapy is applied, patients are ideally questioned, whether they consider QOL maintenance at all costs worth the possibility of an earlier deterioration.
Other issues, like the impact of immunotherapy on OS, what the subgroups of GBM patients benefit most from upfront bevacizumab therapy, the influence of the new genetic stratification of glial tumors or the impact of QOL and neurological status in disease assessment need further research.
Conclusions
Overall survival is generally considered the main endpoint of clinical studies testing therapeutic agents for GBM. As patients commonly undergo several treatments throughout the course of their disease, the efficacy of a specific treatment modality may be masked by the effect of subsequent therapies. Assessing response to therapy in GBM remains a challenge, because surrogate measures of survival, like PFS, are subject to radiographic misinterpretation. Thus, a solid and reliable definition of progression is needed for both clinical decision-making and for evaluating response within the clinical trials.
Radiotherapy-related radiographic changes, like transient increases in contrast enhancement as in pseudoprogression, can be difficult to interpret. Failure to recognize this phenomenon may lead to premature discontinuation of adjuvant TMZ or to redirect the patient to second-line or salvage therapies. Contrarily, anti-angiogenic medication like bevacizumab is able to normalize vasculature permeability and reduce tumor enhancement soon after its administration, which does not seem to be a true anti-tumor effect. However, both the phenomena may be considered as good prognostic factors.
Acknowledging these phenomena is crucial for adequate response assessment. The widely used RANO criteria are a valuable tool in disease evaluation, both in clinical trials and in daily practice, even though they have not been clinically validated yet. However, all the assessment criteria have limitations that the emerging image techniques try to overcome. Differentiating progressive disease from non-progressive disease is the ultimate goal of these criteria.
References
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;5:459–66.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–20.
Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347.
Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–70.
Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol. 2009;193(6):W515–22.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010;28(11):1963–72.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
Reardon DA, Ballman KV, Buckner JC, Chang SM, Ellingson BM. Impact of imaging measurements on response assessment in glioblastoma clinical trials. Neuro Oncol. 2014;16 suppl 7:Vii24–35.
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–85.
Chang JH, Kim CY, Choi BS, Kim YJ, Kim JS, Kim IA. Pseudoprogression and pseudoresponse in the management of high-grade glioma : optimal decision timing according to the response assessment of the neuro-oncology working group. J Korean Neurosurg Soc. 2014;55(1):5–11.
Huang RY, Neagu MR, Reardon DA, Wen PY. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy—detecting illusive disease, defining response. Front Neurol. 2015;6:33.
Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–46.
Yoo RE, Choi SH. Recent application of advanced MR imaging to predict pseudoprogression in high-grade glioma patients. Magn Reson Med Sci. 2016;15(2):165–77.
Khan MN, Sharma AM, Pitz M, Loewen SK, Quon H, Poulin A, et al. High-grade glioma management and response assessment-recent advances and current challenges. Curr Oncol. 2016;23(4):e383–91.
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–15.
Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–8.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9.
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–26.
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–91.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22.
Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–50.
Provenzale JM, Mancini MC. Assessment of intra-observer variability in measurement of high-grade brain tumors. J Neurooncol. 2012;108(3):477–83.
Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–35.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14.
Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–6. https://doi.org/10.1007/s11912-010-0143-y.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80.
Cairncross JG, Pexman JH, Rathbone MP, DelMaestro RF. Postoperative contrast enhancement in patients with brain tumor. Ann Neurol. 1985;17(6):570–2.
Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–8.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Gállego Pérez-Larraya J, Lahutte M, Petrirena G, Reyes-Botero G, González-Aguilar A, Houillier C, et al. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 2012;14(5):667–73.
Galanis E, Buckner JC, Maurer MJ, Sykora R, Castillo R, Ballman KV, et al. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8(2):156–65.
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, et al. Immunotherapy response assessment in neurooncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–42.
NCCN Guidelines. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#cns. Accessed 13 Sept 2017.
Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–98.
Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: response assessment in neuro-oncology (RANO) working group. Neurosurgery. 2012;70(1):234–43.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
Weller M, Yung WK. Angiogenesis inhibition for glioblastoma at the edge: beyond AVAGlio and RTOG 0825. Neuro Oncol. 2013;15(8):971.
Ellingson BM, Harris RJ, Woodworth DC, Leu K, Zaw O, Mason WP, et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98.
