Abstract
Rice blast, caused by Magnaporthe oryzae, is the most devastating disease of rice and severely affects crop stability and sustainability worldwide. In this study, a total of 63 single spore isolates, collected during 2010–2013 from different cultivars in the different rice growing regions of North-East and Eastern India were used for molecular diversity and mating type analysis. DNA fingerprinting was used to study the diversity among the collections of 63 isolates by using POT2-TIR rep-PCR and MGR586-TIR. Different lineages were detected for 63 M. oryzae isolates by Pot2-TIR and eight for MGR586-TIR fingerprints at 75 % similarity. Among the lineages detected by Pot2-TIR, lineage A and I represented the maximum number of isolates whereas other lineages represent fewer numbers of isolates. Generally all the lineages contained isolates of mixed geographical origin. Isolates from Jharkhand were distributed in all the seven lineages. The MGR586-TIR DNA fingerprinting detected eight lineages, out of which three (Lineages F, G, H) were site specific but were represented only by single isolate. Lineage C contained isolates of Jharkhand only. The lineage A was the largest represented 46 isolates from all the states except Madhya Pradesh. Optimization of the sampling may result in considerable improvement in the results as clustering of isolates from Jharkhand in a few lineages and detection of different lineages with limited isolates from other states could be ascribed to improper sampling. MGR586-TIR fingerprints appeared to differentiate the isolates more strongly compared to POT2-TIR as is obvious from the distance among isolates of the same lineage (Lineage A) arbitrarily grouped together at 75 % similarity. All the 63 isolates were also investigated for MAT1-1 and MAT1-2 mating-type distribution by PCR based molecular markers. Of the 63 M. oryzae isolates collected, 16 (25 %) of the isolates were the mating type MAT1-1 while 35 (56 %) were mating type MAT1-2. The MAT1-2 isolates predominated in Jharkhand and Assam while MAT1-1 is more predominant in the isolates of Odisha. Both MAT1-1 and MAT1-2 were equally distributed in the isolates of Meghalaya and Tripura. Only single isolate from Jharkhand was positive for both the mating type. The results indicated that sexual recombination might be the one reason for lineage diversity in M. oryzae in fields of large rice-growing regions in North-East and Eastern states of India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many high yielding varieties of rice are available today and the yield potential is considerably affected by various biotic and abiotic stresses. Among them rice blast, the most devastating is caused by heterothallic ascomycete Magnaporthe oryzae is a serious problem in rice production globally. In India, rice blast disease is seen in almost all geographical regions wherever rice is grown. About 564,000 tonnes of rice is lost due to blast in Eastern India alone, of which 50 % (246,000 tonnes) in the upland ecosystem. The blast disease is common to most rice-growing areas of Eastern India due to blast-conducive environments during the crop season. Extreme virulence polymorphism in the pathogen and in-sufficient exposure of the breeding material to the full range of virulences present in the pathogen population is responsible for blast resistance breakdown. The plant-pathogen system in agriculture tends to co-evolve, but not simultaneously. The evolution of plant population occurs at a low rate as compared to pathogen which evolves faster and thus breakdown the resistance in plants [1, 2].
Different methods and strategies like elimination of crop residues, use of resistant cultivars, chemical controls and biological controls have been developed to prevent and control the rice blast disease, but without much success because of the rapid and frequent genetic variation of M. oryzae [3–5]. The high genetic diversity in M. oryzae poses a challenge to develop durable strategies for their management. Environmental stress also exerts evolutionary pressure which leads to faster mutation rates which helps in adaptation and survival of M. oryzae [6].
