Abstract
Recently, the nanotechnology-based bacterial immunotherapy emerged as a new combinatory therapeutic approach for the effective treatment of cancer, which combines the bacterial immunotherapy with nanotechnology for treating cancer. Although both bacterial immunotherapy and nanotechnology are very effective and advantageous solely, single treatment system is insufficient for complete eradication of cancer. Combining nanotechnology with bacterial immunotherapy opens new avenues for treating various diseases, abates the complication of bacterial immunotherapy, and overcomes the deficiency of both systems. Nanotechnology is helpful in targeting deep into the cancer cell due to its small size, enhanced permeability and retention (EPR) effect, and immunomodulatory activity. It also plays an important role in thermal and radio immunotherapy and cancer diagnostic. In this chapter, we highlighted the role of immunity in cancer and the role of bacteria in cancerogenesis, how bacterial immunotherapy is used in combating cancer, and how nanotechnology-based bacterial immunotherapy works on cancer regression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Immunotherapy is a therapy system used to combat cancer by stimulating the inherent defense mechanism of the body. Immunostimulants are used to boost the defense system to fight with cancer cells and destroy them. Immunotherapy is a kind of natural treatment; it is comprised of white platelets, organs, and tissues of the lymph framework. Biological therapy is a sort of treatment that utilizes substances produced using living life-forms to treat malignancy. Immunotherapy therapy attracted attention as an emergent therapy system in cancer treatment due to its potential to shatter immune tolerance and induce immune effects on selected cancer cell without any side effect (Hu et al. 2015). Immunotherapy can slow down the progress of cancer or arrest the spread of cancer. Bacteria-based immunotherapy for cancer was initiated way back in the nineteenth century (McCarthy 2006). William Coley in 1983 prepared bacterial endotoxin called “Coley toxin,” found its regressing effect on carcinoma, and then successfully treated various types of carcinomas (Kaimala et al. 2018). For the treatment of cancer using bacterial immunotherapy, a thorough knowledge of bacterial immunity against cancer cells is to be explored in a more aggressive way. In the last 10 years, various researches have been performed for exploring the relationship between bacteria and cancer (Linnebacher et al. 2011). The bacterial and microbial flora system maintains a balance; the imbalance between microbial floras induces the carcinogenesis by various ways, e.g., bacteria induced inflammation or immunomodulation (Song et al. 2019). Bacteria are causative agents for various communicable diseases, but they also have contribution in causing cancer, e.g., H. pylori-associated gastric cancer; mutagenic bacterial metabolite is also thought to be a main factor for cancer due to the inflammatory potential of the H. pylori that is associated with gastric cancer (Parsonnet 1995). The chronicity of the infection is directly proportional to the cancer; as long as the inflammation occurs, the chances of cancer development also enhanced (Singh et al. 2019). The intestinal microflora contains microbes that show symbiotic relationship and support the health system by enhancing the immunity against microbial pathogenesis (Zheng et al. 2020). The microflora interactions maintain the balance by protecting from detrimental microbes and provide a biological fencing on the membrane for infiltrating infectious or immunogenic molecule into the blood and also enhance forbearance toward the antigens available on the mucosa (Linnebacher et al. 2011). This process impedes pathogenic colonization, influences the mucosal barrier, and induces the immune response.
The basic concept of immunotherapy was to exploit the patient’s immune system as an effective tool for cancer treatment by stimulating the immune system to attack cancer cells. Some old reports demonstrated that an inflammation-related cancer was reduced by accidental infection by erysipelas (Patyar et al. 2010). The anaerobic bacteria can grow in cancer cell, but being pathogenic, they are not good for cancer treatment. Further, studies show that obligate anaerobic bacteria, e.g., Clostridia, can proliferate in necrotic or anaerobic region of solid tumor and regress the cancer (Barbé et al. 2006). Various in vivo studies show that Clostridium, Salmonella, Bifidobacterium, and Escherichia can be accumulated in cancer cell than the normal cell and can germinate spores in the cancer cell (Oelschlaeger 2010).Various studies suggest that microbes influence the oncogenesis and immunotherapy; they can have the inhibitory or stimulatory effect on the initiation of cancer and progression (Inamura 2020). The bacteria generate toxin and carcinogenic substance that stimulate inflammation or immunosuppression and promote oncogenesis (Nath et al. 2010). E. coli causes inflammation and stays in inflammatory cells that influence the microflora composition that promote the carcinogenesis. Beside these carcinogenic effects, several corroborations suggest bacteria can influence the chemotherapeutic and immunotherapeutic potential (Song et al. 2019).
Bacterial infection stimulates the immune system by modifying various cellular components, e.g., CD4, CD8 T cell, myeloid-derived suppressor cell (MDSC), regulator T cell (Treg), and tumor-associated macrophages (TMA). They also influence inherent immune receptors, e.g., Toll-like receptor (TLR), that are responsible for secretion of pro-inflammatory cytokines (Kaimala et al. 2018). Some bacterial exotoxins also stimulate immune response against the cancer cell and directly destroy cancer cell (Zahaf and Schmidt 2017). Immunotherapy is an impressive strategy for cancer treatment, but the effectiveness is very limited due to their inadequate assemblage at the cancer cell and various unwanted effects. Recently, nanotechnology has gained popularity for conquering these technical shortcomings due to their physicochemical property and versatility. The use of nanotechnology in bacterial immunotherapy can enhance the immunotherapy potential multifolds with their target specificity and intensify the delivery of tumor vaccine, immunomodulator checkpoint inhibitors, etc. (Gupta et al. 2010; Li et al. 2019).
