Abstract
The molecular biomarkers human epidermal growth factor receptor-2 (HER2) and trefoil factor 3 (TFF3) are reported to play important roles in the pathogenesis of gastric cancer (GC). In this study, we investigated the clinicopathological and prognostic significance of TFF3 and HER2 expression in GC and explored the correlation between these two biomarkers. Ninety-two patients who were diagnosed with GC were enrolled. TFF3 and HER2 expression was determined on tumor tissues. The results showed that TFF3 and HER2 were positively expressed in 42.7 and 10.9 % of the cases, respectively. There were significantly higher rates of TFF3 positivity in patients with deep invasive tumors and advanced stage ones. Patients with negative TFF3 staining survived longer than those with the presence of TFF3, with 5-year overall survival (OS) rates of 57.1 ± 7.1 and 39.5 ± 7.5 %, respectively (P = 0.033). However, HER2 positivity was not significantly associated with OS (P = 0.262). Multivariate analysis demonstrated TFF3 expression to be an independent indicator for short-term survival, with a hazard ratio of 2.327 (95 % confidence interval (CI), 1.202–4.507, P = 0.012). There was a trend that the expression of TFF3 was more frequent in HER2 negative tumors than in HER2 positive ones (positive rates: 16.3 vs. 4.7 %, P = 0.098). Patients with HER2-negative/TFF3-negative GC presented higher OS than those with other phenotypes (P = 0.009). This study suggests that TFF3 is an independent indicator for survival in GC, while HER2 is not associated with the outcome. Patients with HER2-negative/TFF3-negative GC have the best outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignant tumors and the second leading cause of cancer death worldwide [1]. The pathogenesis of GC is associated with multiple factors. Helicobacter pylori infection, smoking, alcohol, diet, genetics, and epigenetic factors are all thought to play roles in the occurrence of this disease [2]. However, their clinical relevance has not been clearly confirmed. A better understanding of the biological basis of GC would be helpful.
The human epidermal growth factor receptor-2 (HER2) is one of the most explored molecular markers in GC. The reported rates of HER2 positivity in patients with GC range from 4 to 53 % [3]. Although HER2 expression based on Trastuzumab for Gastric Cancer (ToGA) criteria was not related to survival in patients with GC [4], the ToGA trial did show that patients with HER2-expressing advanced or metastatic cancer benefited from anti-HER2 therapy, underscoring the importance of molecular biological profile testing in cancer diagnosis and treatment.
Trefoil factor 3 (TFF3) is another biological marker which is less studied in GC. It belongs to a family of small mucin-associated polypeptides which plays an important function in maintaining mucosal integrity [5]. It is mainly expressed in the gastrointestinal tract and other epithelial tissues [6]. Recently, TFFs have been reported to play a significant role in the oncogenic transformation, growth, and metastatic extension of human solid tumors, including GC [7, 8], and the positivity of TFF3 is related to a short survival in GC patients [9, 10]. As the pathogenesis of GC is a multi-step process and is related to multiple biological factors, the interaction between different genes needs to be further investigated. It has been reported that the co-expression of HER2 and TFF3 can be a useful predictor for recurrence of GC [11]. However, clinical data in this field are very limited. In the present study, we investigated the expression levels of HER2 and TFF3 in GC, and analyzed their potential correlations with clinical features and overall survival (OS) in patients with GC.
Materials and methods
Patients and tissue samples
A total of 92 consecutive patients with GC who underwent curative gastrectomy at the Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine between December 2006 and October 2008 were examined for the present study. The medical records and surgical specimens of these patients were retrospectively evaluated after obtaining approval from the Medical Ethics Committee of this hospital. The histological classification was according to the 2010 WHO classification criteria [12]. The disease stage was determined according to the International Union Against Cancer (UICC)-TNM classification (seventh edition) [13].
