Abstract
Background
Overexpression of the gene c-erbB2, which encodes a receptor tyrosine kinase, has been associated with prognosis and response to therapy in several solid tumors. This study was designed to test whether c-erb-B2 overexpression can be related to prognosis of patients with metastatic gastric cancer.
Methods
Between 2005 and 2010, 46 cases of metastatic gastric cancer were evaluated immunohistochemically for c-erb-B2 overexpression. Overall survival (OS) and time-to-progression (TTP) served as the main outcome measures.
Results
c-erbB2 was overexpressed in 19 (41.3 %) cases and 8 patients (17.4 %) had a c-erbB2 score of 3+ (a strong complete membrane staining observed in >10 % of the tumor cells). c-erbB2 expression was not associated with the clinicohistological characteristics of the study participants. The mean OS was 11.48 ± 1.03 months, whereas the mean TTP was 8.28 ± 0.8 months. Compared with patients with a score of 2+ or less (n = 38), those with a c-erbB2 score of 3+ (n = 8) had both a significantly lower OS (15.55 ± 1.63 vs. 8.22 ± 0.88 months, respectively, p < 0.05) and TTP (10.72 ± 1.81 vs. 6.11 ± 0.61 months, respectively, p < 0.05). After allowance for potential confounders, Cox regression analysis identified a c-erbB2 score of 3+ as an independent predictor of both OS (hazard ratio = 1.9; 95 % confidence interval = 1.1−3.7, p < 0.05) and TTP (hazard ratio = 1.8; 95 % confidence interval = 1.1−4.1, p < 0.05).
Conclusion
Our results suggest that c-erbB-2 overexpression may have a prognostic significance in patients with metastatic gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite a rapid decline in the global incidence over the recent few decades, gastric cancer remains the second most common cancer in morbidity and mortality globally [1]. In Europe, one of the main issues is that it is frequently detected at later stages, when metastases have developed [2]. Although metastasis is the main cause of death from such tumors, the intricate nature of the metastatic process in gastric cancer is very complex and still not completely understood [3, 4]. It has been well known that the transformation of a normal epithelial cell to a malignant cell results from the accumulation of multiple gene abnormalities. As the gastric epithelium progresses from chronic gastritis to intestinal metaplasia, dysplasia and finally to carcinoma, a progressive accumulation of molecular changes has been observed [5]. Continuous molecular advances have paved the way for new discoveries in the mechanisms of metastasizing, potentially offering better diagnostic and prognostic indications for patients with gastric cancer [3, 4].
c-erbB2 or human epidermal growth factor receptor-2 (HER2) gene is a proto-oncogene mapped to chromosome 17q21 and encodes a 185-kDa transmembrane glycoprotein belonging to the human epidermal growth factor receptor family [6, 7]. c-erbB2 is known to play an important role in the regulation of a number of key cellular processes such as cell growth, survival, and differentiation [8]. c-erbB2 is known to be overexpressed, amplified or both in several human tumors, including gastric cancer [9, 10]. Evidence suggests that c-erbB2 gene amplification or protein overexpression plays a pivotal role in oncogenic transformation, tumorigenesis and metastasis [11]. Numerous—but not all—studies have shown that a positive c-erbB2 status is an independent predictor of prognosis in patients with gastric cancer [12]. Positive c-erbB2 status is estimated to be between 15 and 20 % of gastric cancer and is generally associated with more aggressive disease, leading to shortened survival rates [12, 13]. In metastatic gastric cancer, chemotherapy is the standard treatment because it prolongs survival when compared to best supportive care alone [2, 3]. However, recent years have witnessed an increasing awareness of novel targets in gastric cancer therapy. For example, the Phase III ToGA trial reported an increase in overall survival for patients with human c-erbB2-positive gastric cancer treated with chemotherapy and the therapeutic monoclonal anti-c-erbB2 antibody trastuzumab compared to chemotherapy alone [14]. Because trastuzumab has recently been approved for treatment of advanced gastric cancer, c-erbB2 testing is gaining increasing importance for optimizing additional and more effective targeted therapies [15–17]. The prognostic significance of c-erbB2 overexpression metastatic gastric cancer is assuming increasing importance given the therapeutic advances in the use of trastuzumab in advanced gastric cancer. In this report, a study examining whether c-erbB2 overexpression evaluated immunohistochemically can be related to prognosis or clinicohistological characteristics of Turkish patients with metastatic gastric cancer was carried out.
Patients and methods
The study was performed according to the Declaration of Helsinki, and approval was granted by the Institutional Review Board of the Uludag University School of Medicine. All participants provided their written informed consent.
