Abstract
A series of new sulfonamides have been synthesized from Ampyrone with different benzene sulfonyl chlorides to yield the N-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) benzenesulfonamides (4a–e). All synthesized compounds were characterized on the basis of FTIR, 1H NMR, and 13C NMR, and also by the aid of mass spectral data. Further, all synthesized compounds have studied for their in vitro antimicrobial activities against selected bacterial as well as fungal strains by the agar well diffusion method. Free radical scavenging activity has been investigated by using DPPH method. Among all the synthesized compounds, 4b, 4d, and 4e exhibited significant antimicrobial and antioxidant activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic compounds are the organic compounds having a wide range of applications in pharmaceuticals, agrochemicals, and veterinary products synthesis. Their nitrogen and sulfur containing derivatives are considered as extraordinarily important, unique and valuable sources for developing the new antibacterial as well as antifungal agents [1,2,3,4]. Literature survey shows that one-third of the known organic heterocycles with nitrogen and sulfur are biologically active molecules having the broad spectrum of reactivity and stability in the medicinal fields [5, 6]. Pyrazoles are one of the most prominent five-member nitrogen containing heterocycles by virtue of their wide appearance in a large number of biological activities such as antimicrobial [7], anticancer [8], anti-inflammatory [9], antidiabetic and analgesic compounds [10], etc. Ampyrone is a pyrazole congener which is used to protect oxidative stress [11] as well as preventive for many of diseases including cancer [12], hence received great attention for research. Besides, several derivatives of Ampyrone have been evaluated as the analgesic, anti-inflammatory, antimicrobial, anticancer activity, etc. [13,14,15,16].
Undoubtedly, research on different heterocyclic molecules has been done extensively, but Sulfonamides are still considered as privileged scaffolds in modern medicinal chemistry for developing new anti-tumoural, antibacterial and anti-inflammatory agents [17, 18]. Their structures are similar to p-aminobenzoic acid which is essential for folate synthesis in bacterial cells [19] hence shows various pharmacological activities like anticancer [20], antimicrobial [21], anti-inflammatory [22], antidepressant [23], etc. Several sulfonamide drugs developed from heterocyclic compounds have shown the significant therapeutic applications. For example, Celecoxib is a nonsteroidal anti-inflammatory sulfa drug inhibits prostaglandin-endoperoxide synthase (PTGS) which is responsible for the formation of prostanoids [24], sildenafil is a PDE5 inhibitor that is significantly more potent and selective than other inhibitors [25] (Fig. 1).
Nowadays, Bacterial biofilms are the one of the most serious problems for human health especially in chronic infections, as they are responsible for diseases associated with surfaces, along with medical devices for internal or external use [26]. Hence, microbiologists are continuously working on methods for detection [27] and combating the bacterial biofilm infections [28]. Sulfathiazole used as an anti-biofilm agent against E. coli strains for targeting nucleotide synthesis [29].
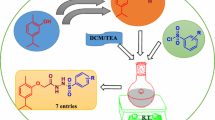
Therefore looking at the vast pharmacological profile of sulfonamides, we have attempted to synthesize the new sulfonamide derivatives from ampyrone with various sulfonyl chlorides and investigate them for biological properties viz., in vitro antimicrobial activities by agar well diffusion method against the selected strains and antioxidant activities by using the DPPH assay method. The results indicate that some newly synthesized compounds show promising biological activities whereas remaining shows moderate.
Materials and Methods
Melting points (m.p.) were recorded by the one- end- open capillary method and are uncorrected. IR absorption spectra’s were recorded on a Shimadzu FTIR 8400 S in KBr pellets. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance II 400 NMR spectrometer in deutero chloroform (CDCl3) and DMSO using TMS as internal standards. Mass spectra (EI, 70 eV) were recorded on a Waters, Q-TOF MICROMASS (ESI–MS) mass spectrometer. The completions of reactions were monitored via TLC analysis using silica gel 60 F-254 plate. All laboratory grade reagents were procured and used as purchased. The starting materials (aromatic compounds), chlorosulfonic acid and Ampyrone having a purity of 99.5% were purchased from S.D. Fine Chemicals.
Synthesis of Compounds 2a–e
Synthesis of sulfonyl chloride derivatives from various aromatic compounds 1 by HSO3Cl is carried out as reported in the literature. [30, 31].