DNA fingerprinting has been widely used for studying the population structure of M. oryzae. DNA fingerprinting analyses of M. oryzae population from different rice growing regions of the world with probes MGR-586, Pot2 rep-PCR, RAPD, Pot2-TIR rep-PCR and SSR clearly reveals that the populations are organized into discrete groups or lineages [7–10]. The diversity of blast pathogen using MGR586 probe in southern region and using Pot2-rep PCR in the eastern region [11] has been demonstrated. Pot2 rep-PCR has advantages with respect to the ease of application for the analysis of a large number of samples in population studies excepting that it requires a long electrophoretic run time. Suzuki et al. [9] demonstrated that using Pot2-TIR rep-PCR with outwardly directed primers complementary to sequences in terminal inverted repeats (TIRs) of the individual transposable elements for rep-PCR fingerprinting, which is simpler and more rapid with high fingerprinting ability.
The ability of M. oryzae isolates to produce perithecia indicating a complex phenotype and mating ability is an excellent criterion for examining the relationship among isolates of the fungus. The genetic relationship between female fertility and pathogenicity to rice in M. oryzae segregates randomly. M. oryzae has single mating type gene having two alleles, MAT1-1 and MAT1-2. The fungus requires both mating types in order for sexual reproduction to occur. The mating type alleles have been used as a marker to measure population diversity in the pathogen [12]. Fertility in M. grisea is a complex phenotype. The degree of fertility ranges from strains that are hermaphroditic (both female and male fertile) to those that behave only as male fertile (female sterility) or female fertile (male sterility) in crosses. While hermaphroditic isolates produce two rows of perithecia when crossed with compatible fertile tester strains, isolates that form a single band of perithecia are either male fertile or female fertile. M. oryzae isolates from rice (Oryza sativa L.) are known to have low fertility even when crossed with fertile tester strains [12].
The purpose of this study was to investigate for the first time the DNA fingerprinting pattern and genetic structure of M. oryzae isolates and the presence of both mating-types and also to measure and assess the spatial diversity existing on commonly grown rice cultivars from the major rice- producing areas in North-East and Eastern India and determine if sexual reproduction is possible in these collected isolates. The main aim of our study was to explore the feasibility of genetic diversity and mating-type results for developing blast-resistant cultivars for the region.
Materials and Methods
Fungal Strains
A collection of 63 isolates of M. oryzae was obtained from sporulating lesions on leaves and panicles of different rice cultivars from North-East and Eastern India during 2010–2013 (Table S1). A single conidial isolate for each of the cultivar sampled was were maintained on sterilized filter paper discs at 4 °C in the refrigerator. The collection of isolates originated from nine regions distributed across six states Jharkhand (Hazaribag, 26; Balumath, 1; Ranchi, 12), Odisha (Semliguda, 4), Assam (Tatabar, 5; Gerua, 6; Golaghat, 2), Tripura (Lembuchera, 4), Meghalaya (Barapani, 2) and Madhya Pradesh (Rewa, 1). The leaf and neck blast samples were collected during the wet season.
DNA Preparation and Rep-PCR with Pot2-TIR and MGR586-TIR
Stored cultures of the fungal isolates were revived by inoculating the colonized filter disc on oat meal agar slant. 10-day-old cultures were inoculated into 100 ml yeast extract and glucose broth in a 250 ml conical flask and kept under constant shaking at 28 °C for 5–7 days. Mycelia were harvested and filtered through Whatman No 1 filter paper. The dry mycelia were ground in liquid nitrogen into a fine powder with a mortar and pestle. Fungal genomic DNA was extracted using DNeasy Plant Mini Kits (QIAGEN) following the manufacturer’s instructions. DNA quality and concentration were determined by electrophoretically in 0.8 % agarose gel and spectrometrically by NanoDrop 2000c (Thermo Scientific, USA). The outwardly directed primers Pot2-TIR (5′ ACAGGGGGTACGCAACGTTA 3′) and MGR586-TIR (5′ TCCGGGGTCCTGATGAACCACGT 3′) designed from the 45-bp TIR sequence of the 1861-bp Pot2 element and the 42-bp TIR sequence of the 1860-bp MGR586 element, respectively were employed for fingerprinting [9]. The PCR amplification was performed in 20 µl volume according to the method described by Suzuki et al. [9]. PCR products were separated by electrophoresis on 1 % agarose gel and visualized with ethidium bromide staining. PCR analysis was done at least twice for each DNA sample to ensure only reproducible bands were scored.