Patients
Between 2005 and 2010, a total of 46 patients with metastatic gastric cancer (35 males and 11 females; mean age: 58.1 ± 11.3 years; age range 19–78 years) were referred to the Department of Oncology, Uludag University Medical School and had tumor specimens available for immunohistochemical staining. We reviewed each medical record and collected demographic and clinicopathologic characteristics including age, sex, histological type, degree of differentiation, distant metastasis, body mass index, loss of weight in previous 6 months, and first-line chemotherapy profile. Patients with severe comorbidities such as active infection and severe cardiopulmonary dysfunction and previous or concurrent other malignancies were excluded.
c-erbB2 immunohistochemistry
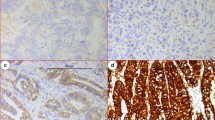
According to the previously described methodology [18], the immunostains were performed using sections cut at 4 pm thick and dried overnight at 37 °C. The sections were deparaffinized in xylene and rehydrated via a series of graded alcohols. Sections were blocked with a specific serum (Ultravision block, Lab Vision Corporation, Fremont, CA, USA) and incubated with the polyclonal antibody for 30 min at room temperature (Rabbit Anti-Human c-erb B-2 oncoprotein, DAKOA 0485, DAKO Corporation, Carpinteria, CA, USA: 1:80 dilution). Antigen retrieval was performed using a retrieval solution from Vector, for 30 min at 98 °C followed by 5 min at room temperature. The other steps of immunohistochemical staining were performed using standard protocols on an automated Lab Vision Autostainer (Lab Vision Corporation Fremont, CA, USA) with a specific kit based on streptavidin–biotin–peroxidase method. Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted. Each run contained negative and positive controls. Quantitation of the immunostaining was performed according to the consensus panel recommendations on Her-2 scoring for gastric cancer, as follows: 0 = negative (no staining is observed or membrane staining is observed in <10 % of tumor cells); 1+ = negative (a faint/barely perceptible membrane staining is detected in >10 % of the tumor cells); 2+ = equivocal (a weak to moderate complete membrane staining is observed in >10 % of the tumor cells); 3+ = positive (a strong complete membrane staining is observed in >10 % of the tumor cells) [18]. Scores of 0/1+ were considered as negative for c-erbB2 overexpression, whereas scores of 2+/3+ were regarded as positive.
Statistical methods
Descriptive statistics are summarized as frequencies, percentages, means, medians, ranges, and standard deviations, as appropriate. For continuous variables, the intergroup differences were evaluated with an unpaired t test or the Mann–Whitney U test, as appropriate. Categorical variables are presented by frequency counts, and intergroup comparisons were analyzed by a χ 2 test. Overall survival (OS) was calculated from the date of biopsy: the endpoint was date of last follow-up or date of death if the patient died. Time to progression was calculated from the date of biopsy to the first documentation of progressive disease. The data were censored at the date of the last follow-up, date of the appearance of a new lesion or date of reactivation or appearance of any lesion. Survival curves were drawn using the Kaplan–Meier method, and the differences between the groups were compared with the log-rank test. Multivariable analyses using Cox regression through a forward selection procedure were used to identify the independent predictors of outcomes. All statistical analyses were performed using the SPSS 14.0 statistical package (SPSS Inc., Chicago, IL, USA). The study power was calculated using the StatMate 2.0 software (GraphPad Inc., San Diego, CA, USA). A two-sided p < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 46 patients with metastatic gastric cancer participated in the study. The primary tumor localization was as follows: antrum (n = 20), corpus (n = 9), fundus (n = 4), and cardia (n = 13). A total of 32 had adenocarcinoma whereas the remaining 14 patients had a histological diagnosis of signet-ring cell carcinomas. The primary tumor was well differentiated in one patient, moderately differentiated in 14 patients, and poorly differentiated in the remaining 31 patients. Twenty-one patients reported a weight loss of more than 10 % whereas the remaining 25 had a weight loss ≤10 %. The most common site of metastases was the liver (n = 20), followed by the peritoneum (n = 15), and other sites (n = 11). Forty-two patients received cisplatin-based chemotherapy, whereas the remaining four received fluoropyrimidine-based chemotherapy.
Significance of c-erbB2 overexpression in metastatic gastric cancer
By immunohistochemistry, c-erbB2 was overexpressed (scores 2+/3+) in 19 (41.3 %) cases, and 8 patients (17.4 %) had a c-erbB2 score of 3+ (a strong complete membrane staining observed in >10 % of the tumor cells). We found no evidence for a correlation between c-erbB2 expression scores and the general characteristics of patients with metastatic gastric cancer (Table 1). The mean OS was 11.48 ± 1.03 months, whereas the mean TTP was 8.28 ± 0.80 months. Compared with patients with a score of 2+ or less (n = 38), those with a c-erbB2 score of 3+ (n = 8) had both a significantly lower OS (15.55 ± 1.63 vs. 8.22 ± 0.88 months, respectively, p < 0.05, Fig. 1) and TTP (10.72 ± 1.81 vs. 6.11 ± 0.61 months, respectively, p < 0.05, Fig. 2). After allowance for potential confounders, Cox regression analysis identified a c-erbB2 score of 3+ as an independent predictors of both OS (hazard ratio = 1.9; 95 % confidence interval = 1.1–3.7, p < 0.05) and TTP (hazard ratio = 1.8; 95 % confidence interval = 1.1–4.1, p < 0.05) (Table 2).