Synthesis of Compounds 4a–e
A mixture of substituted sulfonyl chlorides 2a–e (0.01 mol), Ampyrone 3 (0.01 mol) and TEA as a base in CH2Cl2 (20 mL) was stirred at room temp for 3–5 h. Further, ice-cold water was added into it and again stirred for another 20 min. The product enriched with organic layer was separated and dried over Na2SO4 and concentrated in vacuum. The separated crude solid was dried and recrystallized in aq. methanol to give the pure products 4a–e in 70–80% yield.
Synthesis of N-(1,5-Dimethyl-3-oxo-2-Phenyl-2,3-Dihydro-1H-Pyrazol-4-yl)-4 Methyl Benzene Sulfonamide (4a)
Yellow solid,
Yield = 80%, mp 172 °C.
IR (KBr) cm−1: 3047 (NH), 2821 (CH), 1629 (C = O), 1155, 1313 (SO2).
1H NMR (400 MHz, CDCl3), δ, ppm: 2.34 (3H, s, CH3); 2.38 (3H, s, CH3); 3.08 (3H, s, CH3); 6.94 (1H, s, N–H); 7.16–7.18 (2H, d, H–Ar); 7.22–7.24 (2H, d, H–Ar); 7.28 (1H, t, H–Ar); 7.40–7.44 (2H, t, H–Ar); 7.67–7.69 (2H, d, H–Ar).
13C NMR (400 MHz, CDCl3), δ: 11.36, 21.65, 35.63, 106.54, 124.55, 127.30, 127.44, 129.31, 129.34, 134.37, 136.67, 143.56, 153.70, 161.99.
Mass spectrum (EI, 70 eV): C18H19N3O3S: 357.11 found: [M + 1]+ 358.0.
Synthesis of N-(4-(N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) Sulfamoyl) Phenyl) Acetamide (4b)
Pale-yellow solid,
Yield = 74%, mp 185 °C.
IR (KBr) cm−1: 3404 (NH, Sulfonamide), 3159 (NH, acetanilide) 2821 (CH), 1626 (C = O), 1321, 1161 (SO2).
1H NMR (400 MHz, DMSO-d 6 ) δ, ppm: 2.07 (6H, d, CH3); 3.04 (3H, s, CH3); 7.23–7.30 (3H, m, Ar–H); 7.42–7.46 (2H, t, Ar–H); 7.68–7.73 (4H, q, Ar–H); 9.06 (1H, s, Sulfonamide N–H); 10.21 (1H, s, N–H).
13C NMR (400 MHz, DMSO-d 6 ), δ: 14.49, 24.06, 35.50, 38.91, 39.12, 39.33, 39.54, 39.74, 39.95, 40.16, 105.59, 118.04, 123.84, 126.33, 127.84, 134.52, 134.80, 142.79, 154.72, 161.93, 168.72.
Mass spectrum (EI, 70 eV): C19H20N4O4S: 400.12 found: [M + 1]+ 400.87.
Synthesis of N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)naphthalene-1-Sulfonamide (4c)
Pale-yellow solid,
Yield = 78%, mp 178 °C.
IR (KBr) cm−1: 3022 (NH), 2812 (CH), 1649 (C = O), 1149, 1309 (SO2).
1H NMR (400 MHz, DMSO-d 6 ) δ: 2.13 (3H, s, CH3); 3.04 (3H, s, CH3); 7.19 (2H, d, Ar–H); 7.24 (1H, t, Ar–H); 7.38 (2H, t, Ar–H); 7.88 (1H, d, Ar–H); 7.57–7.65 (2H, m, Ar–H); 7.93–8.02 (3H, m, Ar–H); 8.42 (1H, s, Ar–H); 9.29 (1H, s, N–H).
13C NMR (400 MHz, DMSO-d 6 ), δ, ppm: 10.57, 35.40, 38.95, 39.16, 39.37, 39.57, 39.78, 39.99, 40.20, 105.53, 122.73, 123.89, 126.34, 127.01, 127.47, 127.59, 128.22, 128.48, 128.74, 128.88, 131.51, 134.14, 134.67, 138.04, 154.63, 161.87
Mass spectrum (EI, 70 eV): C21H19N3O3S: 393.11 found: [M + 1] + 393.89.
Synthesis of N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)quinoline-8-Sulfonamide (4d)
Pale-yellow solid,
Yield = 80%, mp 193 °C.
IR (KBr) cm−1: 3045 (NH), 2810 (CH), 1662 (C = O), 1147, 1305 (SO2).
