Abstract
Purpose
To explore the expression of tumoral Gal-1 in association with clinical parameters and outcome in a large population with laryngeal squamous cell carcinomas (LSCCs).
Methods
A total of 187 patients with LSCC were retrospectively enrolled. Immunohistochemistry was performed to evaluate the tumoral expression of Gal-1, apoptosis-related proteins and the density of tumor infiltrating lymphocytes (TILs) in tumor tissues before any intervene. Survival curves were estimated by the Kaplan–Meier method, and differences in survival between groups were determined using the log-rank test. Prognostic effects were evaluated by Cox regression analysis.
Results
A total of 102 carcinomas (54.5 %) were identified as high Gal-1 expression, and 85 carcinomas (45.5 %) as low expression. Tumoral Gal-1 expression was not significantly related with clinical stage and histology differentiation. No correlation of Gal-1 expression with apoptosis-related protein was identified. Instead, Gal-1 status was correlated positively with the ratio of FOXP3+/CD8+ TILs (P = 0.024). In multivariate regression analysis, advanced clinical stage and the presence of metastases were identified as the independent predictors for poor survival in entire cohort. Especially, the statistical correlation between the Gal-1 expression and prognosis was particularly due to the late-stage tumors (P < 0.05).
Conclusion
Current results represent valuable advancements in Gal-1 research and provided further support for using Gal-1 as a diagnostic biomarker and immunotherapeutic target for LSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Laryngeal squamous cell carcinomas (LSCCs) comprise the vast majority of laryngeal malignancies. Despite the numerous advances in treatment modalities involving surgery, radiation and chemotherapy over the last 30 years, the 5-year survival period for head and neck squamous cell carcinomas (HNSCCs) patients in general, and for LSCC patients in particular, has remained below 50 % primarily due to local recurrences [1]. The possibility of developing immunotherapeutic approaches for LSCC patients has consequently attracted recent attention mainly because the development of LSCC is notably influenced by the host immune system [2, 3].
The development of malignant tumors is controlled by a complex biologic system that depends on genetic abnormalities as well as the interplay between tumor cells, stromal cells, and host inflammatory cells. The presence of tumor infiltrating lymphocytes (TILs) within the tumor microenvironment is considered to be an indication of the host immune response to tumor antigens and is thought to reflect the dynamic process of “cancer immunoediting” [4]. Recent progress in the field indicates that immune infiltrates of the primary tumors are not only independent prognostic biomarkers, but can also constitute predictive factors in a wide variety of neoplasms [5, 6].
Galectin-1 (Gal-1, also called Galaptin or LGALS1) is one of the 15 evolutionarily conserved β-galactoside-binding proteins. Involvement of Gal-1 in cell–cell and cell–extracellular interactions through binding to the various ligand receptor molecules provide multi-functional capabilities, including modulation of cell adhesion, migration, proliferation, immunomodulation and angiogenesis [7, 8]. Gal-1 has been shown overexpressed in many different types of carcinomas and play important roles in several aspects of cancer biology, including modulation of apoptosis, cell migration and adhesion, and immunoregulation [8]. Tumor cell-produced Gal-1 is a determining factor of tumor cell-induced T cell apoptosis and is important in the generation of immunoprivileged microenvironment [9, 10]. However, the potential correlation of Gal-1 with TIL subtypes has rarely been explored in LSCC.
Considering the emerging role of Gal-1 in autocrine and paracrine communication between stroma and tumor parts in the local microenvironment, here we focused on Gal-1 expression in relation to TILs density in LSCC tissues. The evaluation of clinicopathologic parameters and apoptosis-related proteins expression in relation to tumoral Gal-1 expression was performed. Another purpose was to validate the prognostic value of Gal-1 in a large cohort with LSCC.
Materials and methods
Ethical considerations
The study protocol was reviewed and approved by the institutional review board of Sun Yat-sen University. Owing to its retrospective nature, informed consent was not necessary.
Study protocol
This study was a retrospective analysis of patients consecutively collected at Anhui Provincial Hospital and the third Affiliated Hospital of Sun Yat-sen University, between 1 Jan 2000 and 31 Dec 2005. All patients underwent diagnostic microlaryngoscopy with laryngeal surgical specimens, upper aerodigestive tract endoscopy, esophagoscopy, neck ultrasonography (with or without fine-needle aspiration cytology), contrast-enhanced computerized tomography and/or magnetic resonance imaging of the head and neck. The inclusion criteria were no history of previous malignancies, primary squamous cell carcinoma of the larynx only and no previous radio- or chemotherapy. The records of enrolled patients were reviewed and corresponding paraffin-embedded tissue specimens were retrospectively analyzed.
