Abstract
The human immune system consists of a balance between immune surveillance against non-self antigens and tolerance of self-antigens. CD8+ T cells and CD4+ regulatory T cells (Tregs) are the main players for immune surveillance and tolerance, respectively. We examined immunohistochemically the immunological balance at the tumor site using 94 surgically resected colorectal cancer tissues. Forkhead box P3 (FOXP3)+ cells were considered to be Tregs in the present study. The number of intratumoral FOXP3+ cells (itFOXP3+ cells) was positively correlated with lymph node metastases (P = 0.030). itCD8+ T/itFOXP3+ cell ratio negatively correlated with pathological stages (P = 0.048). Next, relationship between the number of itCD8+ T cells or itFOXP3+ cells and survival prognosis in 94 patients who underwent a curative resection was analyzed. Only itCD8+ T/itFOXP3+ cell ratio positively correlated with disease-free survival (0.023) and overall survival (P = 0.010). Multivariate analysis indicated that itCD8+ T/itFOXP3+ cell ratio is an independent prognostic factor (P = 0.035) of overall survival. The number of itFOXP3+ cells positively correlated with transforming growth factor-beta TGF-β production at the tumor site (P = 0.020). In conclusion, itCD8+ T/itFOXP3+ cell ratio is a predictive marker for both disease-free survival time and overall survival time in patients with colorectal cancer. Importantly, itCD8+ T/itFOXP3+ cell ratio may be an independent prognostic factor. And, tumor-producing TGF-β may contribute to the increased number of itFOXP3+ cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many years, the immune system has been generally considered to be able to control and eliminate tumors that develop spontaneously, so-called immune surveillance [9]. Indeed, tumor cells can express antigens which become targets for a T cell-mediated immune response [4]. To date, there have been many investigations supporting this concept. For example, correlation has been made between the degree of tumor invasion by tumor-infiltrating lymphocytes (TILs) and better survival in patients with various types of tumors, including colorectal cancer [18, 27, 38]. In a report analyzing subpopulations of TILs, a correlation between an increase in CD8+ T cells and better survival has been shown in colorectal cancer [24]. Recently, it has been reported that an increase of memory T cells, especially memory CD8+ T cells, correlates with increased survival in large cohorts of human colorectal cancer patients [12].
On the other hand, cancer cells evade antitumor T cell response by multiple immunosuppressive mechanisms [8]. In the 1980s, several investigators had already proposed an involvement of suppressor T cells in the immune responses against tumors [3]. Recent evidence has revealed the existence of a unique CD4+ T cell population, designated regulatory T cells (Tregs), as an important suppressor T cell population [22, 29, 32]. Tregs were originally identified as CD4+ T cells that constitutively expressed the interleukin (IL)-2 receptor α-chain (CD25) [28]. More recent studies have shown that the transcription factor forkhead box P3 (FOXP3) is not only a key intracellular marker but also a crucial developmental and functional factor for CD4+CD25+ Tregs [17]. Thus, it is now generally considered that CD25+FOXP3+CD4+ T cells are Tregs. Tregs are considered to act as players in immune tolerance against self-antigens. Most tumor-associated antigens (TAAs) are self-antigens. This means that TAAs themselves may induce an increased number of Tregs in cancer patients and that the increased Tregs may negatively control the antitumor immune response. Indeed, increased numbers of Tregs in the peripheral blood or in tumor tissues have been shown in several types of tumors, including colorectal cancer [7, 20, 21, 35], and a possible suppression of antitumor immunity by Tregs has been indicated [30, 37]. It should be noted that the increased numbers of Tregs infiltrating tumors corresponded to poor prognosis in patients with ovarian cancer [7].