Minaya P, Baumstarck K, Berbis J, Goncalves A, Barlesi F, Michel G, et al. The caregiver oncology quality of life questionnaire (CarGOQoL): development and validation of an instrument to measure the quality of life of the caregivers of patients with cancer. Eur J Cancer. 2012;48(6):904–11.
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–84.
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(1):50–9.
Shaw PJ, Bates D. Conservative treatment of delayed cerebral radiation necrosis. J Neurol Neurosurg Psychiatry. 1984;47(12):1338–41.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95.
Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9(4):273–80.
Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis. Neurooncol Pract. 2016;3(4):272–80.
Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir (Wien). 2012;154(2):191–201.
Happold C, Ernemann U, Roth P, Wick W, Weller M, Schmidt F. Anticoagulation for radiation-induced neurotoxicity revisited. J Neurooncol. 2008;90(3):357–62.
Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209(1–2):115–7.
Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200.
Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–66.
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–61.
Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009;72(4):423–8.
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–10.
Pouleau HB, Sadeghi N, Balériaux D, Mélot C, De Witte O, Lefranc F. High levels of cellular proliferation predict pseudoprogression in glioblastoma patients. Int J Oncol. 2012;40(4):923–8.
Gahramanov S, Raslan AM, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–23.
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7.
Ulmer S, Spalek K, Nabavi A, Schultka S, Mehdorn HM, Kesari S, et al. Temporal changes in magnetic resonance imaging characteristics of Gliadel wafers and of the adjacent brain parenchyma. Neuro Oncol. 2012;14(4):482–90.
Colen RR, Zinn PO, Hazany S, Do-Dai D, Wu JK, Yao K, et al. Magnetic resonance imaging appearance and changes on intracavitary Gliadel wafer placement: a pilot study. World J Radiol. 2011;3(11):266–72.
Wick W, Chinot OL, Bendszus M, Mason W, Henriksson R, Saran F, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18(10):1434–41.
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95.
Norden AD, Drappatz J, Muzikansky A, David K, Gerard M, McNamara MB, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92(2):149–55.
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–300.
Lescher S, Jurcoane A, Veit A, Bähr O, Deichmann R, Hattingen E. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology. 2015;57(1):11–20.
Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, Harris RJ, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–10.
Mascalchi M, Filippi M, Floris R, Fonda C, Gasparotti R, Villari N. Diffusion-weighted MR of the brain: methodology and clinical application. Radiol Med. 2005;109(3):155–97.
Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25(2):201–9.
Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005;26(6):1455–60.
Song YS, Choi SH, Park CK, Yi KS, Lee WJ, Yun TJ, et al. True progression versus pseudoprogression in the treatment of glioblastomas: a comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J Radiol. 2013;14(4):662–72.
Chu HH, Choi SH, Ryoo I, Kim SC, Yeom JA, Shin H, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology. 2013;269(3):831–40.
Boxerman JL, Ellingson BM, Jeyapalan S, Elinzano H, Harris RJ, Rogg JM, et al. Longitudinal DSC-MRI for distinguishing tumor recurrence from pseudoprogression in patients with a high-grade glioma. Am J Clin Oncol. 2017;40(3):228–34.
Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, et al. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012;14(7):919–30.
Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004–10.
Hilario A, Sepulveda JM, Hernandez-Lain A, Salvador E, Koren L, Manneh R, et al. Leakage decrease detected by dynamic susceptibility-weighted contrast-enhanced perfusion MRI predicts survival in recurrent glioblastoma treated with bevacizumab. Clin Transl Oncol. 2017;19(1):51–7.
Yun TJ, Park CK, Kim TM, Lee SH, Kim JH, Sohn CH, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology. 2015;274(3):830–40.
Cha J, Kim ST, Kim HJ, Kim BJ, Kim YK, Lee JY, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35(7):1309–17.
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F] fluoroethyl)-l-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–95.
Kebir S, Khurshid Z, Gaertner FC, Essler M, Hattingen E, Fimmers R, et al. Unsupervised consensus cluster analysis of [18F]-fluoroethyl-l-tyrosine positron emission tomography identified textural features for the diagnosis of pseudoprogression in high-grade glioma. Oncotarget. 2017;8(5):8294–304.
Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest regarding the content of this manuscript.
Ethical approval
No humans or animals participated in this study.
Informed consent
Not needed for a literature review.
Rights and permissions
About this article
Cite this article
Delgado-López, P.D., Riñones-Mena, E. & Corrales-García, E.M. Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin Transl Oncol 20, 939–953 (2018). https://doi.org/10.1007/s12094-017-1816-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1816-x