Immunohistochemistry analysis and definition of HER2 and TFF3 positivity
Formalin-fixed, paraffin-embedded tissues were freshly cut (4 μm). The sections were deparaffinized in xylene and rehydration in a graded series of alcohols. The endogenous peroxidase activity was blocked by exposure to 3 % H2O2 for 5~10 min at room temperature. Then, the sections were immersed in sodium citrate buffer (pH 6.0) and steamed at high power for 30 min. The sections were blocked with 1 % BSA, and immunohistochemical reactions were performed using the mouse anti-human antibody detecting HER2 (DakoCytomation, Denmark) or TFF3 (Abcam, Inc.) and HRP-labeled rabbit-anti-mouse IgG. Normal unrelated mouse IgG was used as an isotype antibody control. Color was developed with 3ʹ3-diaminobenzidine (DAB) as chromogen. Sections were counterstained with hematoxylin and were covered with mounting media.
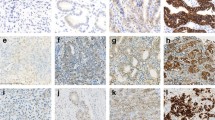
The immunohistochemistry (IHC) results of HER2 were interpreted using the scoring scheme proposed for GC by Hofmann et al. (ToGA score) [14]: 0, no staining or membranous reactivity in <10 % of tumor cells; 1+, weak, barely perceptible membranous reactivity in >10 % of tumor cells; 2+, complete or basolateral membranous reactivity either non-uniform or weak in at least 10 % of cells; and 3+, complete or basolateral membranous reactivity of strong intensity in ≥10 % of cells (Fig. 1a). Cases that scored 2+ were further evaluated HER2 gene amplification by fluorescence in situ hybridization (FISH) using a PathVysion HER2 DNA probe kit (Vysis/Abbott, Abbott Park, Illinois) according to the manufacturer’s instructions. The total numbers of HER2 and chromosome 17 signals were counted in at least 20 tumor cell nuclei in two different areas. The HER2/chromosome 17 ratios were interpreted in accordance with the ToGA FISH scoring scheme as follows: <2.0, HER2 gene not amplified and ≥2.0, HER2 gene-amplified (Fig. 1c). HER2 positivity was defined as IHC3+ or IHC2+ plus gene-amplified. Remaining cases (non-amplified IHC 2+ or IHC 0–1+) were considered HER2 negative.
Kaplan-Meier curves for overall survival (OS) according toTFF3 expression and HER2 status. a Patients with TFF3-positive tumors had a significantly worse survival than those with TFF3-negative ones. b Patients with HER2-positive tumors had a comparable OS than those with HER2-negative ones. c Patients with TFF3-negative/HER2-negative tumors presented higher OS rate than those with other phenotypes. d Patients with TFF3-postive/HER2-negative tumors had comparable OS with those with TFF3-negative/ HER2-positive ones
For TFF3 staining, the IHC result was assessed semi-quantitatively by measuring both the intensity and extent of staining. Five visual fields for each sample were chosen randomly, and the positive cells in each visual field were counted. Briefly, the intensity of staining was scored as follows: 0 for non-staining, 1 for yellow staining, 2 for brown yellow staining, and 3 for brown staining. The number of positive cells were scored as 0, 1, 2, 3, and 4 when ≤5, 6–25, 26–50, 51–75, and >75 % of tumor cells had positive staining, respectively. Then, the scores for the intensity and extent of staining were multiplied to give a final score for each case. The final scores of 0, 1–4, 5–8, and 9–12 were considered as negative (−), weak positive (+), moderate positive (++), and strong positive (+++), respectively (Fig. 1b).
Statistical analysis
To investigate the univariate associations between clinicopathologic variables and the statuses of HER2 and TFF3, the Chi-square or Fisher’s exact test was applied. Overall survival (OS) was defined from the time of surgery to death from any cause, which was estimated by the Kaplan-Meier method, and statistical significance was analyzed using the log-rank test. Hazard ratio (HR) and its 95 % confidence interval (CI) were estimated using Cox’s proportional hazards regression model in multivariate analysis. All statistical analyses were performed using IBM SPSS Statistics 20.0 software. P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
This cohort consisted of 92 patients, including 58 males and 34 females, with an average age of 61.0 ± 10.6 years old. Seventeen patients (18.5 %) had primary tumor located in the gastroesophageal junction (GEJ), while 75 (81.5 %) had tumor in middle to distal stomach. The numbers of patients with stage I, II, and III diseases were 15 (16.3 %), 32 (34.8 %), and 45 (48.9 %), respectively. None of the patients received trastuzumab or other anti-HER2 therapy.