The human immune system consists of an elegant balance between immune surveillance and immune tolerance of self-antigens. Tregs play an important role in the prevention of autoimmune disorders by controlling the activity of autoreactive T cells [22]. On the other hand, Tregs may play critical roles in immune tolerance against cancers [25, 30, 37]. Recently, several studies have shown the importance of a balance between immune surveillance and immune evasion in the tumor microenvironment [11, 13, 31]. First, the tumor-infiltrating CD8+ T cell/Tregs ratio was associated with survival prognosis in ovarian cancer [31]. Second, an inverse relationship between intratumoral CD8+ T cells and intratumoral Tregs in hepatocellular carcinoma (HCC) tissues was shown. In addition, an increased quantity of circulating Tregs was associated with reduced survival time of these patients. However, no significant correlation between numbers of TILs and survival prognosis was found [11]. Third, the possibility of an intratumoral balance of Tregs and activated CD8+ T cells, as an independent predictor of survival in HCC was shown [13].
Transforming growth factor-β (TGF-β) is a potent regulatory cytokine with diverse effects on hemopoietic cells. The role of TGF-β in the induction and maintenance of Tregs has attracted much attention. Importantly, it has been shown that TGF-β1 can convert CD4+CD25− T cells into Tregs in vitro [5]. In addition, a reduced number of peripheral Tregs has been shown in TGF-β1−/− mice [23]. Peng et al. [26] have also shown in mouse that TGF-β may regulate the in vivo expansion of Tregs. Taken together, TGF-β may play an important role in in vivo induction and/or maintenance of Tregs.
In the present study, the absolute numbers of tumor-infiltrating CD8+ T cells and FOXP3+ cells were measured using surgically resected specimens. We indicate for the first time that the tumor-infiltrating CD8+ T/FOXP3+ cell ratio may be a predictive marker for disease-free survival in patients with colorectal cancer. In addition, we suggest that tumor-produced TGF-β contributes to the increased number of intratumoral Tregs.
Patients and methods
Patients and samples
Ninety-five patients with primary colorectal cancer underwent resection at the Department of Surgery and Oncology, Kyushu University (Fukuoka, Japan). All patients who were enrolled in the present study provided informed consent before surgical treatment. Primary colorectal cancer surgical specimens with adjacent normal colonic mucosa were fixed in 10% formalin and embedded in paraffin. Sections were cut serially into 4 μm sections. All tumors were staged according to the TMN classification system of the International Union Against Cancer (UICC).
Immunohistochemistry
Single or double color DAB immunoperoxidase staining was performed as described previously [31]. Anti-human CD8 mouse monoclonal antibody (CM154 BIOCARE, Concord, USA), FOXP3 rabbit monoclonal antibody (ab20034, Abcam, Cambridge, UK), TGF-β rabbit polyclonal antibody (sc-146, Santa Cruz, CA, USA), and CD3 mouse monoclonal antibody (N1617, Dako, Tokyo, Japan) were used. Slides were incubated with each primary antibody at 4°C overnight, washed in phosphate buffered saline (PBS) three times for 5 min each, and incubated with secondary antibodies (goat anti-mouse and rabbit IgG; Nichirei Corp, Ltd, Tokyo, Japan) at room temperature for 30 min. Immunoreactivity was visualized by the development of brown pigment via a standard 3,3′-diaminobenzidine protocol, and of red pigment via a New Fuchsin solution kit (Nichirei Corp., Ltd, Tokyo). Sections were then counterstained lightly with hematoxylin. CD8 and FOXP3 stained cells are described as CD8+ T cells and FOXP3+ cells, respectively.
Evaluation of immunostaining
Evaluation of immunostaining was performed as previously described but with minor modifications [31]. Each entire tumor section was evaluated for TILs by microscopic examination, (×400; BX51; Olympus, Tokyo, Japan) and ten independent areas that had the most abundant numbers of TILs were selected and photographed with a digital camera (Binary Planner 4490; Jenoptil, Jena, Germany). Absolute numbers of labeled tumor-infiltrating cells, excluding tumor cells, were counted manually. The count was performed two times for each photograph by the same investigator without knowledge of the corresponding clinical data. Total numbers of the ten selected areas were represented as intratumoral CD8+ T cells (itCD8+ T cells) or itFOXP3+ cells. A ratio of itCD8+ T cells to itFOXP3+ cells was represented as the itCD8+ T/itFOXP3+ cell ratio.