Analysis of Lineage Structure
The DNA fingerprint profiles generated by Pot2-TIR and MGR586-TIR were compared to determine relatedness among isolates. The presence or absence of each DNA band of particular molecular weight in all the isolates was scored manually. A binary data matrix with 1 indicating the presence of a band of particular molecular weight and 0 indicating its absence was generated for both. A similarity matrix was generated from the binary data using Jaccard’s similarity coefficient in the SIMQUAL program of the NTSYS-pc package [13]. Cluster analysis was performed with the unweighted pair group arithmetic mean method (UPGMA) in the SAHN program of the NTSYS-pc package. The dendrogram with the best fit to the similarity matrix based on cophenetic values (COPH) and matrix comparison (MXCOMP) was chosen.
Mating-Type Analysis
The gene encoding for the mating-type was amplified by the polymerase chain reaction (PCR) using these primers: L1 (5′-ATGAGAGCCTCATCAACGGCA) and L2 (5′-ACAGGATGTAGGCATTCGCAGGAC) for MAT1-1 and T1 (5′ ACAAGGCAACCATCTGGACCCTG) and T2 (5′-CCAAAACACCGAGTGCCATCAAGC) for MAT1-2 [14]. 1 µl (100 ng/µl) of genomic DNA was used as the template in a 10 µl PCR mixture (1 µl of 10× PCR buffer, 10 mM dNTP mix, 5 µM of each primer, and TAQ polymerase). PCR was performed using a Pro-S (Ependroff) thermalcycler. The mixture was subjected to 35 cycles of amplification. Thermocycling conditions consisted of an initial hold at 94 °C for 5 min, followed by 35 cycles of 94 °C (60 s), 60 °C (30 s), and 72 °C (1 min), and a final hold of 72 °C for 10 min. The PCR products were electrophoresed in 1.5 % agarose gel, stained with ethidium bromide and then visualized under UV. M. oryzae Isolates that was not amplified by the MAT1-1 primer was processed again with the MAT1-2 primer.
Results and Discussion
DNA Fingerprinting Based on Rep-PCR with Pot2-TIR and MGR586-TIR Primers
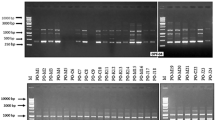
A total of 63 M. oryzae isolates were collected from fields and nursery in the North-East and Eastern India for DNA fingerprint analysis to assess their genomic diversity. DNA polymorphisms of the isolates were detected by rep-PCR with the Pot2-TIR and MGR586-TIR primers. Both the primers yielded distinct DNA banding patterns. The M. oryzae isolates showed 2–25 amplified bands for Pot2-TIR primer and 2–23 amplified bands for MGR586-TIR primer of different molecular weights (Figs. S1, S2). The amplified bands ranged in length from 0.3 to 05 kb for Pot2-TIR primer (Fig. S1) and 0.2 to 5 kb for MGR586-TIR primer (Fig. S2).