2 Role of the Immune System in Cancer
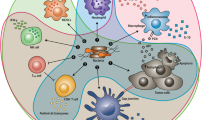
The presence of inflammatory cell in cancer tissue has given the idea of understanding the relation between inflammation and cancer (Singh et al. 2019). Now, the capability of the immune system for initiating and arresting the cancer is established. The imbalance between intrinsic and acquired immunities show immunosuppressant action that instigates the cancerogenesis and proliferation of cancer. Intrinsic immunity generates macrophages against the infection and repairs the tissue (Gonzalez et al. 2018). Macrophages release IFN-γ which destroy the cancer cells and IL-4 and IL-13. TME induces protumorigenic tumor-associated macrophages (TAM) that stimulate angiogenesis, lymphogenesis, and cancer growth (Dhabekar 2011), releases immunosuppressant (IL-10 and TGF-β), prevents maturing of DC cell, and diminishes effector T-cell activity (Taylor et al. 2006). It releases EGF and VEGF that help in cell development, angiogenesis, and restructuring of the ECM by secreting metalloproteinases (MMPs) (Anteby et al. 2004). Damaged and inflammatory tissues contain neutrophils that release the neutrophil extracellular trap (NET), kill microbes, do phagocytosis (Riyapa et al. 2012), and also suppress inflammation. The neutrophils show both protumor and antitumor activities (Brandau et al. 2013). Cancer and stromal cells release chemokines that recruit tumor-associated neutrophils (TANs) to TME. The natural killer (NK) and effector T cells release the TNFα that stimulates the anticancer anti-metastatic hepatocyte growth factor (c-MET). TANs generate new inflammation during cancerogenesis and progression and induce immunosuppression in T cells by PD-L1 expression induced by tumor-derived granulocyte-macrophage CSF (GM-CSF) (Masucci et al. 2019). PMN-MDSCs induce the chronic inflammation and antigen-specific tolerance by T cells (Dorhoi and Du Plessis 2018). NK cells destroy infected cells and induce the apoptosis. The antigen-presenting cells (APCs) or dendritic cells (DCs) interplay between intrinsic and acquired immunities (Steinman 2006) and present internal and foreign antigens to T cells in the context of MHC molecules (Alberts et al. 2003) and DCs found in all tissues throughout the body. During cancer progression, the DCs prime naïve, memory T cells, and antigen presentation cells develop resistance for antigens and evoke effector T-cell response. Various types of cancer cell carry the cancer-infiltrating DCs (Hubert et al. 2019). At the initiatory phase of cancer, T cell is generated, and naïve T cell invades lymph nodes, on enticement migrated to cancer cell environment, exerts immune response, and destroys cancer cells. T effector cell exhibits antigen-dependent anticancer activity. The low immunogenic cancer cells escaped from T cell, and cancer cells develop a system to prevent themselves from destruction from effector T cell (TAMs, NK cells, and TANs). The effector cells regulate immune checkpoints on CTLS and CD4+T and prevent tissue damage. The checkpoint molecules CTLA-4 and PD-1 inhibit T-cell function (Takeuchi and Saito 2017). The programmed cell death protein (PD1) and programmed cell death protein ligand (PDL-1) are expressed by immune cells and cancer cells, inhibit T-cell activity, and suppress the anticancer function such as T-cell migration, proliferation, secretion of cytotoxic mediators, and restriction of cell killing (Han et al. 2020). The cancer cell environment seize the immune checkpoints and suppress anticancer activities by recruiting regulatory CD4+ T cells (Tregs) that suppress cell destroying activity of Th1 CD4 T cells, CTLs, macrophages, NK cells, and neutrophils (Lee et al. 2020). The expression of PDL-1 LAG-3, CD39/73, or PD1 regulates the Treg-derived immunosuppressive function, and LAG-3 and CD39/73 also exhibit immunosuppressant function by contact-independent mechanisms, which sequester IL-2 and produce immunosuppressants IL-10, TGF-β, prostaglandin E2, adenosine, and galectin-1 (Cai et al. 2019). Invariant NK T (iNKT) cells, subset of T cells, identify the NK cell like lipid antigen CD1d; upon activation, it secretes effector cytokines such as IFN-γ, IL-4, and IL-17 (Fujii and Shimizu 2017). B cell exhibits humoral immunity for microbes by extracting specific antibodies that converted into protein and B cells. B cell assists cancer proliferation by releasing IL-35 and enhances immunosuppressive function by secreting IL-10 and TGF-β (Klinker and Lundy 2012). B cell also induces angiogenesis and chronic inflammation by activating myeloid cells via FcRγ (Rivera and Bergers 2015).