To examine whether TGF-β contributes to increased itFOXP+ cell numbers, we analyzed the relationship between TGF-β expression at the tumor site and the numbers of itFOXP+ cells in each specimen. As described above, ten independent areas that were the most abundant in TILs were selected. When TGF-β-expressing areas occupied over 30% of the total of the 10 independent areas, specimens were considered to be high TGF-β-expressing specimens [15].
Cell culture and fluorescence-activated cell sorting (FACS) analysis
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Paque (Life Technologies, Gaithersburg, MD, USA) density gradient centrifugation from five healthy volunteers. CD4+ T cells were purified from PBMCs with a CD4-positive isolation kit (Dynabeads, Dynal Biotech, Oslo, Norway), according to the manufacturer’s instructions. The positive-selection process yielded over 98% CD4+ T cells. CD4+ T cells (5.0 × 105 cells/well) were cultured with GMP-grade RPMI 1640 (Hy-medium; Nipro, Tokyo, Japan) containing 5% human albumin, 100 IU/mL human recombinant IL-2 (Nipro) and immobilized monoclonal antibody to CD3 (10 μg/mL, OKT-3; Jansen-Kyowa, Tokyo, Japan) for 5 days in the presence or absence of recombinant human TGF-β (100-B;R& D systems, Weisbaden, Germany). After 5 days, the cells were harvested and intracellular staining of FOXP3 was conducted using a PE-conjugated anti-human FOXP3 Staining Set (clone PCH101; e-Bioscience, SanDiego, CA, USA) according to the manufacturer’s instructions. Two-color flow cytometry was performed using a FACSCaliburTM (Becton–Dickinson CA, USA). FOXP3+ cells, after gaiting of CD4+ lymphocytes, were analyzed. The percentage of FOXP3+ cells among the total CD4+ T cells was determined.
Statistical analysis
All statistical analyses were performed with JMP Statistical Software (SAS Institute, Inc). The correlations between subsets of TILs (itCD8+ T cells, itFOXP3+ cells, and the itCD8+ T/itFOXP3+ cell ratio) and the clinicopathological features listed were analyzed by Spearman’s test. The statistical significance of difference between the two groups was determined using the Mann–Whitney nonparametric U test. Kruskal–Wallis H nonparametric test was applied to multiple comparisons. All items were treated as dichotomous variables. Survival was measured from the date of operation to the time of death/relapse or the time the patient was last seen. The log-rank test was used to perform univariate analyses and Kaplan–Meier curves were used to estimate survival rates. Survival rates were compared by the log-rank test via the SAS/STAT PHREG procedure. Prognostic factors for survival were evaluated in multivariate analyses by Cox proportional hazards regression. One patient with distant metastasis was excluded from this analysis. In the remaining 94 patients, itCD8+ T cells and itFOXP3+ cells were dichotomized by a cutoff point of 151 and 14, respectively, on the basis of the mean value. Since no itFOXP3+ cells were observed in a few specimens, it was not suitable to divide patients into two groups based on the mean value of the itCD8+ T/itFOXP3+ ratio. Therefore, patients were divided into equal-sized groups. As a result, the itCD8+ T/itFOXP3+ cell ratios were dichotomized by a cutoff point of 12, i.e., ≥12; 47 cases versus ≤12; 47 cases. A P value of <0.05 was considered to be significant.