Data collection
The treatment decision-making was based on the clinical stage and on the presence or not of lymph node metastasis at the time of diagnosis. The treatment modalities consisted of surgery, including partial laryngectomy (PL) and total laryngectomy (TL), alone or combined with radio-, chemo- or radiochemotherapy (RC).
Data regarding patient demographics, smoking and alcohol history, date of initial diagnosis and therapy approaches were retrospectively obtained by medical record review. Details about tobacco and alcohol exposure were obtained by a specific questionnaire systematically enclosed in the medical records. It included questions about smoking habits, age when starting smoking, mean daily cigarette consumption, type of drink consumed, and mean daily consumption of alcoholic beverages. A specific pathologist rechecked the immunostained sections for all patients to confirm the correction of histopathologic diagnosis, grade of tumor differentiation and pathologic tumor stage. The clinical evaluation was obtained by complete examination of the head and neck region by high-resolution computed tomography or magnetic resonance imaging, direct laryngoscopy, pharyngoscopy and esophagoscopy under general anesthesia (with the tumor specimens), chest radiography, and abdominal ultrasonography. T and N categories were retrospectively assigned according to the UICC classification on the basis of the pathology reports. The clinical staging and the anatomic site of the tumors were assessed according to the UICC tumor-node metastasis classification of malignant tumors. All data from patients were reviewed by the authors without knowledge of histopathological status.
Patient follow-up
This report included follow-up data at 60 months. Follow-up time was measured from the date of diagnosis of LSCC until 31 Dec 2010. Follow-up examinations including ENT clinical examination, imaging evaluation and pathological studies were performed in 3-month intervals during the first 2 years and every 6 months thereafter. The overall survival (OS) and disease-free survival (DFS) were calculated as the period from the date of surgery to the date of death or tumor relapse, respectively.
Immunohistochemistry
The tumor specimens of all enrolled patients were processed and evaluated in one pathology institute (Department of Pathology, Cancer Center, Sun Yat-sen University) at the same time. Formalin-fixed paraffin-embedded tissue specimens were cut into 4-μm sections and mounted on to poly-l-lysine-coated slides. For each patient, a representative tissue block containing adequate tumor cells and non-neoplastic larynx tissue was selected. All section was stained with hematoxylin and eosin and reviewed to confirm the histopathologic diagnosis and adequacy of specimens for immunohistochemistry (IHC) analysis.
In brief, sections were deparaffinized in xylene and rehydrated in graded alcohols and water. The slides were then heated in a microwave for 10 min in a 10-mM citrate buffer solution at pH 6.0, and cooled to room temperature for 20 min. After quenching the endogenous peroxidase activity with 0.3 % hydrogen peroxide (in absolute methanol) for 30 min, the sections were blocked for 2 h at room temperature with 5 % bovine serum albumin (Sigma Chemical Co.). Subsequently, duplicate sections were incubated overnight at 4 °C with the primary specific antibodies (Table 1).
After several rinses in phosphate-buffered saline (PBS), the sections were incubated in the biotinylated secondary antibody. Then, sections were incubated with streptavidin linked with peroxidase and visualized with 3,3′-diaminobenzidine tetrahydrochloride as the chromogen (Invitrogen, Carlsbad, CA). Slides were rinsed in PBS, exposed to diaminobenzidine, and counterstained with Mayer’s hematoxylin. The negative controls for these proteins were made by the omission of the primary antibody during the process of immunohistochemical staining. According to the previous reports [11], macrophage and endothelial cells, which are positive for Gal-1, served as internal controls for each case. Positive cells showed a brownish color and negative controls, as well as unstained cells, were blue.
Evaluation of immunohistochemical variables
All of the immunostained sections were analyzed by two pathologists unaware of clinicopathological data. According to our previous report [12], staining intensity of Gal-1 and apoptosis-related proteins in tumor cells were graded into three groups: (1) weak, (2) moderate and (3) strong. Strong intensity corresponded with that in control samples used as standards. Weak intensity was similar to that noted in benign bronchial epithelium. Moderate intensity was classified as a staining intensity between weak and strong. In each case, at least 1,000 cells were counted in 10 different areas using the 40× objective lens. Percentage of the positively stained cancer cells was evaluated using a continuous scale (0–100 %) and graded on a scale of 0–4: 0, none; 1, 1–25 %; 2, 26–50 %; 3, 51–75 %; 4, >75 %, respectively. For further analysis, the extent of staining was defined as the product of grades of the extent and intensity of staining to determine the cutoff value for high expression of the proteins.