3 Role of Bacteria in Immunotherapy
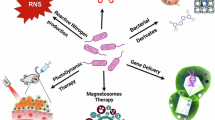
Bacteria functioned as a dual-edge blade for cancer. In one hand, it instigates the cancer, and in other hand, it induces the immune system and blocks cancerogenesis (Linnebacher et al. 2011). In the last 10 years, the killed or attenuated microbes have gained popularity as an important weapon for cancer immunotherapy (Kaimala et al. 2018). The explicit mechanism of bacterial immunotherapy is not so clear till date, but it is believed that weak antigenic cancer with facultative anaerobic bacteria promotes immunogenicity for cancer cell. There are several examples of bacterial immunotherapy using the underlying mechanism (Fig. 1.1). The attenuated bacterial infection harmonizes the activity of various cellular components (CD4+, CD8+ T cells, Treg) and TAM of the immune system to wield their antitumoral immunity (Nelson et al. 2015).
3.1 Effect of Bacteria on Myeloid Cell
3.1.1 Tumor-Associated Macrophages
A study on mice reveals S. typhimurium helps in reversing the cancer by helping in the maturation of myeloid cell (MDCs), reduces the suppressive function, and increases anticancer immune response (Kaimala et al. 2014). Another study revealed that Salmonella shows the immunomodulatory effect by elevating the level of TILs, CD11+myeloid cell, CD4+, and CD8 + T in mice and induces the inhibitory effect on cancer cell. The Salmonella treatment enhances the level of TAM which is responsible for the activation IFN-γ-dependent Sca-1 and MHC class II proteins. Leschner and colleague have shown that S. typhimurium SL7207 on i.v. administration activated the TNF-α and invades the tumor cell by disrupting the vasculature (Leschner et al. 2009). Recombinant attenuated Salmonella enterica serovar typhimurium vaccine arrests cancer development by inhibiting the HER-2/neu expression. The vaccine shows anticancer effect by diminishing the immunosuppressive function in cancer environment, increases the level of CD11b+Gr-1+ myeloid cell MDSC and TNF-α, and modifies Treg, CD4+CD25+ Foxp3+ Tregs (Hong et al. 2013).
3.1.2 Dendritic Cells and TANs
L. monocytogenes infect the melanoma cell in human or animal, convert them into antigen-presenting cell like DCs, and express CD11c, F4/80, MHCII, CD40, and CD83 similar to mature dendritic cells, and APC manages the host defense mechanism (Gorvel et al. 2014). Toll-like receptors (TLR) are responsible against microbial infection. Salmonella stimulates TLR signaling pathways that increase the expression of co-stimulatory molecules (e.g., CD86 and CD80) on APCs and activate the antigen-specific CD8 T cells and natural killer cells that are responsible for killing and relapse of cancer cell (Hajam et al. 2017). The TLR signal, a cytoplasmic adaptor protein (MyD88a), is responsible for inducing the TLRs and IL-1/IL-18 receptors and activation of downstream of IL-1 receptor-associated kinases (IRAKs) and NF-κB (Cohen 2014). A study show the TLR-MyD88 is necessary for bacterial immunotherapy and mice with MyD88 deficiency were found to be unable to reverse the cancer after Salmonella treatment (Kaimala et al. 2014). Salmonella typhi vaccine also activates and recruits neutrophils at cancer cell that releases the TNFα producing additive cytotoxic effect in the presence IFN-γ (Vendrell et al. 2011).
3.1.3 Effect of Bacteria on Tumor-Associated Lymphoid Cells
3.1.3.1 Natural Killer Cell
The cancer microenvironment nurtures the lymphoid cells, e.g., NK cells, B cells, CD4+ T cells, CD8+ T cells, and Tregs (Kaimala et al. 2018). The lymphoid cells have both inhibitory and stimulatory effects on the cancer (Wagner and Koyasu 2019). The microenvironment can affect the function of lymphoid cells in cancer cells, and the NK cells can identify and remove cancer cell without any previous antigenic exposure (Kaimala et al. 2018). Salmonella infection enhances IFN-γ and NK level in mice model (Harrington et al. 2007). The cancer depresses the host immunity that disturbs the NK cell response for cancer cell (Guerrouahen et al. 2020). Recombinant Salmonella activates NK cell and IL-2 expression. This strain exhibits excellent anticancer activity in vivo against metastatic cancer and also enhances IFN-γ or TNF-α expression that stimulates the anticancer activity (Lin et al. 2021).
3.2 CD4+ and CD8+ T Cells
CD4 T cells exert anticancer activity due the helper cells (Th1, Th2, or Th17) that secrete cytokines (IFN-γ and IL-12, IL-4, IL-5 and IL-13). Th1 cell shows anticancer activity by stimulating the CD8 cell or kills the cancer cell by releasing TNF-α and/or IFN-γ (Bascuas et al. 2018). Th1 ceases the progression of cancer by allowing DCs and macrophages and enhances the antigen-presenting activity that allows TCD8+ cell for strong anticancer activity. Th2 has insignificant effect in cancer treatment, but T17 enhances the cancer progression by secreting IL-17 (Lee et al. 2019). The attenuated Salmonella increases the infiltration of CD4+ and CD8+ T cell about threefold into cancer cell in mice model. M1 secretes macrophage inflammatory protein 1-alpha that recruited stimulated T cell in cancer cell; Salmonella follows this mechanism and increases the T-cell penetration into cancer cell. Salmonella typhi increases the leukocyte count and decreases the cancer progression and mitotic index and increases the life span of mice (Vendrell et al. 2011).