Discussion
The principal findings in this study are: (1) c-erbB2 overexpression in metastatic gastric cancer in Turkish patients is higher (41.3 %) than that estimated frequency (between 15 and 20 %) reported in studies from other countries with mostly western populations; (2) an immunohistochemical score of 3+ for c-erbB2 overexpression in metastatic gastric cancer is associated with poor OS and TTP. This study supports the hypothesis that c-erbB2 overexpression in metastatic gastric tumors from Turkish patients is associated with poor prognosis and overall survival.
c-erbB2 overexpression has been reported to contribute to increased metastatic potential of cancer cells and enhance malignancy [7–9]. Upregulation of matrix metalloproteinases by c-erbB2 can also result in an increased invasiveness, a stronger angiogenic response, and a higher apoptosis resistance [7]. A recent review by Jørgensen and Hersom [12] has examined the results of a total of 12,749 patients with gastric cancer who underwent c-erbB2 testing. The majority of the studies reviewed (71 %) suggested a negative prognostic impact of a c-erbB2-positive status. Concerning metastatic gastric cancer, Kataoka et al. [19] showed that c-erbB2 overexpression is less frequent in resectable gastric cancer than in metastatic gastric cancer. Sawaki et al. [20] demonstrated that the overall survival of Japanese patients with c-erbB2-positive advanced/metastatic gastric cancer who received trastuzumab plus chemotherapy was better than that of patients who received chemotherapy alone. In addition, Weissinger et al. [21] suggested that there is an improved prognosis with addition of trastuzumab to chemotherapy with a platinum compound and a fluoropyrimidin in first-line therapy in c-erbB2-overexpressing gastric cancer. For these reasons, trastuzumab in combination with chemotherapy is the current standard of therapy for patients with metastatic c-erbB2-overexpressing gastric cancers [15, 16]. Future studies should dissect whether the activity of lapatinib, an active agent in advanced c-erbB2-positive breast cancer, could be useful in c-erbB2-overexpressing gastric cancers [10]. In this study, we found a statistically significant association between c-erbB2 overexpression and prognosis in patients with metastatic gastric cancer; this finding, however, was chiefly confined to patients who expressed this molecule at the highest levels (i.e., 3+ expression by immunohistochemistry). It can be hypothesized that c-erbB2 overexpression is, at least for some gastric tumors, one of the steps in the multistep process towards metastatic spread and a progressive increase in c-erbB2 expression therefore might result in an increased biological aggressiveness.
The caveats of this study are the homogenous ethnic background of the study population and that the patients included in the study could be a selected group and may not represent the general population of patients with metastatic gastric cancer. A recent systematic analysis of data from the literature has shown that the rates of patients with gastric cancer who overexpress c-erbB2 vary widely from 4.4 to 53.4 % [12]. The observed rate in our present study (41.3 %) is therefore in accordance with these data. It is noteworthy that the histological features of gastric cancer (Lauren’s classification) may influence the rate of c-erbB2 overexpression [12]. In particular, patients with the intestinal phenotype seem to have a significant higher rate of c-erbB2 overexpression compared with the diffuse or mixed types. Therefore, it can be speculated that the relatively high number of patients who overexpress c-erbB2 can be due to a relative prevalence of patients with the intestinal phenotype. Unfortunately, our retrospective pathological data did not include the results of Lauren’s classification to corroborate this possibility. Concerning the methodology used for determining c-erbB2 overexpression, we used a monoclonal antibody against p185 (the protein encoded by the c-erbB2 gene) coupled with a highly sensitive streptavidin–biotin-elite kit. This methodology has been previously used in a highly cited study [22]. Concerning the potential influence of ethnic factors of the extent of c-erbB2 overexpression in patients with gastric cancer, there are three published studies conducted in Turkey [23–25]. In these reports, c-erbB2 was overexpressed in 17, 10, and 24 % of the cases, respectively. For this reason, we believe that ethnic factors are not a major explanation for the observed rate of c-erbB2 overexpression in our study. In addition, it could be argued that we found no association between c-erbB2 overexpression and the clinicopathological characteristics of the study participants. Different experimental designs, different number and characteristics of subjects recruited, and different ways by which histology samples were handled might at least partially explain the differences among the studies. Finally, we acknowledge the small sample size as a major limitation of our study. However, our study had a 95 % power to detect an overall survival difference of 0.309 with a significance level (alpha) of 0.05 (two-tailed) between patients with metastatic gastric cancer with and without a c-erbB2 score of 3+.