The evaluation of TILs was performed by two independent observers in a blinded fashion. Discrepancies in enumeration, within a range of 5 %, were re-evaluated and a consensus decision was made. As previous report [13], to ensure representativeness and homogeneity, ten different high-power fields (HPF, 400×) representing the densest lymphocytic infiltrates, were selected for each sample and photographed with a digital camera (Nikon Eclipse 80i, Japan). The absolute number of TILs within the neoplastic nests and immediately adjacent stroma, excluding tumor cells, was counted and the average in these 10 HPF digital images was determined for statistical analysis. No attempt was made to evaluate the various tumor compartments separately (e.g., stroma, tumor cell nests). In addition, FOXP3+/CD8+(FOXP3+ T cell count divided by CD8+ T cell count) and FOXP3+/CD4+ ratios were calculated for each specimen using the mean number of total fields, and the averages were compared.
Statistical analysis
Given that there were no widely accepted standard cut points to define the clinical outcome, we selected the median value to be the cutoff for definition of TILs subgroups according to previous reports [13, 14]. All items were treated as dichotomous variables. Comparisons between two groups of patients according to Gal-1 status were done using the Chi-square test or Fisher’s exact test as appropriate for categorical data. The correlations of Gal-1 expression with the density of TIL subtypes and the expression of apoptosis-related proteins were examined using Pearson’s correlation analysis.
Time was defined as the period starting from the date of diagnosis to the date of disease relapse (event) or that of last follow-up visit (censored) for DFS, and as the period starting from the date of diagnosis to the date of death (event) or that of last follow-up visit (censored) for OS. Survival curves were estimated by the Kaplan–Meier method, and differences in OS and DFS between groups were determined using the log-rank test. Cox proportional hazards regression analysis was used to measure the association of clinicopathologic variables to overall survival. In all tests, a P < 0.05 was considered statistically significant. All analyses were done with SPSS for Windows 11.0 software package (SPSS Inc., Chicago, IL).
Results
Baseline characters of patients
Within the research period, a total of two hundred and sixteen patients were diagnosed as primary LSCC in two hospitals. Twenty patients were lost during follow-up and nine patients were omitted from the final calculation due to uncompleted medical records. As a result, one hundred and eighty-seven patients were included in this retrospective study and went to the final analysis. The median age was 52.4 years (range 37–85 years) and 54.0 % (n = 101) were over 60 years. The majority of patients were males (95.7 %, n = 179). Most of the patients had been exposed to risk factors, such as tobacco smoke (87.2 %, n = 163) and/or alcohol consumption (66.3 %, n = 124).
Clinicopathologic characteristics of the enrolled patients are shown in Table 2. The anatomic subsites of primary lesions were the glottis in 92 cases (49.2 %) and the supraglottis in 65 patients (34.8 %). The pathological classification of the primary laryngeal lesions was T1 in 23 cases (12.3 %), T2 in 42 cases (22.5 %), T3 in 95 cases (50.8 %), and T4 in 27 cases (14.4 %). For the regional lymph nodes, it was N0 in 71 cases (38.0 %), N1 in 62 cases (33.2 %), and N2 in 45 cases (24.1 %), N3 in 9 cases (4.8 %). As for pathological differentiation, 83 cases (44.4 %) were well, 75 cases (40.1 %) were moderate. According to the TNM staging system, 20 cases were in stage I, 58 in stage II, 88 in stage III and 21 in stage IV, respectively. In 187 patients investigated, 88 cases (47.1 %) were treated with surgery alone (PL in 51 cases, TL in 37 cases). 43 cases (23.0 %) received PL combined with RC and 30 cases (16.0 %) received TL combined with RC. In addition, 26 patients (13.9 %) were treated with radio- and/or chemotherapy alone.
Association of Gal-1 expression with clinicopathologic variables
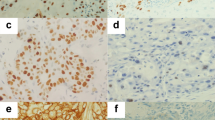
Representative pictures of Gal-1 expression in LSCC tissue are shown in Fig. 1. As shown in Fig. 1a, e, Gal-1 staining appeared in the form of a heterogeneous cytoplasmic staining pattern in tumor cells and surrounding tumor stroma, which was most pronounced at the luminal surface. Here, we focused on the Gal-1 expression in tumor cells, not in immune cells or tumor stroma.
Representative immunohistochemical staining of laryngeal squamous cell carcinomas. a High expression of tumoral Gal-1 (×400). b High expression of Bax (×400). c Low expression of Fas (×400). d High density of CD8+ TILs (×200). e Low expression of tumoral Gal-1 (×400). f Low expression of Bcl-2 (×400). g High expression of FasL (×400). h High density of FOXP3+ Tregs (×200)
By visual estimation, tumors were grouped into two categories. 102 carcinomas (54.5 %) were identified as high Gal-1 expression, and 85 carcinomas (45.5 %) were low expression. Clinicopathologic variables stratified by Gal-1 status are listed in Table 2. Although the statistic levels remained insignificant (P = 0.053), there was a trend seen between Gal-1 expression and clinical stages. High expression of Gal-1 tended to be more frequent in advanced-stage (III–IV) subgroup comparing with early-stage (I–II) subgroup; in addition, tumoral Gal-1 expression was not significantly correlated with histology differentiation (P > 0.05). As shown, no correlations were found between Gal-1 expression and T stage (P = 0.164), or N stage (P = 0. 079) of the tumors, or the presence of metastases (P = 0.236) and recurrence (P = 0.568). Overall, the status of intratumoral Gal-1 expression was not associated with other clinicopathologic parameters.