3.3 Gamma-Delta (γδ) T Cells
Gamma-delta T cells belong to T-lymphocyte cell, having a surface antigen recognition complex type 2. During stimulation, it exhibits excellent anticancer potential due to the release of ample amount of IFN-γ and TNF-α (Silva-Santos et al. 2015). Mycobacterium vaccae, M. obuense, and BCG stimulate the activation of γδ T cells that result in upregulation of granzyme expression and synthesis of Th1 cytokines (IFN-γ and TNF-α) (Fowler et al. 2012).
3.4 Effect of Bacteria on Immunosuppressor Cells
3.4.1 Myeloid-Derived Suppressor Cells
Cancer or autoimmune diseases can modify the myeloid-derived suppressor cells (MDSCs), found in the bone marrow, peripheral blood, and spleen, that help cancer cell in evading from the host’s immune system (Gabrilovich and Nagaraj 2009). MDSCs do nitration of T cell that represses the CD4 and CD8 T-cell-induced anticancer effect (Ostroumov et al. 2018). MDSCs can be a good target for cancer treatment. Salmonella triggers the differentiation of intratumoral MDSCs and improves immunomodulatory properties. L. monocytogenes LLO reduces the immunosuppressant activities of MDSCs and Tregs in surroundings of cancer tissues. MDSCs reduce Arg1 expression and IL-10 by Tregs. It both causes the above action (Wallecha et al. 2013) and also depresses the expression of T-cell receptor and immunosuppressive cytokines (Lindau et al. 2013).
4 Regulatory T Cells (Tregs)
Tregs are involved in autoimmunity and cancer diseases. Generally, CD4+CD25+Foxp3+ has the preventive immunogenic reaction for autoantigen. These cells inhibit the tumor antigen-specific CLTs and decrease the anticancer immunity (Manzo et al. 2015). Study shows attenuated S. typhi reduces Tregs cells in cancer-draining lymph node that diminish lung metastasis and cancer progression, improve animal life (Vendrell et al. 2013), and also decrease the proportion of CD25+FoxP3+ cells in the spleen and CD4+ T cells in a colon cancer model. It also elicits the conversion of immunosuppressive MDSCs into TNF-α-secreting neutrophils and reduces the generation of Treg cells, especially in the presence of tumor-specific CTLs (Hong et al. 2013). Salmonella vaccine also acts on the downregulation of CD44, a key cell surface molecule on Tregs as well as tumor cells, and contributes to angiogenesis and proliferative potential (Liu and Chopra 2010).
5 Nanotechnology in Immunotherapy
The cancer immunotherapy shows promising results, but developing a low toxic dosage form with high targeting efficiency and excellent efficacy is a difficult task. Nanotechnology has the revolutionary potential to reform the cancer therapy. Nanoparticles have already established its significance in the modern drug delivery system; it ameliorates the immunostimulation, cancer cell targeting, bio-distribution, and release kinetics. All the nanoparticulated system (nanosized vesicular system, e.g., nanoparticles, liposomes, etc.) could be utilized for passive and active immunotherapy (Sharma et al. 2017; Keservani et al. 2017a, b). Nanotechnology improves the cancer immunotherapy by protecting the burden during circulation, targeting the immunotherapeutics to the cancer tissues, inducing the immune system, and modulating the immunosuppressant action for cancer cell (Qian et al. 2018; Keservani et al. 2017a, b). The nanosized immunotherapeutic system can improve the therapeutic techniques and conquer the difficulties related to the poor therapeutic responses, for example, insufficient stimulation, side effects, and suppressed pharmacological activity. Researchers are continuously evolving the nanomaterials with improved structural properties and surface modification ability that can be exploited for guiding nanotechnology-based immunotherapeutic into the vasculature, avoidance of opsonization, and assemblage of nanoparticles at the cancer cell due to enhanced permeability and retention (EPR) properties. A controlled drug release can be used to design nanoparticle systems specific for microbiome intervention in cancer. However, this represents a generally unexplored area (Song et al. 2019). The first cancer vaccine, Sipuleucel-T was approved by the US Food and Drug Administration (FDA) in the year 2010 for prostate cancer treatment (Cheever and Higano 2011).
5.1 Nanoparticle-Based Bacterial Immunotherapy
Recently, new combinatory therapeutic approaches emerged for the effective treatment of cancer because single-treatment method is insufficient for complete eradication of cancer. The advantages of bacterial immunotherapy and nanoparticle-based immunotherapy are combined together to overcome the deficiency of both systems and also exploit its advantages. Both systems together work effectively with enhanced cancer targeting and excellent efficacy; recently, the researcher has prepared various vaccine and outer membrane vesicles (OVM) and nanobodies for the therapeutic and diagnostic purpose as well; some example are given here. Figure 1.2 shows the nanotechnology-based immunotherapy.)
5.2 Nano-vaccine
Live or attenuated bacterial oral vaccine is unable to penetrate into the cancer vasculature. To enhance the penetrability, Hu et al. (2015) prepared oral nano-vaccine containing attenuated Salmonella bacteria; the cationic nanosized enwrapped bacterial vectors efficiently deliver the vaccine into the cancer cell. The nano-vaccine protects bacteria from phagocytosis and enhances the circulatory time and acid tolerability in GIT. The nano-vaccine stimulates the T cell, increases the secretion of cytokine, and inhibits the cancer growth by suppressing the angiogenesis. In 2019, Terán-Navarro and team synthesized gold nanoparticles containing a peptide (listeriolysin O) derived from bacteria for treating skin cancer. The vaccine works on dendritic cell, modifies the cancer cell sensitivity toward the immune system, and activates the cell killing function that ameliorates the cell viability. The anti-PD-1 or anti-CTLA-4 checkpoint inhibitors when incorporated into the nano-vaccine enhance the cancer eradicating function manyfold. The study shows the nano-vaccine is a safe therapeutic system and enhances the immunity against the cancer cell.