The dendrogram of DNA fingerprint groups by two primers obtained from 63 M. oryzae isolates from North-East and Eastern India by using NTSYS. DNA fingerprint data for both the primers yielded high lineage diversity. At 75 % similarity cluster analysis of the PCR banding patterns of the 63 isolates for both the primers showed that haplotypic diversity was very high at all sites. Different lineages were detected for 63 M. oryzae isolates by Pot2-TIR and eight for MGR586-TIR fingerprints (Fig. S3a, b). Among the lineages detected by Pot2-TIR, lineage A and I represented the maximum number of isolates from different states whereas other lineages represent fewer numbers of isolates. Clustering of isolates from Hazaribagh and Ranchi is a result of unequal sampling. Stratified or hierarchical sampling of isolates based on geographichic, temporal, and/or genotypic variation would reduce the anomalies and improve the reliability of results. Further, the possible of differentiation of isolates from a single lineage into sub-groups consisting of one to two isolates cannot be ignored as it would suffice to contribute to different virulence patterns. Generally all the lineages contained isolates of mixed geographical origin. Isolates from Jharkhand were distributed in seven lineages. The MGR586-TIR DNA fingerprinting detected eight lineages, out of which three (Lineages F, G, H) were site specific but were represented only by single isolate. Lineage C contained isolates of Jharkhand only. The lineage A was the largest represented 46 isolates from all the states except Madhya Pradesh (Table S2). The DNA fingerprinting analysis of 63 isolates from North-East and Eastern India clearly indicated that in general, the M. oryzae isolates cannot be delineated into region-specific groups.
Mating-Type Analysis
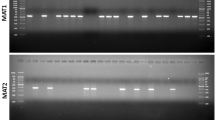
Assessment of mating types was made in the collected isolates from North-East and Eastern India. Both mating types were identified in the surveyed fungal isolates. MAT1-1 (male) isolates generated a 522 bp amplicon and the MAT1-2 (female) isolates generated a 390 bp amplicon. An example of MAT1-1 and MAT1-2 is depicted in Fig. S4. The figure represents the amplification of band of respective size of both mating types. Each isolate produced a single fragment of the expected size. Of the 63 M. oryzae isolates analysed, 16 (25 %) of the isolates were of the mating type MAT1-1 while 35 (56 %) were of the mating type MAT1-2 (Table S3). Eleven (11) isolates did not produce a PCR product with either of these two mating types. The results clearly revealed the presence of both mating type in the isolates from all the states except one single isolates from Madhya Pradesh. The MAT1-2 isolates predominated in Jharkhand and Assam while MAT1-1 was more predominant in the isolates of Odisha. Both MAT1-1 and MAT1-2 were equally distributed in the isolates of Meghalaya and Tripura. Only single isolate from Jharkhand was positive for both the mating types.
Genotype and pathotype diversity of M. grisea was the focus of previous investigations in different rice growing regions of India [8]. The work reported here represents the first attempt to characterize M. oryzae isolates in North-East and Eastern India using single primers complementary to sequences in the terminal inverted repeat (TIR) of Pot2 and MGR586, transposable elements found in M. oryzae. A total of 63 isolates of M. oryzae were obtained from the fields of different states of North-East and Eastern India was subjected to DNA fingerprint analysis to assess their genomic diversity. Pot2-TIR rep-PCR fingerprinting differentiated nine fingerprint lineages designated A, B, C, D, E, F, G, H and I while MGR586-TIR differentiated eight lineages among 63 field isolates. Previously also the diversity of rice blast populations was examined by rep-PCR using Pot2 primers and pathotyping [15, 16]. Multilocus microsatellite typing has also been widely used to identify the genetic diversity of M. oryzae population [17].