Association of Gal-1 expression with apoptosis-related proteins
Representative pictures of immunohistochemical staining of Bax, Bcl-2, Fas and FasL expression in LSCC are shown in Fig. 1. Among the 187 carcinomas studied, a high expression of Bax, Bcl-2, Fas and FasL was observed in 137 (73.3 %), 104 (55.6 %), 97 (51.9 %), and 126 (67.4 %) patients, respectively. We next studied the Gal-1 expression in relation to mentioned apoptosis-related proteins in LSCC. In brief, Gal-1 expression has no obvious correlation with the expression of Fas, FasL, Bcl-2 and Bax, respectively (Table 3).
Relationship between Gal-1 expression and tumoral lymphocytes infiltration
Immune cells infiltrated tumor tissue in a disseminated manner as scattered solitary cells, as shown by hematoxylin staining, and displayed low level of homogeneity and broad inter-individual differences for stained cell density, the number of CD8+ and FOXP3+ cells varying significantly among samples. The CD8 immunostaining demonstrated cytomembrane staining in a subset of TIL around the tumor nests (Fig. 1d). The median number of CD8+ cells was 30.89 cells/high-power field (HPF) and the range was 6.89–98.71 cells/HPF. The FOXP3 immunostaining demonstrated nuclear staining in a subset of lymphocytes around tumor tissues (Fig. 1h). The median number of FOXP3+ cells was 15.25 cells/HPF, and the range was 2.64–56.49 cells/HPF. Among the 187 carcinomas studied, 107 cases (57.2 %) were identified as low density of FOXP3+ TILs, and 80 cases (42.8 %) were classified as high proportion. By general logical linear regression analysis, no significant correlation was found between the densities of any two types of lymphocytes in the present study (data not shown). As shown in Table 3, tumoral Gal-1 expression was correlated with neither CD8+ nor FOXP3+ TILs alone (P > 0.05). Instead, it was positively associated with the ratio of FOXP3+/CD8+ TIL (P = 0.024).
Prognostic significance of Gal-1 in LSCC
The follow-up period ranged from 4 to 93 months with a mean of 42.0 months for all patients (SD = 22.8). Mean disease-free survival (DFS) for the patients included in our study was 21.3 months (range 2–49 months). At the time of data analysis, a total of 52 (27.8 %) tumors relapsed. Sixty-nine (36.9 %) patients died of the disease and 12 (6.4 %) died of other causes. For the 106 (56.7 %) surviving patients, 103 (55.1 %) were disease free and 3 (1.6 %) was alive with disease.
Next, we focus on the impact of tumoral Gal-1 expression on OS and DFS in LSCC patients. In entire cohort (n = 187), the 5-year survival was 60.0 % (n = 51) in patients with low Gal-1 expression and 53.9 % (n = 55) in patients with high Gal-1 expression (P = 0.079). In subgroup with 78 early-stage LSCC (stages I and II), the 5-year survival was 64.1 % (n = 25) in patients with low Gal-1 expression and 69.2 % (n = 27) in patients with high Gal-1 expression (P = 0.153). In subgroup with 109 late-stage LSCC (stage III and IV), the 5-year survival was 56.5 % (n = 26) in patients with low Gal-1 expression and 44.4 % (n = 28) in patients with high Gal-1 expression (P = 0.035).
In the entire cohort, the mean overall survival time was 44.89 months (95 % CI 19.46–83.32 months) for 85 patients with low Gal-1 expression, and 39.74 months (95 % CI 15.51–73.95 months) for 102 patients with high Gal-1 expression (x 2 = 1.280, P = 0.258). The mean disease-free survival for patients with low Gal-1 expression was 21.69 months (95 % CI 18.79–46.07 months), and 20.93 months (95 % CI 15.45–43.94 months) for patients with high Gal-1 expression (x 2 = 0.199, P = 0.655).