1H NMR (400 MHz, CDCl3) δ, ppm: 2.45 (3H, s, CH3); 3.04 (3H, s, CH3); 7.09 (2H, d, Ar–H); 7.19 (1H, t, Ar–H); 7.27 (2H, q, Ar–H); 7.98 (1H, d, Ar–H); 8.20 (1H, d, Ar–H); 8.25–8.28 (2H, d, Ar–H); 9.07 (1H, s, N–H).
13C NMR (400 MHz, CDCl3), δ, ppm: 11.35, 35.62, 107.13, 122.23, 124.54, 125.11, 126.99, 128.81, 129.14, 129.62, 133.57, 134.50, 136.99, 137.15, 143.78, 151.09, 154.17, 161.66.
Mass spectrum (EI, 70 eV): C20H18N4O3S: 394.45 found: [M] + 394.90.
Synthesis of 2,4-Dichloro-N-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) Benzene Sulfonamide (4e)
White solid,
Yield = 75%, mp 145 °C.
IR (KBr) cm−1: 3030 (NH), 2812 (CH), 1635 (C = O), 1155, 1315 (SO2).
1H NMR (400 MHz, DMSO-d 6 ) δ, ppm: 2.19 (3H, s, CH3); 3.08 (3H, s, CH3); 7.21–7.23 (2H, d, Ar–H); 7.29 (1H, t, Ar–H); 7.41–7.45 (3H, t, Ar–H); 7.62 (1H, d, Ar–H); 8.09 (1H, d, Ar–H); 9.47 (1H, s, N–H)
13C NMR (400 MHz, DMSO-d 6 ), δ, ppm: 10.48, 35.27, 38.94, 39.15, 39.36, 40.19, 104.43, 123.96, 126.48, 126.85, 128.82, 130.80, 131.64, 132.77, 134.53, 137.68, 137.77, 154.82, 161.83
Mass spectrum (EI, 70 eV): C17H15Cl2N3O3S: 411.02 found: [M + 1] + 411.83.
Antimicrobial Activity
Antimicrobial activity for the compounds 4a–e was carried out by using agar well diffusion method [32, 33]. Bacterial culture i.e., two gram positive (Bacillus subtilis, Staphylococcus aureus) and two gram negative (Escherichia coli, Pseudomonas aeruginosa) along with two fungi (Aspergillus niger and Aspergillus flavus) were selected to evaluate antibacterial activity and antifungal activity. Ofloxacin (20 mg/mL) and Fluconazole (20 mg/mL) were used as the standards for antibacterial and antifungal study respectively. The experiments were carried out by inoculating each culture with different nutrient agar plates followed by incubation at 37 °C for 48 h. About 25–30 mL of Mueller–Hinton inoculated agar was poured on different sterile Petri plates and permitted to solidify. After solidifying, the surface of each agar plate was streaked by a sterile cotton swab with the reference bacterial strain and wells of 6 mm were made with a sterile borer. All test samples were prepared in DMSO (20 mg/mL) and 40 μL of each concentration was added to the respective wells with a micropipette. DMSO served as negative control and the standards as positive controls. The plates were then incubated at 35 ± 2 °C for 24 h for bacteria and at 27 ± 2 °C for 48 h for fungi. After appropriate incubation, a clear zone around each well clearly shows the inhibition to organism growth due to the inhibitory action of test compounds. Measurements were carried out by measuring the diameter of the zone of inhibition of each well.
Antioxidant Activity
The antioxidant activity of the synthesized compounds 4a–e was investigated by DPPH free radical scavenging method [34,35,36]. Stock solutions of testing compounds (1 mg/mL) for the antioxidant activity were prepared in DMSO. Addition of 50 μL of each prepared stock solution into 1 mL of a 200 μM solution of DPPH in methanol was done and all samples were kept for 2 h in the dark at room temperature and then measurement of absorbance was carried out at 517 nm. Ascorbic acid was used as a reference.
The % scavenging activity was determined as follows:
where ‘A blank’ is the absorbance of the control reaction (only DMSO) and ‘A sample’ is the absorbance of the test compounds. The analyses were performed in triplicate. The % radical scavenging activity has been calculated from the initial and final absorbance of each test sample.