Both sexual and asexual stages in their life cycles are seen in ascomycetous fungi. In many fungi, including M. oryzae sexual cycle is controlled by mating type genes. M. oryzae can only mate when two fertile opposite mating-types are present. Sexual propagation in M. oryzae is controlled by a single mating-type (MAT) locus, which is represented by two idiomorphs known as MAT1-1 and MAT1-2 [18–20]. The M. oryzae isolates collected from different cultivars may have different level of fertility and genetic differences in compatibility of isolates determined the nature of fertility in population. Therefore, the fertility status and mating-type of an unknown M. oryzae isolate can be better assessed by using PCR-based method rather than using tester strain for in vitro crosses. The PCR-based method will allow determining the mating-type independent of fertility and incompatibility factors [14]. In this study two primer pairs LI, L2 and T1, T2 were used for amplification of MAT1-1 and MAT1-2 idiomorphs to determine mating-type alleles in M. oryzae isolates in North-East and Eastern India. The results revealed the presence both mating-types and the MAT1-2 mating-type dominated in all the sampled isolates. If a single mating-type predominates in a particular rice growing region, M. oryzae may not be sexually reproducing in that region and this may be important in the population dynamics of this pathogen. In past studies, MAT1-1 has been identified as the dominant mating-type associated with rice [21]. For example, only MAT1-1 mating-type was observed in Karnataka, Andhra Pradesh, Andaman Islands, Haryana, and Punjab [21]. The presence of only MAT1-1 in the pathogens is not uncommon as it was also detected in the M. oryzae population in Japan and other regions [22]. The presence of both mating-types has also been reported from the states of Meghalaya and Himachal Pradesh, but only two out of 90 isolates were MAT1-2 [21]. Thailand and in Central Himalayan Region of India higher frequency of occurrence of both mating-type has also been observed [23, 24]. Rathour et al. [25] also recovered sexually fertile isolates in high frequency from in vitro mating among M. grisea isolates from different hosts including rice.
DNA fingerprinting of M. grisea rice population of Uttaranchal has revealed high genetic diversity and lack of discrete DNA fingerprint groups. Since the blast fungus originates from South-East Asia and has invaded almost all the continents through infected seed exchange, the chances of finding diverse M. oryzae population decreases [1]. The high genetic diversity of M. oryzae helps in determining the partial resistance of the rice variety [2]. Kumar and Ziegler [24] suggested the possibility of sexual recombination in M. grisea population of Himalayan regions. Both mating type and sexually fertile M. grisea rice isolates have also been reported from the hilly areas of the Yunnan province of China and Thailand.
The observation that both mating-types were present in the states of Jharkhand, Odisha, Assam, Meghalaya and Tripura, suggests the possibility of sexual recombination in nature and this can affect diversity and dissemination. In this study, the degree of genetic diversity in terms of lineage composition observed in the small population of M. oryzae isolates analyzed and presence of both mating-types appears to support this suggestion. This high lineage diversity might indicate the co-evolution of rice and M. oryzae for long time. As stated earlier, genetic diversity and mating-type studies formed the basis of suggestion that M. oryzae exists as a recombinant population. However, detection of sexual form in natural field conditions and the pathogenicity of its progenies on rice still remain elusive [26] despite the existence of firm populations. The presence of both mating-type as detected by PCR based molecular markers are basically useful to identify the mating type locus in the field isolates of M. grisea but not effective to identify the fertile M. grisea strains because fertility is also influenced by genes other than the mating-type genes [21].
Conclusion
The data on mating type distribution and lineage diversity presented in this study indicate that sexual recombination might be the one reason for lineage diversity in M. oryzae in fields of large rice-growing regions in North-East and Eastern states of India. The other reason that may shape or influence the genotypic variation of M. oryzae could be evolutionary forces like mutation, vegetative compatibility, parasexual recombination, random genetic drift, migration and gene flow. Further research is needed to determine the fertility status and the significance of various evolutionary forces in shaping of genetic structure of the M. oryzae populations from North-East and Eastern states of India.