In a subgroup with late-stage LSCC, the mean survival time for 46 patients with low Gal-1 expression was significant longer than that in patients with high Gal-1 expression (49.06 vs. 36.83, x 2 = 4.527, P = 0.033). Similarly, the mean disease-free survival for patients with low Gal-1 expression was obviously improved when compared with the patients with high Gal-1 expression (24.74 vs. 19.18, x 2 = 5.693, P = 0.017). Figure 2 illustrates patient survival over time according to Gal-1 distribution in entire cohort and subgroups with late-stage, respectively. In a word, the results of Kaplan–Meier and log-rank test analyses showed that the tumoral expression of Gal-1 had a prognostic significance for OS and DFS in cohort with late-stage LSCC (P < 0.05).
Gal-1 expression was included as a covariate for Cox proportional hazards analyses together with conventional clinicopathologic and therapeutic variables, and the significances of their prognostic association were assessed by the multivariate assessment. As shown in Table 4, advanced clinical stage and presence of metastases were identified as the independent predictors for poor survival (both DFS and OS) in entire cohort. Differences in treatment modalities were included in this model and did not change the significance of these variables (data not shown). It is important to note the difference between the low-stage and the high-stage population. Although the statistical correlation between the Gal-1 expression and prognosis was particularly due to the late-stage tumors (P < 0.05), the correlation was not significant in the cases of the low-stage LSCC.
Discussion
This study explored the clinical implication of tumoral Gal-1 in LSCC. Tumoral Gal-1 expression was significantly related with advanced clinical stage, but not with the presence of metastases and recurrence. A close correlation of tumoral Gal-1 expression with the ratio of FOXP3+/CD8+TILs was found in our cohort. More importantly, the overexpression of Gal-1 in tumor is apparent associated with poor survival in our cohort, especially in advanced LSCC.
Gal-1 has been studied in numerous neoplasms, and different expression profiles have been reported for the neoplastic and the tumor-associated stromal cells [8]. Significantly stronger immunostaining for Gal-1 was reported in most fibroblasts and inflammatory cells in the tumor-associated stroma, such as breast [15], prostatic [16], pancreatic [17], ovarian cancer [18] and colorectal [19]. From our results in LSCC, Gal-1 protein is obviously overexpressed in both tumor cells and the surrounding stroma, as compared to normal tissues. This observation is consistent with the results form OSCC [20–22], tongue SCC [23] and nasopharyngeal carcinoma (NPC) [24]. Moreover, the patterns of Galectins expression in diverse subtype of HNSCC and separated area of tumor may be quite different [22]. In brief, although the specific profile of Gal-1 expression in LSCC was unclear, these results indicated that Gal-1 expression may serve as a potential diagnostic biomarker for LSCC.
Gal-1 and clinical parameters in LSCC
Cumulative evidence proved that Gal-1 expression was correlated with aggressive phenotypes in many types of tumors [25, 26]. Both Gillenwater et al. [20] and Zhong et al. [21] identified that Gal-1 expression was conversely correlated with the tumor pathologic differentiation grades in 35 HNSCC and 44 OSCC, respectively. As compared, Chiang et al. [22] found that Gal-1 was more frequently expressed in less differentiated tumors and in cells of the invasion front of early-stage OSCC. We did not find a relationship of Gal-1expression with pathologic differentiation, the presence of recurrence or metastases in our cohort.
In current study, intratumoral expression of Gal-1 tends to be correlated with advanced stage in LSCC patients. The frequence of high Gal-1 expression in the patients at late (III–IV) stage is higher than that at early (I–II) stage, although the difference is insignificant (P = 0.053). Similar results have been seen in patients with HNSCC [21, 27]. Inconsistently, Alves et al. [23] reported that Gal-1 expression was negatively correlated with metastasis and clinical stage in 65 cases of SCC of tongue, with the observation of strong expression of this protein in cases without metastases and in early-stage tumors. Beside inconsistence in histological types and clinical stages of studied population, the apparent discrepancies were due to the different techniques of tissue analysis and immune characterization, as well as the diverse scoring systems to calculate the prevalence of Gal-1. Moreover, it might be explained by disparities in the embryological origins of LSCC or HNSCC tissue, these differences translating directly into significantly different patterns of galectin expression and clinical behavior profiles seen for subtypes. Previous results underline the importance of assessing the precise distribution and specific function of Gal-1 for particular tumor type.
Gal-1 and TILs in LSCC
Leukocyte infiltrates into or around tumor cell nests are found in the context of protumorigenic inflammation and anticancer immunosurveillance. Indeed, Gal-1 has shown to have an inverse relation with CD4+ and CD8+ T cells infiltration in other tumor [28]. Knockdown Gal-1 expression can increase the frequency of CD4+ and CD8+ T cells infiltrating tumors from mice as compared to the controls. High Gal-1expression was associated with reduced CD8+ T cell infiltration at the tumor site in Hodgkin lymphoma [29]. Moreover, it has been recently found that Gal-1 is a key regulator of CD4+CD25+FOXP3+ regulatory T cells which play an essential role in the suppression of anticancer immunity [30]. It is proved that Gal-1 treatment [31] or cancer-derived Gal-1 [32] could increase the number of CD4+CD25+FOXP3+ Tregs in vitro. Recently, Wu et al. [33] found that Gal-1 level was positively related to the number of tumor-infiltrating FOXP3+ Tregs in 386 patients with hepatocellular carcinoma. In brief, regulating immune responses via Tregs modulation is one of the many immunological activities attributed to Gal-1 [34].