The outer membrane vesicles (OMVs) derived from Gram-negative bacteria are effective vaccine platforms with precedence for safe use in humans (Davitt et al. 2016). Unlike most subunit antigens and synthetic nanoparticles, OMVs contain endogenous immunostimulatory ligands and deliver the antigens in their native orientation. OMVs could induce antigen-presenting cells by engaging the intrinsic receptors and CMI by activating the APC, co-stimulation, and cytokine production. After the uptake, the DCs enhance the surface MHC classes I and II, CD80 and CD86 expressions. OMV-induced DCs produce the T-cell polarizing cytokines IL-1β, IL-6, IL-18, and IL-12 and their expression contributed by TLR4, whereas the NLRP3 inflammasome was required for OMV-induced production of IL-1β and IL-18. Following vaccination of mice, Th1- and Th17-type T-cell responses were observed after ex vivo restimulation with OMVs and HK bacteria. In addition, OMV vaccination produced functional CD8+ T cells capable of killing bacteria-infected cells. Collectively, these results support the utility of the OMV platform as a nonliving vaccine that can lead to protective CMI against bacterial pathogens (Davitt et al. 2016).
In another study, the nanosized outer membrane vesicles (OVM) were prepared from the Gram-positive and Gram-negative (E. coli Δmsb B and S. aureus) bacteria (Kim et al. 2017). The bacterial cell allows genetic modification that can be utilized to attach targeting moieties on the surface and enhance the safety feature by eliminating the endotoxins. Bacterial cell can also load various chemotherapeutic agents. Such system can be utilized for effective anticancer immunotherapy. These extracellular vesicles induce IFN-γ generation, and the results exhibit the potential of OVM as a cancer immunotherapeutic without side effects. The nanosized OVM assemblage in the cancer cells produces the IFN-γ and cytokine CXCL10 in the cancer microenvironment and elicits the anticancer effect. This system also enhances the memory cell production for longer anticancer effects (Kim et al. 2017).
5.3 Nanoparticulate System for Cancer Therapy
In 2016, Lizotte and his team member prepared self-aggregating nano-based inhalant system containing cowpea mosaic virus (CPMV) for treating lung cancer; the inhalant system produces effective systemic cancer immunotherapy against the B16F10 in the skin. The nano-based inhalant system stimulates cytokines and enhances the potency of the immune system against lung cancer, ovarian cancer, metastatic breast cancer, and colon cancer.
As we know, phagocytosis minimizes the circulation time of nanoparticles in the body. To overcome this problem, the attenuated Salmonella bacterial membrane vesicles were combined with nanoparticles, and the nanoparticle surface was modified with polyethylene glycol (PEG) and also attached to the Arg-Gly-Asp (RGD) peptide in which the Asp (RGD) peptide enhances the circulatory time and cancer targetability and stability (Chen et al. 2020). Tegafur is loaded to this system that removes MDSc and synergistically enhanced the anticancer effect and prevents metastasis (Chen et al. 2020). This system also induces the intrinsic immunity and accumulated into the cancer tissue due to the enhanced permeability and retention (EPR) and active targeting effects.
Probiotic bacteria were utilized to deliver the nanoparticulated checkpoint blocker to the affected area. The probiotic bacterial nanoparticulated system used a synchronized lysing integrated circuit (SLIC) using E. coli Nissle 1917 strain for releasing the target program cell death ligand and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) after intratumoral injection. The computerized SLIC model was used for validating the lysis mechanism for higher therapeutic efficiency. The formulation exhibited higher therapeutic efficiency over the other conventional system and suppressed the cancer in mouse by potentiating the immunity; the formulation enhanced the activation of T cells, an abscopal effect, and enhanced T memory cell in mice. The formulation enhances the cytokine granulocyte-macrophage colony-stimulating factor.
The swimming ability of Salmonella could be utilized for effective targeting of cancer cell by incorporating the nanomedicine; it freed the drug by degrading itself at cancer environment; for this purpose, doxorubicin incorporated nanosized liposome into bacterial cell (Zoaby et al. 2017); and the bacterial motility is pH- and glucose-dependent, which favors the cancer environment. The nanoparticles are incorporated into the bacterial cell by incubation or electroporation. Electroporation builds ephemeral aperture on the bacterial cell wall that provides entry to the nanosized liposomes inside the bacteria. The metabolic process causes the drug release from the liposome; mainly, ammonia causes the osmotic imbalance in the liposome and releases the drug that destroys the bacterial carrier and kills the cancer cell. This process enhances in situ therapeutic efficacy (Zoaby et al. 2017).