Results
Immunohistochemical characteristics
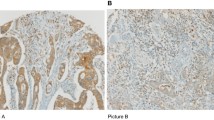
In this study, we used surgically resected tumor specimens. By hematoxylin and eosin staining, TILs distribution was found to be relatively homogenous within a tumor, excluding necrotic and fibrotic areas, although TILs were usually more abundant in stromal areas compared with epithelial areas, in agreement with previous reports [19, 21]. To estimate the number of TILs, ten independent areas that were the most abundant in TILs were selected and total number of labeled cells was expressed as itCD8+ T cells or as itFOXP3+ cells. ItFOXP3+ cells exhibited distinct nuclear staining (Fig. 1a). When TILs were doubly stained with anti-FOXP3 and anti-CD3 antibodies, most itFOXP3+ cells were positive for CD3 (date not shown), indicating that itFOXP3+ cells are T cells. itCD8+ T cells exhibited homogeneous cytoplasmic staining, while nuclei were not stained (Fig. 1b). When TILs were doubly stained with anti-FOXP3 and anti-CD8 antibodies, itFOXP3+ cells and itCD8+ T cells could be counted separately as different cells (Fig. 1c). When the correlation between the number of itCD8+ T and itFOXP3+ cells was analyzed, no significant correlation was detected (Fig. 1d).
Immunohistochemical staining shows that FOXP3+ lymphocytes are distinguishable from CD8+ lymphocytes in colorectal cancer. a FOXP3 positive cells are stained brown, as shown by asterisks (×400). b CD8 positive cells are stained red, as shown by arrows (×400). c Double staining of FOXP3 (asterisks, in cell nucleus) and CD8 (arrows, on cell membrane) (×400). d The correlation between CD8+ T cells and FOXP3+ cells is shown (Mann–Whitney U test). P values less than 0.05 were considered significant (color figure online)
Correlation of TIL subtypes with clinicopathological features
The relationships of itCD8+ T and itFOXP3+ cells with traditional pathological measures for the 94 colorectal cancer specimens were analyzed statistically (Table 1). The number of itCD8+ T cells showed no correlation with any pathological parameters. On the other hand, the number of itFOXP3+ cells was positively correlated with lymph node metastases (P = 0.030). The ratio of the number of itCD8+ T cells to the number of itFOXP3+ cells (itCD8+ T/itFOXP3+ cell ratio) was negatively correlated with pathological stages (P = 0.048).
Correlation of TIL subtypes with survival prognosis
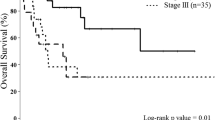
To determine whether itCD8+ T and itFOXP3+ cell numbers are significant prognostic factors for survival, 95 patients who underwent a curative resection were analyzed (Fig. 2). One patient was excluded from this analysis, because he had distant metastases and thus underwent a non-curative resection. The 94 patients were divided into two groups by cutoff points of 151 and 14 for the number of itCD8+ T cells and itFOXP3+ cells, respectively, as described in the “Patients and methods” section. The number of itCD8+ T cells did not significantly correlate with disease-free survival (Fig. 2a) and overall survival (Fig. 2b). The number of itFOXP3+ cells also showed no significant correlation with disease-free survival (Fig. 2c) and overall survival (Fig. 2d). Interestingly, when the itCD8+ T/itFOXP3+ cell ratio was dichotomized by a cutoff point of 12, itCD8+ T/itFOXP3+ cell ratio showed a significant positive correlation with disease-free survival (P = 0.023; Fig. 2e) and overall survival (P = 0.010; Fig. 2f).