By far, only two reports in the literature have investigated the Gal-1 expression in relation with CD3+TILs density in head and neck cancer. Saussez et al. [35] observed a significant negative correlation between the number of tumor-infiltrating CD3-positive lymphocytes and the percentage of Gal-1-immunopositive tissue in 20 patients with high-stage LSCC. Comparable result was found by Le et al. [36] in 101 HNSCC patients. In current cohort, the significant correlations of Gal-1 expression with the distribution of CD4+, CD8+, CD3+ and FOXP3+ TILs were not found. Instead, we detected a positive association of intratumoral expression of Gal-1 with the ratio of FOXP3+/CD8+TILs.
It is projected that the immunological data (the type, density, and location of immune cells within the tumor samples) is a better predictor of patient survival than the histopathological methods currently used to stage cancer [37]. Our data may be compatible with this new theory. Actually, the ratio of intratumoral CD8+/Treg was proved to be associated with outcome in ovarian [38], CRC [39] and breast cancer [14]. The ratio of CD8+/FOXP3+ TILs may reflect a specific effective anti-tumor reaction of the effector cells against the tumor, while absolute numbers reflect the general activation. Indeed, Gal-1 was shown to influence the Th1/Th2 cytokine balance by selectively inducing Th1 apoptosis and promoting Th2 function in several mouse models [40, 41]. Therefore, the balance or interplay between various types of immune cells within the tumor bed may be more precise in depicting the immune reaction in the tumor microenvironment.
Caution should be exercised in interpreting the current results because the immune system in reaction with tumor cells and/or stoma cells in tumor microenvironment are extremely complicated. Tumor cell-derived Gal-1 might efficiently contribute to tumor self-defense, which is one of mechanism for tumor cell-induced T cell apoptosis [42]. The activity of Gal-1 might be regulated by other defined and yet not defined microenvironmental components, the elucidation of which will be helpful in understanding tumor escape mechanisms, giving rise to further therapeutic approaches. More studies are necessary to elucidate the functional status of Gal-1 itself and/or to microenvironment interactions.
Gal-1 and prognosis in LSCC
Actually the prognostic value of Gal-1 in human tumor has attracted crescent attention in last decade. The overexpression of Gal-1 has been associated with poor prognosis in epithelial ovarian cancer [43], gastric adenocarcinoma [44] and hepatocellular carcinoma [33], Hodgkin lymphoma [45], prostate carcinoma [46] and malignant melanomas [47]. To date, there was very limited paper in the literature concerning the prognostic value of intratumoral Gal-1 expression in LSCC or HNSCC, and the results were paradoxical and incomparable. Saussez et al. [35] identified that high levels of Gal-1 determined by IHC is predictive of worse prognoses in a series of 62 LSCCs including only 20 high-stage cases. In another cohort with 81 stage IV hypopharyngeal SCCs, they determined that high levels of galectin-7, but not galectins-1 and -3, are associated with dismal prognoses [48]. Le et al. [49] identified high immunohistochemical staining of Gal-1 closely correlated with poor cancer-specific survival in 101 HNSCC patients. However, Chiang et al. [22] did not prove an independent prognostic significance of Gal-1 overexpression in 64 primary OSCC.
We here reported a prognostic value of intratumoral Gal-1 expression for OS and DFS in patients with late-stage LSCC, but not in early-stage subgroup. It may be due to a relatively high proportion (almost 60 %) of late-stage LSCC in our cohort. This observation indicated that the prognostic value of Gal-1 may be inconsistent in different stage of tumor. Although some patients with advance stage presented a high Gal-1 expression and worse prognosis, we did not find a significant relationship between Gal-1 expression and clinical stage in LSCC patients. The functions of Gal-1 depend on its binding to specific ligands, which are localized either on the cell surface or in the extracellular matrix. Tumor Gal-1 may be more critical than host Gal-1 in promoting tumor growth and metastasis [43]. Collectively, these results indicate that the origin, location, and activation modes of Gal-1 may vary according to diverse tumor types, and the prognostic implication of Gal-1 may be evolutive accompanying with aggravating progression of tumors. However, it should be pointed out that inevitable flaws exist due to the retrospective nature. We did not analysis the relationship between Gal-1 expression and cause-specific survival rate in current research. In the further, the particular role of Gal-1 warrant more thorough and explicit investigations in a prospective setting.