Results and Discussion
We have designed and synthesized a series of target compounds, (4a–e), depicted in Scheme 1 from Ampyrone with different benzene sulfonyl chlorides by condensation method. The structures of all compounds were characterized by IR, 1H and 13C NMR, and Mass spectral data. N–H asymmetric stretching vibrations of all compounds are observed around 3159–3022 cm−1. Asymmetric and symmetric SO2 stretching vibrations appear as strong absorption lines in the ranges, 1321–1305 and 1161–1147 cm−1, respectively. The appearances of the band near 900 cm−1 region suggest the presence of S–N group of sulfonamide. All the IR absorption signals of the synthesized compounds 4a–e clearly confirmed the formation of SO2–NH group in structure. All other peaks are in good agreement with the synthesized molecules. The 1H NMR spectra were recorded in DMSO-d6 at room temperature using TMS as an internal reference. The spectra showed characteristic singlet around 6.94–9.47 ppm for –NH moiety in the structures. Similarly, the presence of doublet and triplet around 7.09–8.20 ppm reveals the presence of Ar–H on the ring of the structures. The other signals and peaks of 1H NMR and IR are in complete agreement with the assigned structures. The mass spectra of these compounds displayed an expected molecular ion peak at appropriate m/z values. The proton and carbon signals for other characteristics groups were observed according to the expected chemical shift and integral values. The NMR spectral data coupled with mass spectra strong support the proposed structures of each synthesized compounds.
Synthesized compounds were screened for in vitro activity against two gram positive (B. subtilis, S. aureus) and two gram negative (E. coli, P. aeruginosa) for antibacterial activity and were compared with the standard antibiotic, Ofloxacin.
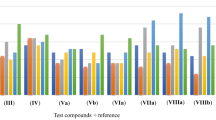
The data in average MIC values at 20 mg/mL is illustrated in Fig. 2 which indicates that amongst all compounds, 4b and 4e exhibited significant antibacterial activities to bacterial strains in respect of standard antibiotic Ofloxacin.
The same series have been investigated for in vitro antifungal activity against two fungi (A. niger and A. flavus) with standard reference, Fluconazole. The data in average MIC values at 20 mg/mL is illustrated in Fig. 3 indicated that compounds having quinoline ring and dichloro substitution i.e., 4d and 4e exhibited superior antifungal activity to both fungal strains in respect of standard control Fluconazole, while other showed moderate activities.
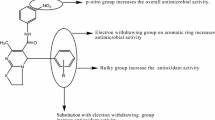
Results of antibacterial and antifungal activity shows, the compound 4e having dichloro substitution on benzene ring carrying electron-withdrawing groups have shown potential activity against the bacterial and fungal strains compared to all compounds. Compound 4b and compound 4d also exhibit good activity due to the presence of acetamide and quinoline ring, respectively. However, other compounds do not show the zone of inhibition similar to that of the standards.
All synthesized compounds (4a–e) evaluated for in vitro antioxidant activity by DPPH assay method have shown excellent to moderate activity compare with standard i.e., Ascorbic acid. Free radical scavenging activity was carried out by using an alcoholic solution of the stable free radical DPPH which possesses deep purple color with absorption maxima at 517 nm. If the antioxidant material is added into an alcoholic solution of DPPH, its purple color disappears and it turns into a colorless solution. Thus, the antioxidant molecule present in solution quenches the DPPH free radical and become responsible for the change in the absorbance at 517 nm. Therefore, activity potential of the compounds is proportional to rapidness of absorbance decrease due to the formation of the non-radical form of DPPH-H by the reactions. Compound 4e (92.64%) with dichloro substitution on benzene moiety shows higher % inhibition value among all compounds indicating the excellent scavenging activity Fig. 4 because the electronegative effect can be responsible for the formation of a non-radical form of DPPH [37].
Conclusion
In summary, the N-1,5-dimethyl-3-oxo-2-phenyl-2, 3-dihydro-1H-pyrazol-4-yl) benzenesulfonamides molecules (4a–e) have been synthesized by condensation of Ampyrone with different benzene sulfonyl chlorides in good yield, characterized by spectral analyses and investigated for their in vitro antimicrobial and antioxidant activity. The compounds (4b), (4d) and (4e) were found to be potent antibacterial and antifungal agents compared with Ofloxacin and Fluconazole as standards respectively. Furthermore, the compound 4e shows excellent antioxidant activity (92.64%) whereas, compounds 4b and 4d shows moderate (63.14) and (72.25%) respectively when compared with standard ascorbic acid (96.69%). In general, compounds having chloro substitution along with the acetamide and quinoline ring exhibited good antimicrobial and antioxidant activities.