These limitations notwithstanding, our data have provided additional and updated information on the frequency and prognostic value of c-erbB2 overexpression in patients with metastatic gastric cancer. Some of the findings presented will prove immediately clinically useful for prediction of prognosis and risk stratification. Hopefully, these data can make some contribution towards improving the prediction of the efficacy of trastuzumab-based therapy in gastric cancer. Beyond clinical applicability, future work must address mechanistic questions about the functional role of c-erbB2 overexpression in determining the outcome of metastatic gastric cancer.
References
Kelley JR, Duggan JM (2003) Gastric cancer epidemiology and risk factors. J Clin Epidemiol 56:1–9
Kang H, Kauh JS (2011) Chemotherapy in the treatment of metastatic gastric cancer: is there a global standard? Curr Treat Options Oncol 12:96–106
Zagouri F, Papadimitriou CA, Dimopoulos MA et al (2011) Molecularly targeted therapies in unresectable-metastatic gastric cancer: a systematic review. Cancer Treat Rev 37:599–610
Croxtall JD, McKeage K (2010) Trastuzumab: in HER2-positive metastatic gastric cancer. Drugs 70:2259–2267
Jang BG, Kim WH (2011) Molecular pathology of gastric carcinoma. Pathobiology 78:302–310
Popescu NC, King CR, Kraus MH (1989) Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12–21.32. Genomics 4:362–366
Yarden Y (2001) Biology of HER2 and its importance in breast cancer. Oncology 61:1–13
Ross JS (2011) Update on HER2 testing for breast and upper gastrointestinal tract cancers. Biomark Med 5:307–318
Hicks DG, Whitney-Miller C (2011) HER2 testing in gastric and gastroesophageal junction cancers: a new therapeutic target and diagnostic challenge. Appl Immunohistochem Mol Morphol 19:506–508
Tsang RY, Finn RS (2012) Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br J Cancer 106:6–13
Bianco AR (2004) Targeting c-erbB2 and other receptors of the c-erbB family: rationale and clinical applications. J Chemother 16(Suppl 4):52–54
Jørgensen JT, Hersom M (2012) HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer 3:137–144
Stern HM (2012) Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med 4:127rv2
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Okines AF, Cunningham D (2010) Trastuzumab in gastric cancer. Eur J Cancer 46:1949–1959
Fornaro L, Lucchesi M, Caparello C et al (2011) Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol 8:369–383
Lorenzen S, Lordick F (2011) How will human epidermal growth factor receptor 2-neu data impact clinical management of gastric cancer? Curr Opin Oncol 23:396–402
Mammano E, Belluco C, Sciro M et al (2006) Epidermal growth factor receptor (EGFR): mutational and protein expression analysis in gastric cancer. Anticancer Res 26:3547–3550
Kataoka Y, Okabe H, Yoshizawa A et al (2012) HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. doi:10.1007/s10120-012-0150-9
Sawaki A, Ohashi Y, Omuro Y et al (2011) Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the trastuzumab for gastric cancer (ToGA) study. Gastric Cancer. doi:10.1007/s10120-011-0118-1
Weissinger F, Reymond M, Dumke K et al (2011) Successful treatment of a patient with HER2-positive metastatic gastric cancer with third-line combination therapy with irinotecan, 5-fluorouracil, leucovorin and trastuzumab (FOLFIRI-T). Onkologie 34:548–551
Allgayer H, Babic R, Gruetzner KU et al (2000) c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol 18:2201–2209
Dursun A, Poyraz A, Celik B et al (1999) Expression of c-erbB-2 oncoprotein in gastric carcinoma: correlation with histopathologic characteristics and analysis of Ki-67. Pathol Oncol Res 5:104–106
Gürel S, Dolar E, Yerci O et al (1999) The relationship between c-erbB-2 oncogene expression and clinicopathological factors in gastric cancer. J Int Med Res 27:74–78
Satiroglu-Tufan NL, Bir F, Calli-Demirkan N (2006) Investigation of HER-2 codon 655 single nucleotide polymorphism frequency and c-ErbB-2 protein expression alterations in gastric cancer patients. World J Gastroenterol 12:3283–3287
Conflict of interest
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayrak, M., Olmez, O.F., Kurt, E. et al. Prognostic significance of c-erbB2 overexpression in patients with metastatic gastric cancer. Clin Transl Oncol 15, 307–312 (2013). https://doi.org/10.1007/s12094-012-0921-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0921-0