Conclusion
In conclusion, the current observations made in a retrospective, population-based cohort require confirmation in independent clinical data sets that include both quantitative and qualitative assessment of Gal-1. Nevertheless, the current results represent valuable advancements in Gal-1 research and provided further support for using Gal-1 as a diagnostic biomarker and immunotherapeutic target for LSCC. Although the detail mechanism is not yet fully understood, our observation indicated that tumor-produced Gal-1 may affect microenvironmental immunologic milieu by modulating immunological balance of TILs. It will help offer mechanistic insights into Gal-1-mediated cytokine regulation, and hopefully translate into effective therapies for tumor and inflammatory disorders.
Abbreviations
- DFS:
-
Disease-free survival
- FOXP3:
-
Forkhead box P3
- HPF:
-
High-power field
- HNSCC:
-
Head and neck squamous cell carcinomas
- IHC:
-
Immunohistochemistry
- LSCCs:
-
Laryngeal squamous cell carcinomas
- NPC:
-
Nasopharyngeal carcinoma
- OS:
-
Overall survival
- PL:
-
Partial laryngectomy
- RC:
-
Radiochemotherapy
- SCCs:
-
Squamous cell carcinomas
- TIL:
-
Tumor infiltrating lymphocytes
- TL:
-
Total laryngectomy
- Treg:
-
Regulatory T lymphocytes
References
Forastiere A, Koch W, Trotti A, Sidransky D (2001) Head and neck cancer. N Engl J Med 345:1890–1900
Mao L, Hong WK, Papadimitrakopoulou VA (2004) Focus on head and neck cancer. Cancer Cell 5:311–316
Allen CT, Judd NP, Bui JD, Uppaluri R (2012) The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope 122:144–157
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570
Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH (2010) Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29:1093–1102
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Salatino M, Croci DO, Bianco GA, Ilarregui JM, Toscano MA, Rabinovich GA (2008) Galectin-1 as a potential therapeutic target in autoimmune disorders and cancer. Expert Opin Biol Ther 8:45–57
Demydenko D, Berest I (2009) Expression of galectin-1 in malignant tumors. Exp Oncol 31:74–79
Sioud M (2011) New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol 73:79–84
Rabinovich GA, Ilarregui JM (2009) Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev 230:144–159
Juszczynski P, Rodig SJ, Ouyang J, O’Donnell E, Takeyama K, Mlynarski W, Mycko K, Szczepanski T, Gaworczyk A, Krivtsov A, Faber J, Sinha AU, Rabinovich GA, Armstrong SA, Kutok JL, Shipp MA (2010) MLL-rearranged B lymphoblastic leukemias selectively express the immunoregulatory carbohydrate-binding protein galectin-1. Clin Cancer Res 16:2122–2130
Liu H, Zhang T, Li X, Huang J, Wu B, Huang X, Zhou Y, Zhu J, Hou J (2008) Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci 99:2185–2192
Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J (2012) Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother 61:1849–1856
Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L (2011) CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 130:645–655
Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC, Choi SK, Ha WS, Kim JW, Lee CW, Lee JS, Park ST (2007) Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer 120:2331–2338
Van Den Brule FA, Waltregny D, Castronovo V (2001) Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol 193:80–87
Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Büchler MW (2001) Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem 49:539–549
van den Brule FA, Califice S, Garnier F, Fernandez PL, Berchuck V, Castronovo V (2003) Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest 83:377–386
Barrow H, Rhodes JM, Yu LG (2011) The role of galectins in colorectal cancer progression. Int J Cancer 129:1–8
Gillenwater A, Xu XC, el-Naggar AK, Clayman GL, Lotan R (1996) Expression of galectins in head and neck squamous cell carcinoma. Head Neck 18:422–432
Zhong LP, Wei KJ, Yang X, Pan HY, Ye DX, Wang LZ, Zhang ZY (2010) Overexpression of Galectin-1 is negatively correlated with pathologic differentiation grade in oral squamous cell carcinoma. J Cancer Res Clin Oncol 136:1527–1535
Chiang WF, Liu SY, Fang LY, Lin CN, Wu MH, Chen YC, Chen YL, Jin YT (2008) Overexpression of galectin-1 at the tumor invasion front is associated with poor prognosis in early-stage oral squamous cell carcinoma. Oral Oncol 44:325–334
Alves PM, Godoy GP, Gomes DQ, Medeiros AM, de Souza LB, da Silveira EJ, Vasconcelos MG, Queiroz LM (2011) Significance of galectins-1, -3, -4 and -7 in the progression of squamous cell carcinoma of the tongue. Pathol Res Pract 207:236–240