References
Sonia G, Thachil KK, Parameswaran MK, Kochupappy RT (2014) Synthesis of some benzoxazinyl pyrazolone arylidenes as potent antimicrobials and antioxidants. Med Chem Res 23:1320–1326. https://doi.org/10.1007/s00044-013-0719-9
García-Valverde M, Torroba T (2005) Sulfur–nitrogen heterocycles. Molecules 10:318–320. https://doi.org/10.3390/10020318
Kvasnica M, Urban M, Dickinson NJ, Sarek J (2015) Pentacyclic triterpenoids with nitrogen-and sulfur-containing heterocycles: synthesis and medicinal significance. Nat Prod Rep 32:1303–1330. https://doi.org/10.1039/c5np00015g
Naim MJ, Alam O, Farah Nawaz M, Alam J, Alam P (2016) Current status of pyrazole and its biological activities. J Pharm Bioallied Sci 8:2–17. https://doi.org/10.4103/0975-7406.171694
Gaba M, Mohan C (2016) Development of drugs based on imidazole and benzimidazole bioactive heterocycles: recent advances and future directions. Med Chem Res 25:173–210. https://doi.org/10.1007/s00044-015-1495-5
Feng M, Tang B, Liang SH, Jiang X (2016) Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem 16:1200–1216
Ahn M, Gunasekaran P, Rajasekaran G, Kim EY, Lee SJ, Bang G, Cho K, Hyun JK, Lee HJ, Jeon YH, Kim NH (2017) Pyrazole derived ultra-short antimicrobial peptidomimetics with potent anti-biofilm activity. Eur J Med Chem 125:551–564. https://doi.org/10.1016/j.ejmech.2016.09.071
Nitulescu GM, Draghici C, Olaru OT, Matei L, Ioana A, Dragu LD, Bleotu C (2015) Synthesis and apoptotic activity of new pyrazole derivatives in cancer cell lines. Bioorg Med Chem 23:5799–5808. https://doi.org/10.1016/j.bmc.2015.07.010
El-Moghazy SM, Barsoum FF, Abdel-Rahman HM, Marzouk AA (2012) Synthesis and anti-inflammatory activity of some pyrazole derivatives. Med Chem Res 21:1722–1733. https://doi.org/10.1007/s00044-011-9691-4
Datar PA, Jadhav SR (2015) Design and synthesis of Pyrazole-3-one derivatives as hypoglycaemic agents. Int J Med Chem. https://doi.org/10.1155/2015/670181
Ghorab MM, El-Gazzar MG, Alsaid MS (2014) Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles. Int J Mol Sci 15:7539–7553. https://doi.org/10.3390/ijms15057539
Sigroha S, Narasimhan B, Kumar P, Khatkar A, Ramasamy K, Mani V, Mishra RK, Majeed AB (2012) Design, synthesis, antimicrobial, anticancer evaluation, and QSAR studies of 4-(substituted benzylidene-amino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-ones. Med Chem Res 21:3863–3875. https://doi.org/10.1007/s00044-011-9906-8
Mohanram I, Meshram J (2014) Synthesis and biological activities of 4-aminoantipyrine derivatives derived from betti-type reaction. ISRN Org Chem. https://doi.org/10.1155/2014/639392
dos Santos GG, Dias EV, Teixeira JM, Athie MC, Bonet IJ, Tambeli CH, Parada CA (2014) The analgesic effect of dipyrone in peripheral tissue involves two different mechanisms: neuronal K ATP channel opening and CB 1 receptor activation. Eur J Pharmacol 741:124–131. https://doi.org/10.1016/j.ejphar.2014.07.019
Rubtsov AE, Makhmudov RR, Kovylyaeva NV, Prosyanik NI, Bobrov AV, Zalesov VV (2002) Synthesis, antiinflammatory and analgesic activity of 4-antipyrine derivatives. Pharm Chem J 36:608–612. https://doi.org/10.1023/A:1022669432631
Santos PM, Antunes AM, Noronha J, Fernandes E, Vieira AJ (2010) Scavenging activity of aminoantipyrines against hydroxyl radical. Eur J Med Chem 45:2258–2264. https://doi.org/10.1016/j.ejmech.2010.01.071
Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL (2002) COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci 99:13926–13931. https://doi.org/10.1073/pnas.162468699
Malvar DD, Soares DM, Fabrício AS, Kanashiro A, Machado RR, Figueiredo MJ, Rae GA, de Souza GE (2011) The antipyretic effect of dipyrone is unrelated to inhibition of PGE2 synthesis in the hypothalamus. Br J Pharmacol 162:1401–1409. https://doi.org/10.1111/j.1476-5381.2010.01150.x