References
Saleh D, Milazzo J, Adreit H, Fournier E, Tharreau D (2013) South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol 201:1440–1456. doi:10.1111/nph.12627
Gallet R, Bonnot F, Milazzo J, Tertois C, Aldreit H, Ravigne V, Tharreau D, Fournier E (2014) The variety mixture strategy assessed in a G × G experiment with rice and the blast fungus Magnaporthe oryzae. Front Microbiol 312:1–11. doi:10.3389/fgene.2013.00312
Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Asfaliza R, Latif MA (2013) Blast resistance in rice: a review of conventional breeding to molecular approaches. Mol Biol Rep 40:2369–2388. doi:10.1007/s11033-012-2318-0
Yu Q, Liu Z, Lin D, Zhang W, Sun Q, Zhu J, Lin M (2013) Characterization and evaluation of Staphylococcus sp. Strain LZ16 for the biological control of rice blast caused by Magnaporthe oryzae. Biol Control 65:338–347. doi:10.1016/j.biocontrol.2013.03.016
Taniguchi S, Miyoshi S, Tamaoki D, Yamada S, Tanaka K, Uji Y, Tanaka S, Akimitsu K, Gomi K (2014) Isolation of jasmonate-induced sesquiterpene synthase of rice: product of which has an antifungal activity against Magnaporthe oryzae. J Plant Pathol 171:625–632. doi:10.1016/j.jplph.2014.01.007
Chadha S, Sharma M (2014) Transposable elements as stress adaptive capacitors induce genomic instability in fungal pathogen Magnaporthe oryzae. PLoS One 9:e94415. doi:10.1371/journal.pone.0094415
George MLC, Nelson RJ, Zeigler RS, Leung H (1998) Rapid population analysis of Magnaporthe grisea by using rep-PCR and endogeneous repetitive DNA sequences. Am Phytopathol Soc 88:223–229. doi:10.1094/PHYTO.1998.88.3.223
Rathour R, Singh M, Sharma TR, Chauhan RS (2004) Population structure of Magnaporthe grisea from North-western Himalayas and its implication for blast resistance breeding of rice. J Phytopathol 152:304–312. doi:10.1111/j.1439-0434.2004.00846.x
Suzuki F, Arai M, Yamaguchi M (2006) DNA fingerprinting of Pyricularia grisea by rep-PCR using a single primer based on the terminal inverted repeat from either of the transposable elements pot2 and MGR586. J Gen Plant Pathol 72:314–317. doi:10.1007/s10327-006-0290-z
Babu TK, Sharma R, Upadhyaya HD, Reddy PN, Deshpande SP, Senthilvel S, Sarma NDRK, Thakur RP (2013) Evaluation of genetic diversity in Magnaporthe grisea populations adapted to finger millet using simple sequence repeats (SSRs) markers. Physiol Mol Plant Pathol 84:10–18. doi:10.1016/j.pmpp.2013.06.001
Sridhar R, Singh UD (2001) Genetic and pathogenic diversity of the rice blast pathogen. In: Sreenivasaprasad S, Johnson R (eds) Major fungal disease of rice. Kluwer Academic Publisher, Dordrecht
Viji G, Uddin W (2002) Distribution of mating type alleles and fertility status of Magnaporthe grisea causing gray leaf spot of perennial ryegrass and St Augustinegrass turf. Plant Dis 8:827–832. doi:10.1094/PDIS.2002.86.8.827
Rohlf FJ (1993) NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System Version 1.80. Exeter Software, Setauket
Tredway LP, Stevenson KL, Burpee LL (2003) Mating type distribution and fertility status in Magnaporthe grisea populations from turf grasses in Georgia. Plant Dis 87:435–441. doi:10.1094/PDIS.2003.87.4.435
Ashkani S, Rai MY, Sarias M, Siti Nor Akmar A, Rusli I, Abdul Rahim H, Latif MA (2011) Analysis of simple sequence repeat markers linked with blast disease resistance genes in a segregating population of rice (Oryza sativa). Genet Mol Res 10:1345–1355. doi:10.4238/vol10-3gmr1331
Wang JC, Jia Y, Wen JW, Liu WP, Liu XM, Li L, Jiang ZY, Zhang JH, Guo XL, Ren JP (2013) Identification of rice blast resistance genes using international monogenic differentials. Crop Prot 45:109–116. doi:10.1016/j.cropro.2012.11.020