5.4 Thermotherapy
Several cancer therapies are available, but cancer monotherapy can result in reappearance of cancer due to partial destruction of cancer tissue. To overcome these problems, Park et al. (2020) developed nanoemulsion containing anaerobic Clostridium novyi-NT spores for synergistic image-guided combinational cancer therapy. The nanoemulsion also carries scintillators for guiding MRI and for an image-guided X-ray photodynamic therapy (PDT) of the normoxic peripheral cancer. The nanoemulsion was used for the treatment of the hypoxic central tumor tissues. Photosensitizer-coated NSs (PS-NSs) and C. novyi-NT spores are emulsified with clinically available ethiodized oil (Lipiodol) to be the nanoemulsion and injected into the tumor with computed tomography image guidance. Following the image-guided X-ray PDT and anaerobic C. novyi-NT combination treatment, apoptotic cell death in cancer tissues, including both peripheral and central tumor regions, is significantly higher than in the control groups. This nanoemulsion system can overcome the limitations of conventional cancer therapy, resulting in increased cancer therapeutic efficacy.
5.5 Radiotherapy and Diagnostic Purpose
Nanoparticles coated with bacterial wall was prepared that also contains the immunostimulatory PC7A/CpG Polyplex core and imide groups. The bacterial nanoparticles with various attachments can ameliorate the antigen salvage, improve the affinity of antigen toward the MHC-1, and also augment the innate immunity by proliferation of the T cells. Nano-bacterial system on combining with radiotherapy arrests the neoantigens and increases their absorption in DCs and from there delivered to MHC-presenting cell and activated the effector T cell; PC7A and CpG assist in modulating the antigen uptake in DCs, raise the expression of MHC-I in TME, and increase the effector T cell and type I IFN. Treating mice with this system exhibits 100% relapse of primary cancer with high survival rate and low metastasis rate. For improving the immunotherapeutic efficiency of the nano-bacterial system, the immune checkpoint can also be combined with the nano-system that possibly impedes the immunosuppressive signals. Such nano-bacterial system can ameliorate cancer immunity and long-term immune response (Patel et al. 2019). In another study, melanin-containing OMVs were prepared from E. coli for therapeutic and diagnostic purpose (Gujrati et al. 2019). OMVs show good biocompatibility, biodegradability, and low-cost manufacturing. The strong acoustic waves are converted into optical image; OMVs show superiority over fluorescence method due to high resonating efficiency. OMVs can be utilized for monitoring and imaging of cancer in vivo. The nanosized bacterial cell escapes out from the phagocytosis and the EPR effect; both the two events enhance the residence time in the cancer cell. OMVs could penetrate into deep tissue due to high absorption coefficient. NIR irradiation killed the cancer cell. OMVs also induce the production of anticancer cytokines, including IFN-γ. Dosing for longer time induces IFN-γ production in a sustained manner (Gujrati et al. 2019).
6 Challenges and Opportunities for Nanotechnology-Based Immunotherapy
Designing the nanotechnology-based bacterial immunotherapy faces several challenges, e.g., understanding cancer site, microenvironment, microbial flora, and inflammation-derived cancerogenesis and bacterial efficacy against cancer with the toxicological effect or side effects of microflora, and their relationships with cancerogenesis, progression, and treatment of cancer (Song et al. 2019), in the last 10 years of research, several preclinical and clinical studies have been performed on nanotechnology-based immunotherapy, but just a few approaches are so far clinically approved. For example, attenuated herpes simplex virus (genetically modified)-based cancer treatment successfully passed phase III (Shukla and Steinmetz 2016). There is a lacuna between the understanding of cancerogenesis and immunology and in vivo behaviors of nanoparticles that include long-time assemblage at the nontarget site and their safety concern. They can cause toxicity, e.g., hollow silica nanoparticles induce the release of pro-inflammatory cytokines that damages the hepatic tissues. Nanoparticles can influence the constitution of microflora; a depth understanding of nanoparticles and microflora is required for developing new strategy (Song et al. 2019). The intersubject variability should also be considered. The microflora is patient-specific that changes with the food intake, drugs, and other environmental factors, and the nanoparticles can modify the composition of microflora that cannot be predicted in vivo or by clinical investigation. Other challenges may include the production, targeting efficiency, and distribution. Targeting the nanoparticles to the specific organ or tissues is affected by surface morphology, surface properties, and physicochemical properties of nanoparticles (Song et al. 2019).
7 Advantage and Disadvantage
The distinct characteristics of nanoparticulated system and bacterial membrane make them an excellent drug delivery system; nanoparticle-based bacterial immunotherapy has excellent immunomodulatory properties, penetrability, biocompatibility, and targeting ability to specific cells and sustained release (Li et al. 2013). The nanosized bacterial system enhances the circulatory time and targeting into deep vasculature because of EPR and also augments the efficacy of thermotherapy and radiotherapy (Park et al. 2020; Patel et al. 2019). The nanoparticle surface also allows surface modification and attachment of ligands that improve the targeting (Li et al. 2013). The nanoparticle-based bacterial system not only improves cancer targeting but also helps in cancer diagnosis. Nanoparticles that may cause immunogenic response have short residence time due to rapid blood clearance.