The itCD8+ T/itFOXP3+ cell ratio predicts poor disease-free survival and overall survival in 94 colorectal cancer patients. itCD8+ T cells (a and b) and itFOXP3+ cells (c and d) correlated with neither disease-free survival nor with overall survival. Disease-free survival and overall survival of the patients showing high an itCD8+ T/itFOXP3+ cell ratio were significantly better compared with those of the patients showing a low itCD8+ T/itFOXP3+ cell ratio (e and f). P values less than 0.05 were considered significant
To estimate the value of TIL numbers as a prognostic factor, the correlation of traditional clinicopathological factors, such as lymph node metastases, with disease-free survival (Table 2) and overall survival (Table 3) was analyzed. Venous invasion, lymph node metastases, pathological stages, and itCD8+ T/itFOXP3+ cell ratio correlated with disease-free survival in this cohort (Table 2, upper panel). We next examined whether the itCD8+ T/itFOXP3+ cell ratio is able to be an independent prognostic indicator for disease-free survival. Multivariate analysis was performed between these four factors (Table 2, lower panel). Only venous invasion significantly correlated with disease-free survival (P = 0.045). On the other hand, venous invasion, pathological stages, and itCD8+ T/itFOXP3+ cell ratio correlated with overall survival (Table 3, upper panel). When multivariate analysis was performed between these four factors, venous invasion (P = 0.050) and itCD8+ T/itFOXP3+ cell ratio (P = 0.035) significantly correlated with overall survival (Table 3, lower panel).
Correlation between TGF-β distribution within tumor tissues and the number of itFOXP3+ cells
We tried to evaluate quantitatively TGF-β expression in 31 out of the 94 specimens. TGF-β expression was estimated by a ratio (%) of the TGF-β-stained area to the total area of ten independent tumor areas that had the most abundant number of TILs. When this ratio was over 30% [15], the specimens were considered to be high TGF-β-expressing specimens (Fig. 3a), and other specimens were considered to be low TGF-β-expressing specimens (Fig. 3b). Specimens were doubly stained with anti-TGF-β antibody and anti-FOXP3 antibody (Fig. 3c). When the numbers of itFOXP3+ cells were over 14, the specimens were considered to be high itFOXP3+ cells number specimens as described in “Patients and methods”. A ratio of TGF-β-expressing area positively correlated with the number of itFOXP3+ cells (P = 0.020; Table 4).
Representative pictures of immunohistochemical double staining. a A high TGF-β-expressing specimen (red) (×400). b A low TGF-β-expressing specimen are shown (×400). c There are a high number of FOXP3+ cells (brown) indicated by asterisks, in the high TGF-β expressing area (×400) (color figure online)
Discussion
We show for the first time that a balance of itCD8+ T and itFOXP3+ cells, the itCD8+ T/itFOXP3+ cell ratio, is an independent indicator for overall survival in patients with colorectal cancer who have undergone curative surgery. In addition, itCD8+ T/itFOXP3+ cell ratio shows a positive correlation with disease-free survival time and overall survival time. We also suggest that tumor-producing TGF-β at least partly contributes to the increased number of itFOXP3+ cells.
A correlation of increased memory CD8+ T cells with increased survival in large cohorts of colorectal cancer patients has been shown [12], and importantly, our data may recapitulate these findings (Fig. 2a, b).
Many investigators have observed that Tregs are significantly higher in a number in tumor tissues, especially in stromal areas, compared with normal tissues [7, 13, 20, 21], and that tumor-infiltrating Tregs (itTregs) may play an important role in the suppression of the antitumor immune response [7, 11, 19, 30, 37]. If this is so, numbers of itTregs should correlate inversely with survival prognosis. In fact, there are several studies indicating an inverse correlation of itTregs with survival in certain kinds of tumors, including colorectal cancer [2, 7, 13, 16, 36]. On the other hand, there are also some investigations indicating no significant correlation between itTregs and survival [11, 14, 21]. In most of these studies, FOXP3+ T cells are classified as itTregs [2, 7, 11, 14, 16, 19, 21, 31, 36]. In the present study, most itFOXP3+ cells were also positive for CD3, indicating that itFOXP3+ cells are T cells (data not shown). Thus, we also considered itFOXP3+ cells to be itTregs.