Correlation between HER2 status and clinicopathological features
HER2 expression in GC tissues was determined by IHC and FISH. Of these tumor tissues, 78 cases (84.8 %) were scored 0; 3 cases (3.3 %) were scored 1+; 3 cases (3.3 %) were scored 2+; and 8 cases (8.7 %) were scored 3+. Among 3 cases with IHC 2+, 2 cases were positive for HER2 gene amplification. Therefore, HER2 was positively expressed in 10 cases (10.9 %). The correlations between HER2 status and clinicopathological features were summarized in Table 1. HER2 status was associated with gender and TNM stage. Male patients and those with advanced stage cancer (TNM stage III) presented higher rates of HER2 positivity. However, HER2 positivity was not significantly associated with age, tumor location, lymph node status, differentiation degree, and depth of invasion.
Correlation between TFF3 status and clinicopathological features
Expression of TFF3 and its association with clinicopathological parameters were assessed in this cohort. TFF3 was positively expressed in 42 (45.7 %) cases. As shown in Table 1, there were significantly higher rates of TFF3 positivity in patients with deep invasive tumors and advanced stage ones. No significant associations were noted between TFF3 expression and other clinicopathological parameters including age, gender, tumor location, lymph node involvement, and tumor differentiation status.
Correlation between TFF3 expression and HER2 status
The correlation between TFF3 expression and HER2 status in GC was investigated. Only two HER2-positive tumors exhibited TFF3 expression, while the other eight samples with HER2 overexpression did not react to TFF3 antibody. There was a trend that the expression of TFF3 was more frequent in HER2 negative tumors than in HER2 positive ones (16.3 vs. 4.7 %). However, the P value of 0.098 did not reach statistical significance, which was warranted to be confirmed in studies with larger sample size.
Correlation between TFF3 expression and survival
The median follow-up period for this cohort was 49.5 months (range 2.2 to 87.8 months). Forty-seven patients died during this period and the median OS interval for the entire cohort was 49.6 months. The 3- and 5-year OS rates for this cohort were 60.1 ± 5.9 and 48.9 ± 5.2 %, respectively. As shown in Table 2, depth of invasion, TNM stage, lymph node status, and TFF3 expression were significantly related to OS. Tumors with deep invasion, advanced stage, lymph node metastasis, and TFF3 expression were related to short OS intervals. Patients without TFF3 staining survived longer than those with the presence of TFF3 (median survival intervals, >66 vs. 28 months; 5-year OS, 57.1 ± 7.1 and 39.5 ± 7.5 %; P = 0.033) (Fig. 2a). We further studied how the interactions between TFF3 and other clinicopathological characteristics affected long-term survival. The results showed that TFF3 expression was predictive of inferior outcome in female patients, patients with old age, and those presenting lymph node metastasis. In multivariate analysis which includes age, gender, depth of invasion, lymph node status, location, differentiation status, HER2 status, and TFF3 expression, only female gender, lymph node involvement, and TFF3 expression were independent risk factors, with hazard ratios of 1.907 (95 % CI, 1.001–3.632), 5.741 (95 % CI, 2.319–14.212), and 2.327 (95 % CI, 1.202–4.507), respectively (Table 3).
HER2 and TFF3 status and HER2 amplification in tumor samples from patients with gastric cancer (GC). a The upper panel showed the results of immunohistochemical staining of HER2 in GC tissues. The figures from left to right represented IHC 0, 1+, 2+, 3+ (×200). b The middle panel showed the results of immunohistochemical staining of TFF3 in GC tissues. The figures from left to right represented –, +, ++, and +++ (×200). c HER2 gene amplification evaluation by fluorescence in situ hybridization (FISH). The left figure showed HER2 gene non-amplification (HER-2/CSP17 = 1.54) and the right figure showed HER2 gene amplification
The combined effect of HER2 and TFF3 on the outcome was determined. Positive expression of HER2 had little impact on survival in this cohort (Fig. 2b), while expression of TFF3 was related to short OS in both HER2 positive and negative GCs (Table 2). We then combined the two markers to investigate their roles on survival in GC. Patients with HER2-negative/TFF3-negative GC presented higher OS than those with single gene expression, and the two patients with HER2-positive/TFF3-positive GC both died (Fig, 2c). For patients with single gene expression (either HER2 or TFF3), those with positive TFF3 expression did not show any difference on OS when compared with those with positive HER2 expression (Fig. 2d).