8 Conclusion
The understanding of the role of immunity in cancer pushes scientist to make new system that plays an important role in cancer. Cancer immunotherapy holds great promise in cancer therapy. Collaboration between cancer immunologists and engineers will further the understanding of the complex and underlying immunology, therefore driving technological development. With emerging nanotechnological interventions, the efficacy of many such immunotherapies could be drastically improved, and a merger of these two rapidly growing fields of science could facilitate clinical translation of many cancer immunotherapies.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2003) Molecular biology of the cell. 4th edn. Ann Bot 91:401
Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S (2004) Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod 10:229–235
Barbé S, Mellaert LV, Anné J (2006) The use of clostridial spores for cancer treatment. J Appl Microbiol 101:571–578
Bascuas T, Moreno M, Grille S, Chabalgoity JA (2018) Salmonella immunotherapy improves the outcome of CHOP chemotherapy in non-Hodgkin lymphoma-bearing mice. Front Immunol 9:7
Brandau S, Dumitru CA, Lang S (2013) Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol 35:163–176
Cai J, Wang D, Zhang G, Guo X (2019) The role of PD-1/PD-L1 axis in Treg development and function: implications for cancer immunotherapy. Onco Targets Ther 12:8437–8445
Cheever MA, Higano CS (2011) Provenge (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 17:3520–3526
Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y (2020) Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett 20:11–21
Cohen P (2014) The TLR and IL-1 signalling network at a glance. J Cell Sci 127:2383–2390
Davitt CJ, Petersen HE, Kikendall NL, Lavelle EC, Morici LA (2016) Naturally-derived bacterial nano-particles engage diverse innate receptors, driving the activation of dendritic cells and leading to the establishment of potent adaptive immune responses. J Immunol 196:76.11
Dhabekar GS (2011) Role of macrophages in malignancy. Ann Maxillofac Surg 1:150
Dorhoi A, Du Plessis N (2018) Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol 8:1895
Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD (2012) Mycobacteria activate γδ T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother 61:535–547
Fujii S-I, Shimizu K (2017) Exploiting antitumor immunotherapeutic novel strategies by deciphering the cross talk between invariant NKT cells and dendritic cells. Front Immunol 8:886
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–1284
Gorvel L, Textoris J, Banchereau R, Ben Amara A, Tantibhedhyangkul W, von Bargen K, Ka MB, Capo C, Ghigo E, Gorvel J-P, Mege J-L (2014) Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PLOS One 9:e99420
Guerrouahen BS, Maccalli C, Cugno C, Rutella S, Akporiaye ET (2020) Reverting immune suppression to enhance cancer immunotherapy. Front Oncol 9:1554
Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G, Aichler M, Walch A, Ntziachristos V (2019) Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun 10:1114
Gupta S, Yadav BS, Kesharwani RK, Mishra KP, Singh NK (2010) The role of nanodrugs for targeted drug delivery in cancer treatment. Arch Appl Sci Res 2(1):37–51
Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH (2017) Bacterial flagellin—a potent immunomodulatory agent. Exp Mol Med 49:e373–e373
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10:727–742
Harrington L, Srikanth CV, Antony R, Shi HN, Cherayil BJ (2007) A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol Med Microbiol 51:372–380
Hong E-H, Chang S-Y, Lee B-R, Pyun A-R, Kim J-W, Kweon M-N, Ko H-J (2013) Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine 31:1377–1384
Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, Chu PK, Ping Y, Tang G (2015) Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett 15:2732–2739
Hubert M, Gobbini E, Bendriss-Vermare N, Caux C, Valladeau-Guilemond J (2019) Human tumor-infiltrating dendritic cells: from in situ visualization to high-dimensional analyses. Cancers 11:1082
Inamura K (2020) Roles of microbiota in response to cancer immunotherapy. Semin Cancer Biol 65:164–175
Kaimala S, Mohamed YA, Nader N, Issac J, Elkord E, Chouaib S, Fernandez-Cabezudo MJ, Al-Ramadi BK (2014) Salmonella-mediated tumor regression involves targeting of tumor myeloid suppressor cells causing a shift to M1-like phenotype and reduction in suppressive capacity. Cancer Immunol Immunother 63:587–599
Kaimala S, Al-Sbiei A, Cabral-Marques O, Fernandez-Cabezudo MJ, Al-Ramadi BK (2018) Attenuated bacteria as immunotherapeutic tools for cancer treatment. Front Oncol 8:136
Keservani RK, Sharma AK, Kesharwani RK (2017a) Drug delivery approaches and nanosystems, Drug targeting aspects of nanotechnology, vol 2. Apple Academic Press, Toronto. isbn:9781771885843
Keservani RK, Sharma AK, Kesharwani RK (2017b) Drug delivery approaches and nanosystems, Novel drug carriers, vol 1. Apple Academic Press, Toronto. isbn:9781771885836, E-Book isbn:978131522537
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee S-W, Gho YS (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun 8:626
Klinker MW, Lundy SK (2012) Multiple mechanisms of immune suppression by B lymphocytes. Mol Med 18:123–137
Lee HL, Jang JW, Lee SW, Yoo SH, Kwon JH, Nam SW, Bae SH, Choi JY, Han NI, Yoon SK (2019) Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci Rep 9:3260
Lee WS, Yang H, Chon HJ, Kim C (2020) Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 52(9):1475–1485
Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, Lienenklaus S, Falk W, Gekara N, Loessner H, Weiss S (2009) Tumor invasion of Salmonella enterica serovar typhimurium is accompanied by strong hemorrhage promoted by TNF-α. PLOS One 4:e6692
Li XD, Gao JY, Yang Y, Fang HY, Han YJ, Wang XM, Ge W (2013) Nanomaterials in the application of tumor vaccines: advantages and disadvantages. Onco Targets Ther 6:629
Li Y, Ayala-Orozco C, Rauta PR, Krishnan S (2019) The application of nanotechnology in enhancing immunotherapy for cancer treatment: current effects and perspective. Nanoscale 11:17157–17178
Lin Q, Rong L, Jia X, Li R, Yu B, Hu J, Luo X, Badea SR, Xu C, Fu G, Lai K, Lee M, Zhang B, Gong H, Zhou N, Chen XL, Lin S, Fu G, Huang J-D (2021) IFN-γ-dependent NK cell activation is essential to metastasis suppression by engineered Salmonella nature communications. Nat Commun 12:2537
Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138:105–115
Linnebacher M, Maletzki C, Klier U, Klar E (2011) Bacterial immunotherapy of gastrointestinal tumors. Langenbecks Arch Surg/Dtsch Gesell Chirurg 397:557–568
Liu T, Chopra AK (2010) An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: the critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene Ther 17:97–108
Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S (2016) In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol 11:295
Manzo T, Heslop HE, Rooney CM (2015) Antigen-specific T cell therapies for cancer. Hum Mol Genet 24:R67–R73
Masucci MT, Minopoli M, Carriero MV (2019) Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 9:1146
McCarthy EF (2006) The toxins of William B. coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 26:154
Nath G, Gulati AK, Shukla VK (2010) Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterol 16:5395–5404
Nelson MH, Diven MA, Huff LW, Paulos CM (2015) Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res 2015:e368736
Oelschlaeger TA (2010) Bacteria as tumor therapeutics? Bioeng Bugs 1:146–147
Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N (2018) CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci 75:689–713
Park W, Cho S, Kang D, Han J-H, Park J-H, Lee B, Lee J, Kim D-H (2020) Tumor microenvironment targeting nano-bio emulsion for synergistic combinational X-ray PDT with oncolytic bacteria therapy. Adv Healthcare Mater 9:e1901812
Parsonnet J (1995) Bacterial infection as a cause of cancer. Environ Health Perspect 103(Suppl 8):263–268
Patel RB, Ye M, Carlson PM, Jaquish A, Zangl L, Ma B, Wang Y, Arthur I, Xie R, Brown RJ, Wang X, Sriramaneni R, Kim K, Gong S, Morris ZS (2019) Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv Mater 31:e1902626
Patyar S, Joshi R, Byrav DP, Prakash A, Medhi B, Das B (2010) Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci 17:21
Qian H, Liu B, Jiang X (2018) Application of nanomaterials in cancer immunotherapy. Mater Today Chem 7:53–64
Rivera LB, Bergers G (2015) Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol 36:240–249
Riyapa D, Buddhisa S, Korbsrisate S, Cuccui J, Wren BW, Stevens MP, Ato M, Lertmemongkolchai G (2012) Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect Immun 80:3921–3929
Sharma AK, Keservani RK, Kesharwani RK (2017) Nanobiomaterials, Applications in drug delivery. Apple Academic Press, Toronto. isbn:9781771885911
Shukla S, Steinmetz NF (2016) Emerging nanotechnologies for cancer immunotherapy. Exp Biol Med (Maywood) 241:1116–1126
Silva-Santos B, Serre K, Norell H (2015) γδ T cells in cancer. Nat Rev Immunol 15:683–691
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB (2019) Inflammation and cancer. Ann Afr Med 18:121–126
Song W, Anselmo AC, Huang L (2019) Nanotechnology intervention of the microbiome for cancer therapy. Nat Nanotechnol 14:1093–1103
Steinman RM (2006) Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp 279:101–109. discussion 109-113, 216–219
Takeuchi A, Saito T (2017) CD4 CTL, a cytotoxic subset of CD4+ T cells, their differentiation and function. Front Immunol 8:194
Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA (2006) Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology 117:433–442
Vendrell A, Gravisaco MJ, Pasetti MF, Croci M, Colombo L, Rodríguez C, Mongini C, Waldner CI (2011) A novel Salmonella typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine 29:728–736
Vendrell A, Gravisaco MJ, Goin JC, Pasetti MF, Herschllik L, De Toro J, Rodríguez C, Larotonda G, Mongini C, Waldner CI (2013) Therapeutic effects of Salmonella typhi in a mouse model of T-cell lymphoma. J Immunother 36:171–180
Wagner M, Koyasu S (2019) Cancer immunoediting by innate lymphoid cells. Trends Immunol 40:415–430
Wallecha A, Singh R, Malinina I (2013) Listeria monocytogenes (Lm)-LLO immunotherapies reduce the immunosuppressive activity of myeloid-derived suppressor cells and regulatory T cells in the tumor microenvironment. J Immunother 36:468–476
Zheng D, Liwinski T, Elinav E (2020) Interaction between microbiota and immunity in health and disease. Cell Res 30:492–506
Zoaby N, Shainsky-Roitman J, Badarneh S, Abumanhal H, Leshansky A, Yaron S, Schroeder A (2017) Autonomous bacterial nanoswimmers target cancer. J Control Release 257:68–75
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Arya, R.K.K., Sati, D., Bisht, D., Keservani, R.K. (2022). Nanotechnology-Based Bacterial Immunotherapy. In: Kesharwani, R.K., Keservani, R.K., Sharma, A.K. (eds) Nutraceuticals and Functional Foods in Immunomodulators. Springer, Singapore. https://doi.org/10.1007/978-981-19-2507-8_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-2507-8_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2506-1
Online ISBN: 978-981-19-2507-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)