Choi J, Kim H, Lee YH (2013) Comparative analysis of the Korean population of Magnaporthe oryzae by multilocus microsatellite typing. Plant Pathol J 29:435–439. doi:10.5423/PPJ.NT.04.2013.0042
Consolo VF, Cordo CA, Salerno GL (2005) Mating-type distribution and fertility status in Magnaporthe grisea populations from Argentina. Mycopathology 160:285–290. doi:10.1007/s11046-005-4333-3
Leslie JF, Klein KK (1996) Female fertility and mating type effects on effective population size and evolution in filamentous fungi. Genetics 144:557–567
Zeng J, Feng S, Cai J, Wang L, Lin F, Pan Q (2009) Distribution of mating type and sexual status in Chinese rice blast populations. Plant Dis 93:238–242. doi:10.1094/PDIS-93-3-0238
Dayakar BV, Narayanan NN, Gnanamaickam SS (2000) Cross-compatibility and distribution of mating type alleles of the rice blast fungus Maganporthe grisea in India. Plant Dis 84:700–704. doi:10.1094/PDIS.2000.84.6.700
Notteghem JL, Silule D (1992) Distribution of mating type alleles in Magnaporthe grisea populations pathogenic on rice. Phytopathology 82:421–424. doi:10.1094/Phyto-82-421
Roumen E, Luangsaard J, Sirthunyal P et al. (1998) Population genetic studies on the rice blast pathogen, Magnaporthe grisea in Thailand. In: Abstract second international rice blast conference, Montpellier, France
Kumar J, Zeigler RS (1995) Mating behavior of Magnaporthe grisea from central Himalayas of India. Phytopathology 85:1201 (Abstract)
Rathour R (2000) DNA fingerprinting of Magnaporthe grisea from rice for virulence diversity. PhD Thesis, Himachal Pradesh Krishi Vishvavidyalaya, Palampur, India
Saleh D, Milazzo J, Adreit H, Tharreau D, Fournier E (2012) Asexual reproduction induces a rapid and permanent loss of sexual reproduction capacity in rice fungal pathogen Magnaporthe oryzae: results of in vitro experimental evolution assays. BMC Evol Biol 12:42. doi:10.1186/1471-2148-12-42
Acknowledgments
This research was supported by grants from National Agriculture Innovative Project- Component 4 (C1071), Indian Council of Agricultural Research (ICAR), on Allele mining of blast resistance genes.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
12088_2014_504_MOESM1_ESM.jpg
Figure S1 DNA fingerprinting pattern of 63 Magnaporthe oryzae isolates with Pot2-TIR primer. Lane 1–63 corresponds to the numbers assigned to M. oryzae isolates. Lane M: Molecular marker (1 kb Plus Ladder) (JPEG 256 kb)
12088_2014_504_MOESM2_ESM.jpg
Figure S2 DNA fingerprinting pattern of 63 Magnaporthe oryzae isolates with MGR586-TIR primer. Lane 1–63 corresponds to the numbers assigned to M. oryzae isolates. Lane M: Molecular marker (1 kb Plus Ladder) (JPEG 234 kb)
12088_2014_504_MOESM3_ESM.jpg
Figure S3 Dendogram of 63 M. oryzae isolates from North-East and Eastern India by using NTSYS with a Pot2-TIR b MGR586-TIR (JPEG 595 kb)
12088_2014_504_MOESM5_ESM.jpg
Figure S4 An example of PCR assay to determine the mating-type in Magnaporthe oryzae isolates. Lane M: Molecular marker (100 bp Ladder). Lane 1–6: MAT1-1 isolates. Lane 7–11: MAT1-2 isolates. Lane 12: M. oryzae isolate possessing both MAT1-1 and MAT1-2. (JPEG 47 kb)
Rights and permissions
About this article
Cite this article
Imam, J., Alam, S., Mandal, N.P. et al. Molecular Diversity and Mating Type Distribution of the Rice Blast Pathogen Magnaporthe oryzae in North-East and Eastern India. Indian J Microbiol 55, 108–113 (2015). https://doi.org/10.1007/s12088-014-0504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-014-0504-6