Tang CE, Tan T, Li C, Chen ZC, Ruan L, Wang HH, Su T, Zhang PF, Xiao ZQ (2010) Identification of Galectin-1 as a novel biomarker in nasopharyngeal carcinoma by proteomic analysis. Oncol Rep 24:495–500
Danguy A, Camby I, Kiss R (2002) Galectins and cancer. Biochim Biophys Acta 1572:285–293
Liu FT, Rabinovich GA (2005) Galectins as modulators of tumour progression. Nat Rev Cancer 5:29–41
Choufani G, Nagy N, Saussez S, Marchant H, Bisschop P, Burchert M, Danguy A, Louryan S, Salmon I, Gabius HJ, Kiss R, Hassid S (1999) The levels of expression of galectin-1, galectin-3, and the Thomsen-Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer 86:2353–2363
Soldati R, Berger E, Zenclussen AC, Jorch G, Lode HN, Salatino M, Rabinovich GA, Fest S (2012) Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int J Cancer 131:1131–1141
Gandhi MK, Moll G, Smith C, Dua U, Lambley E, Ramuz O, Gill D, Marlton P, Seymour JF, Khanna R (2007) Galectin-1 mediated suppression of Epstein–Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood 110:1326–1329
Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30:636–645
Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA (2007) The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA 104:13134–13139
Kuo PL, Hung JY, Huang SK, Chou SH, Cheng DE, Jong YJ, Hung CH, Yang CJ, Tsai YM, Hsu YL, Huang MS (2011) Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol 186:1521–1530
Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX, Sun TW, Zhou J, Shi YH, Yang XR, Jin JJ, Cheng YF, Fan J, Qiu SJ (2012) Overexpression of galectin-1 associates with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol 27:1312–1319
Uppaluri R, Dunn GP, Lewis JS Jr (2008) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun 8:16
Saussez S, Decaestecker C, Lorfevre F, Cucu DR, Mortuaire G, Chevalier D, Wacreniez A, Kaltner H, André S, Toubeau G, Camby I, Gabius HJ, Kiss R (2007) High level of galectin-1 expression is a negative prognostic predictor of recurrence in laryngeal squamous cell carcinomas. Int J Oncol 30:1109–1117
Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ, Giaccia AJ, Koong AC (2005) Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol 23:8932–8941
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci 102:18538–18543
Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, Katano M (2010) Intratumoral CD8(+) T/FOXP3(+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 59:653–661
Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC (2008) Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol 38:3015–3027
Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA (2006) Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol 176:6323–6332
Cedeno-Laurent F, Dimitroff CJ (2012) Galectin-1 research in T cell immunity: past, present and future. Clin Immunol 142:107–116
Kim HJ, Jeon HK, Cho YJ, Park YA, Choi JJ, Do IG, Song SY, Lee YY, Choi CH, Kim TJ, Bae DS, Lee JW, Kim BG (2012) High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. Eur J Cancer 48:1914–1921
Bektas S, Bahadir B, Ucan BH, Ozdamar SO (2001) CD24 and galectin-1 expressions in gastric adenocarcinoma and clinicopathologic significance. J Pathol 193:80–87
Kamper P, Ludvigsen M, Bendix K, Hamilton-Dutoit S, Rabinovich GA, Møller MB, Nyengaard JR, Honoré B, d’Amore F (2011) Proteomic analysis identifies galectin-1 as a predictive biomarker for relapsed/refractory disease in classical Hodgkin lymphoma. Blood 117:6638–6649
van den Brûle FA, Waltregny D, Castronovo V (2010) Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. Pathol Oncol Res 16:569–577
Lefranc F, Mathieu V, Kiss R (2011) Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior. World J Biol Chem 2:193–201
Saussez S, Cucu DR, Decaestecker C, Chevalier D, Kaltner H, André S, Wacreniez A, Toubeau G, Camby I, Gabius HJ, Kiss R (2006) Galectin 7 (p53-induced gene 1): a new prognostic predictor of recurrence and survival in stage IV hypopharyngeal cancer. Ann Surg Oncol 13:999–1009
Le QT, Kong C, Lavori PW, O’byrne K, Erler JT, Huang X, Chen Y, Cao H, Tibshirani R, Denko N, Giaccia AJ, Koong AC (2007) Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 69:167–175
Acknowledgements
We gratefully thank Dr. Jinghui Hou and his colleagues (Department of Pathology, Cancer Center, Sun Yat-sen University) for technical assistance in pathological evaluations.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, J., Liu, H., Hu, Y. et al. The clinical implication of tumoral Gal-1 expression in laryngeal squamous cell carcinomas. Clin Transl Oncol 15, 608–618 (2013). https://doi.org/10.1007/s12094-012-0975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0975-z