Discussion
TFF3 is a soluble peptide and a member of the trefoil peptide family, which is conserved among species and has trefoil domain and C-terminal dimerization domain [15]. TFF3 was first recognized in rat intestine, and was then shown to be primarily expressed and secreted onto the intestinal surface by goblet cells of the human small and large intestinal mucosa [16, 17]. TFF3 plays an important role in the reconstitution of the mucosal barrier to protect the epithelial layer against environmental injury [18, 19]. In colon cancer cell lines, TFF3 is found to inhibit cell adhesion and promote cell invasion [20, 21]. In breast tumors, TFF3 is a possible role in tumor angiogenesis [22]. In GC, the expression level of TFF3 is much higher in gastric biopsies with intestinal metaplasia than that in those without metaplasia, and is significantly increased from non-neoplastic gastric mucosa to GC [23]. Furthermore, the expression of TFF3 is much more common in diffuse-type GC than in the intestinal-type one [11]. As diffuse-type GC is more aggressive than the intestinal-type one, it is believed that TFF3 may play roles in an early stage of gastric carcinogenesis and may contribute to the aggressiveness of GC. It has been reported that the combination of serum TFF3 and pepsinogen testing could be a biomarker for predicting the presence of GC [24].
Our results showed that TFF3 was expressed in 46.7 % of GC cases and was correlated with more aggressive clinicopathological features. For example, TFF3 expression level was higher in patients with deeply invasive tumor and advanced stage ones. More importantly, TFF3 expression was an independent negative prognostic indicator for OS in patients with GC. N stage of the seventh edition of TNM classification is an intensive variable for prediction of OS in GC [25]. TFF3 was the second highest risk factor next to lymph node involvement for postoperative survival in this cohort. All the clinical results were consistent with the role of TFF3 in tumor progression and metastasis.
HER2 gene is a well-described proto-oncogene where high amplification of this gene induces protein overexpression in the cellular membrane, conferring oncogenic properties to a malignant cell [26]. Although HER2 overexpression has been mostly studied in breast cancer, a wide overexpression variation has been demonstrated in other malignancies [27]. The role of HER2 as a prognostic factor in GC remains controversial. A recent systematic review assessing the impact of HER2 overexpression on survival found that 20 studies (57 %) reported no difference in OS, 2 (6 %) showed significantly longer OS in patients with HER2 overexpression, and 13 (37 %) found significantly worse OS in patients with HER2 overexpression [3]. Due to the significant heterogeneity of the approaches and criteria used for HER2 assessment among different studies, we performed a meta-analysis enrolling only studies that defined HER2 expression using ToGA criteria and found that HER2 overexpression based on ToGA criteria was not associated with poor OS in GC [4]. This was consistent with the conclusion drawn from this study.
An important purpose of this study is to assess the association and interaction between TFF3 and HER2. As shown in this study, TFF3 was a prognostic factor independent from HER2 status, which was predictive of OS in both HER2-positive and HER2-negative GC. We found that there was a trend that TFF3 expression was inversely correlated with HER2 status, which has been confirmed in Xu et al.’s study [11]. TFF3 was rarely expressed in HER2-positive GC and only 2 % of patients had phenotype of TFF3-positive/HER2-positive in this cohort. Patient with TFF3-negative/HER2-positive GC presented similar OS with those with TFF3-positive/HER2-negative, and both were inferior to those with TFF3-negative/HER2-negative GC. As both the two biomarkers were related to aggressive clinicopathological characteristics, this result seemed to be reasonable. But in Xu et al.’s study, patients with TFF3-positive/HER2-negative had longer disease-free survival (DFS) than those with TFF3-negative/HER2-positive, and patients with TFF3-positive/HER2-positive phenotype had the highest DFS [11], which seemed to be inconsistent to our results. However, the OS status was not compared among these groups in Xu et al.’s study. Furthermore, HER2 overexpression was related to better DFS in this study, which was contrary to results from most clinical studies in this field [3]. On the other hand, as the analysis in this study was conducted in a small population, the small sample size might contribute to the inconsistence with the previous reports. Prospective study with large sample size should be done to confirm the role of TFF3 expression in gastric cancer.