Our present data did not show a significant correlation between itFOXP3+ cells and survival (Fig. 2c, d). Lymph node metastases, which is one of the important prognostic indicators in colorectal cancer [6], did correlate with disease-free survival time (P = 0.018; Table 2, upper panel) and overall survival time (P = 0.069; Table 3, upper panel). Thus, although cohort used in the present study is small, it seems that this cohort may be relatively suitable for analysis of prognostic factors. However, it is still controversial as to whether the number of itFOXP3+ cells is a prognostic indicator in colorectal cancer. Nevertheless, it is noteworthy that the number of itFOXP3+ cells showed a significant positive correlation with the status of lymph node metastases (P = 0.030; Table 1). This finding indicates that the number of itFOXP3+ cells may be a predictive factor for lymph node metastases.
A direct link between itFOXP3+ cells and prognostic survival remains unclear in the present study. However, recent evidence indicates that a balance of itTregs and itCD8+ T cells is a more sensitive predictor for recurrence and survival than itTregs or itCD8+ T cells alone [13, 31]. Our data may be compatible with this new theory. Namely, itFOXP3+ cells alone were not an indicator for survival, whereas the itCD8+ T/itFOXP3+ cell ratio correlated well with disease-free survival (Fig. 2e) and overall survival (Fig. 2f).
The next question is why a balance of itFOXP3+ and itCD8+ T cells is a more sensitive marker of recurrence or survival compared with itFOXP3+ cells or itCD8+ T cells alone. One possible answer is that antitumor immunity is determined by the immunological balance in the microenvironment of the tumor site. The molecular mechanisms governing the intratumor accumulation of CD8+ T cells may be different from those regulating Tregs. Namely, CD8+ T cells and FOXP3+ cells are likely to accumulate independently at the tumor site. In the present study, the number of itFOXP3+ cells correlated with lymph node metastases (P = 0.030), whereas the number of itCD8+ T cells did not (P = 0.940) (Table 1). This finding may indirectly indicate an independent accumulation of CD8+ T cells and of FOXP3+ cells at the tumor site. Thus, we suggest that the CD8+ T/FOXP3+ cell ratio at the tumor site reflects more strongly the patient’s total antitumor immunity than simply the numbers of CD8+ T or FOXP3+ cells.
It is very rare to detect FOXP3+ cells in normal colorectal tissues (data not shown), therefore, why does the number of FOXP3+ cells increase within tumor tissues? A unique mechanism of specific recruitment of Tregs in ovarian cancer tissues has been proposed [7], whereby chemoattractants for Tregs are produced by tumors and/or by tumor-infiltrating macrophages. Similar chemoattractants may be produced within the colorectal cancer tissues; however, this suggestion requires further investigation. Another possibility is the conversion of FOXP3− T cells to FOXP3+ T cells or the expansion of the FOXP3+ T population within the tumor microenvironment [1, 34]. It has been shown that TGF-β, which is present at high levels in the tumor microenvironment, can mediate this conversion or expansion [5, 10, 33]. Indeed, we confirmed the production of TGF-β in the colorectal cancer tissues examined. Importantly, a larger number of FOXP3+ cells were found in high TGF-β-expressing specimens compared with low TGF-β expressing specimens (Table 4). In addition, co-culture of peripheral CD4+ T cells with recombinant TFG-β1 increased the number of FOXP3+ cells (data not shown). Although these findings support a possible conversion of FOXP3− T cells to FOXP3+ T cells or the expansion of the FOXP3+ T cell population in the colorectal cancer microenvironment, it is difficult to precisely evaluate this possibility from our surgically resected specimens.
In conclusion, we want to stress that the CD8+ T/FOXP3+ cell ratio may be an independent prognostic factor for colorectal cancer patients who have undergone curative resection. This finding will improve our understanding of clinical significance of immune cells existing in the tumor microenvironment.