Thiede JM, Kordus SL, Turman BJ, Buonomo JA, Aldrich CC, Minato Y, Baughn AD (2016) Targeting intracellular p-aminobenzoic acid production potentiates the anti-tubercular action of antifolates. Sci Rep 6:38083. https://doi.org/10.1038/srep38083
Ghorab MM, Ragab FA, Hamed MM (2009) Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem 44:4211–4217. https://doi.org/10.1016/j.ejmech.2009.05.017
Genç Y, Özkanca R, Bekdemir Y (2008) Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob 7:17. https://doi.org/10.1186/1476-0711-7-17
Zhang ZJ, Tian J, Wang LT, Wang MJ, Nan X, Yang L, Liu YQ, Morris-Natschke SL, Lee KH (2014) Design, synthesis and cytotoxic activity of novel sulfonylurea derivatives of podophyllotoxin. Bioorg Med Chem 22:204–210. https://doi.org/10.1016/j.bmc.2013.11.035
Badgujar J, More D, Meshram J (2017) Synthesis, characterization, in vitro antimicrobial, and anthelmintic evaluations of N-(4,6-dimethylpyrimidin-2-yl)-4-((2-hydroxynaphthalen-1-yl) diazenyl) benzene sulfonamide and its metal (II) complexes. Mod Org Chem Res 2:33–40. https://doi.org/10.22606/mocr.2017.22001
Kumar V, Kaur K, Gupta GK, Gupta AK, Kumar S (2013) Developments in synthesis of the anti-inflammatory drug, celecoxib: a review. Recent Pat Inflamm Allergy Drug Discov 7:124–134
Leibovitch L, Matok I, Paret G (2007) Therapeutic applications of sildenafil citrate in the management of paediatric pulmonary hypertension. Drugs 67:57–74. https://doi.org/10.2165/00003495-200767010-00005
Srivastava S, Bhargava A (2016) Biofilms and human health. Biotechnol Lett 38:1–22. https://doi.org/10.1007/s10529-015-1960-8
Wu H, Moser C, Wang HZ, Høiby N, Song ZJ (2015) Strategies for combating bacterial biofilm infections. Int J Oral Sci 7:1–7. https://doi.org/10.1038/ijos.2014.65
Kalia VC, Prakash J, Koul S, Ray S (2017) Simple and rapid method for detecting biofilm forming bacteria. Indian J Microbiol 57:109–111. https://doi.org/10.1007/s12088-016-0616-2
Bordi C, de Bentzmann S (2011) Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care 1:19. https://doi.org/10.1186/2110-5820-1-19
Furniss BS (1989) Vogel’s textbook of practical organic chemistry. Pearson Education India, London
Tan S, Yang Y, Luo Z, Zhao S, Huang D, Zhang J, Dong L, Wang G (2011) An alternative synthetic process of p-acetaminobenzenesulfonyl chloride through combined chlorosulfonation by HClSO3 and PCl5. Chem Pap 65:510–518. https://doi.org/10.2478/s11696-011-0026-1
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Bagul SD, Rajput JD, Tadavi SK, Bendre RS (2017) Design, synthesis and biological activities of novel 5-isopropyl-2-methylphenolhydrazide-based sulfonamide derivatives. Res Chem Intermed 43:2241–2252. https://doi.org/10.1007/s11164-016-2759-5
Sanna D, Delogu G, Mulas M, Schirra M, Fadda A (2012) Determination of free radical scavenging activity of plant extracts through DPPH assay: an EPR and UV–Vis study. Food Anal Methods 5:759–766. https://doi.org/10.1007/s12161-011-9306-1
Burda S, Oleszek W (2001) Antioxidant and antiradical activities of flavonoids. J Agric Food Chem 49:2774–2779. https://doi.org/10.1021/jf001413m
Soare JR, Dinis TC, Cunha AP, Almeida L (1997) Antioxidant activities of some extracts of Thymus zygis. Free Radical Res 26:469–478. https://doi.org/10.3109/10715769709084484
Pamerla M, Reddy DR, Rao BS, Bodipati N, Murthy YL (2015) Antimicrobial evaluation of 1,4-benzoxazine derivatives. Med Chem Res 24:611–615. https://doi.org/10.1007/s00044-014-1159-x
Acknowledgements
We are thankful to the University Grants Commission (UGC), New Delhi for finical assistance under UGC—BSR fellowship scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Badgujar, J.R., More, D.H. & Meshram, J.S. Synthesis, Antimicrobial and Antioxidant Activity of Pyrazole Based Sulfonamide Derivatives. Indian J Microbiol 58, 93–99 (2018). https://doi.org/10.1007/s12088-017-0689-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-017-0689-6