In conclusion, the results of this study show that expression of TFF3 is related to more aggressive clinicopathological features and inferior outcome in GC, which is an independent predictor for OS; while HER2 positivity has little impact on survival. There is a trend that TFF3 is rarely expressed in HER2-positive GC. Patients with HER2-negative/TFF3-negative GC present superior outcome than those with other phenotypes.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Gonzalez CA, Agudo A. Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int J Cancer. 2012;130:745–53.
Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes–a systematic review. Int J Cancer. 2012;130:2845–56.
Gu J, Zheng L, Wang Y, et al. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35:5315–21.
Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–8.
Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319–34.
John R, El-Rouby NM, Tomasetto C, et al. Expression of TFF3 during multistep colon carcinogenesis. Histol Histopathol. 2007;22:743–51.
Dhar DK, Wang TC, Tabara H, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res. 2005;11:6472–8.
Meng JR, Tang HZ, Zhou KZ, et al. TFF3 and survivin expressions associate with a lower survival rate in gastric cancer. Clin Exp Med. 2013;13:297–303.
Ding A, Zhao W, Shi X, et al. Impact of NPM, TFF3 and TACC1 on the prognosis of patients with primary gastric cancer. PLoS One. 2013;8:e82136.
Xu CC, Yue L, Wei HJ, et al. Significance of TFF3 protein and Her-2/neu status in patients with gastric adenocarcinoma. Pathol Res Pract. 2013;209:479–85.
Lauwers GY, Carneiro F, Graham DY. Gastric carcinoma. In: Bowman FT, Carneiro F, Hruban RH, editors. Classification of tumours of the digestive system. Lyon: IARC; 2010.
Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. Chichester: Wiley; 2010.
Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Muskett FW, May FE, Westley BR, et al. Solution structure of the disulfide-linked dimer of human intestinal trefoil factor (TFF3): the intermolecular orientation and interactions are markedly different from those of other dimeric trefoil proteins. Biochemistry. 2003;42:15139–47.
Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A. 1991;88:11017–21.
Podolsky DK, Lynch-Devaney K, Stow JL, et al. Identification of human intestinal trefoil factor Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:12230.
Dignass A, Lynch-Devaney K, Kindon H, et al. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–83.
Xian CJ, Howarth GS, Mardell CE, et al. Temporal changes in TFF3 expression and jejunal morphology during methotrexate-induced damage and repair. Am J Physiol. 1999;277:G785–95.
Efstathiou JA, Noda M, Rowan A, et al. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci U S A. 1998;95:3122–7.
Emami S, Le Floch N, Bruyneel E, et al. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J. 2001;15:351–61.
Ahmed AR, Griffiths AB, Tilby MT, et al. TFF3 is a normal breast epithelial protein and is associated with differentiated phenotype in early breast cancer but predisposes to invasion and metastasis in advanced disease. Am J Pathol. 2012;180:904–16.
Leung WK, Yu J, Chan FK, et al. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J Pathol. 2002;197:582–8.
Huang Z, Zhang X, Lu H, et al. Serum trefoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: a monocentric cohort study in China. BMC Gastroenterol. 2014;14:74.
Deng J, Zhang R, Pan Y, et al. N stages of the seventh edition of TNM Classification are the most intensive variables for predictions of the overall survival of gastric cancer patients who underwent limited lymphadenectomy. Tumour Biol. 2014;35:3269–81.
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12.
Scholl S, Beuzeboc P, Pouillart P. Targeting HER2 in other tumor types. Ann Oncol. 2001;12 Suppl 1:S81–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianchun Gu and Leizhen Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gu, J., Zheng, L., Zhang, L. et al. TFF3 and HER2 expression and their correlation with survival in gastric cancer. Tumor Biol. 36, 3001–3007 (2015). https://doi.org/10.1007/s13277-014-2933-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2933-6