Abbreviations
- Tregs:
-
CD4+ regulatory T cells
- FOXP3:
-
Forkhead box P3
- itCD8+ T cells:
-
Intratumoral CD8-positive T cells
- itFOXP3+ cells:
-
Intratumoral FOXP3-positive cells
- itCD8+ T/itFOXP3+ cell ratio:
-
Ratio of number of intratumoral CD8-positive T cells to number of intratumoral FOXP3-positive cells
- TILs:
-
Tumor-infiltrating lymphocytes
- PBMCs:
-
Peripheral blood mononuclear cells
- TGF-β:
-
Transforming growth factor-beta
References
Akbar AN, Vukmanovic-Stejic M, Taams LS et al (2007) The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol 7:231–237
Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Berendt MJ, North RJ (1980) T-cell-mediated suppression of anti-tumor immunity An explanation for progressive growth of an immunogenic tumor. J Exp Med 151:69–80
Boon T, Cerottini JC, Van den Eynde B et al (1994) Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 12:337–365
Chen W, Jin W, Hardegen N et al (2003) Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886
Cohen AM, Tremiterra S, Candela F et al (1991) Prognosis of node-positive colon cancer. Cancer 67:1859–1861
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Drake CG, Jaffee E, Pardoll DM (2006) Mechanisms of immune evasion by tumors. Adv Immunol 90:51–81
Dunn GP, Bruce AT, Ikeda H et al (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998
Fantini MC, Becker C, Monteleone G et al (2004) Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172:5149–5153
Fu J, Xu D, Liu Z et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328–2339
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Gao Q, Qiu SJ, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593
Grabenbauer GG, Lahmer G, Distel L et al (2006) Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 12:3355–3360
Hashimoto K, Nio Y, Sumi S et al (2001) Correlation between TGF-b1 and p21 (WAF1/CIP1) expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. Pancreas 22:341–347
Hiraoka N, Onozato K, Kosuge T et al (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12:5423–5434
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Jass JR (1986) Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 39:585–589
Ling KL, Pratap SE, Bates GJ et al (2007) Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun 7:7
Liyanage UK, Moore TT, Joo HG et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Loddenkemper C, Schernus M, Noutsias M et al (2006) In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J Transl Med 4:52
Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2:816–822
Marie JC, Letterio JJ, Gavin M et al (2005) TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+ CD25+ regulatory T cells. J Exp Med 201:1061–1067
Naito Y, Saito K, Shiiba K et al (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Nomura T, Sakaguchi S (2005) Naturally arising CD25+ CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol 293:287–302
Peng Y, Laouar Y, Li MO et al (2004) TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+ CD25+ regulatory T cells responsible for protection against diabetes. P Natl Acad Sci USA 101:4572–4577
Ropponen KM, Eskelinen MJ, Lipponen PK et al (1997) Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182:318–324
Sakaguchi S, Sakaguchi N, Asano M et al (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25) Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151–1164
Sakaguchi S (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455–458
Sasada T, Kimura M, Yoshida Y et al (2003) CD4+ CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98:1089–1099
Sato E, Olson SH, Ahn J et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. P Natl Acad Sci USA 102:18538–18543
Shevach EM (2002) CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2:389–400
Sundrud MS, Rao A (2007) New twists of T cell fate: control of T cell activation and tolerance by TGF-beta and NFAT. Curr Opin Immunol 19:287–293
Vukmanovic-Stejic M, Zhang Y, Cook JE et al (2006) Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest 116:2423–2433
Wolf AM, Wolf D, Steurer M et al (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Wolf D, Wolf AM, Rumpold H et al (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11:8326–8331
Woo EY, Yeh H, Chu CS et al (2002) Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168:4272–4276
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Acknowledgments
This study was supported by a General Scientific Research Grant (18591440) from Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Kaori Nomiyama for skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, H., Chikazawa, N., Tasaka, T. et al. Intratumoral CD8+ T/FOXP3+ cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 59, 653–661 (2010). https://doi.org/10.1007/s00262-009-0781-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0781-9