Abstract
The study attempted to confirm the efficacy of Saccharomyces cerevisiae as a probiotic in augmenting the overall wellbeing and disease resistance in the Indian Major Carp, rohu (Labeo rohita) juveniles. The growth rate, nutritional quality and immunity of L. rohita fry were studied for 60 days fed with four isocaloric and isonitrogenous diets supplemented with 0.50 % (L1), 0.75 % (L2) and 1.00 % (L3) lyophilized whole yeast (S. cerevisiae) cells. L3-fed fish registered significantly better in wellbeing parameters such as growth, RNA:DNA, lower feed conversion and higher protein efficiency ratios. High intestinal enzyme (protease and α-amylase) activities, high liver serum GOT and GPT activities, and better non-specific immune responses were also demonstrated by them. The blood parameters hemoglobin, total erythrocyte and leukocyte counts, corpuscular volume, corpuscular hemoglobin, and cell hemoglobin concentration were also encouraging. Challenged with Aeromonas hydrophila AH2 (hourly exposure to 105 and 107 CFUs/ml strengths with a week interval) by bath exposure, highest survival percentage (96.66 %) was observed in L3-fed fish, whereas only 30 % in the control, after 10 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture production and aquatic animal health are inseparable propositions. Higher aqua-production is dependent on the enhanced protection against infectious diseases. Besides therapeutics and vaccines, an effective alternate approach to enhance disease resistance is by administering probiotics, a special form of microbe used either as additive or as nutriceutical in feed since long. Probiotics in aquaculture has a dual role, one as a feed additive, and the other role, often referred to as environmental probiotics, helps improve the water quality [1] by balancing the microbial population and restricting the proliferation of the pathogenic microbes [2]. Lactic acid bacteria have been well-researched as probiotics with few similar studies on yeast [3]. Yeast (Saccharomyces cerevisiae) adheres and colonizes the fish intestine [2, 3], a basic trait of a potential probiotic. Besides growth, it enhances nonspecific immunity [4–9]. These spore-formers reportedly enhanced the innate immune system in trout [6, 9] and gilthead seabream [7]. Thus, selecting a suitable probiotic candidate is the key.
Yeast cell wall contains two main polysaccharide classes, mannose polymers covalently linked to peptides or mannoprotein and glucose polymers or glucans, with N-acetyl-glucosamine and chitin as minor constituents. β-glucans and chitin are known powerful immune-stimulants in fish and mammals [10, 11]. β-glucans activate fish macrophages and enhance nonspecific defense mechanism [11], also demonstrating significant adjuvant properties. Chitin polymer reportedly enhances nonspecific defense and resistance to infection in fish [8].
Intraperitoneal injection is the most widely accepted efficient and rapid method to study nonspecific immune response. It elicits an enhanced non-specific immune response through production of acute-phase proteins, and also macrophages activation [8, 10, 12]. However, oral administration of immunostimulants is resorted to [4, 6, 8, 11] in farm situations. Yeast in aqua-feeds as probiotics [3, 7, 9, 12] or as a protein substitute [13] has been studied. The present study examined the effects of feed-incorporated lyophilized whole yeast, S. cerevisiae MTCC 172 on the growth, nutrition, immunity and pathogen-resistance in L. rohita juveniles.
Materials and Methods
Candidate Species
Labeo rohita fry (2.52–2.81 g) were obtained from a farm in Eastern India’s largest IMC cultivation region, on the outskirts of Midnapore (22°25′N and 87°20′E), West Bengal, India. After a 15-day laboratory conditioning (temperature 25–29 °C; pH 7.2–7.8), the test organism was released into the 200 l capacity continuous-flow experimental aquaria.
Preparing the Experimental Feeds
Table S1 details the formulation of the four experimental feeds (LC, L1, L2 and L3). This was based on the ‘square-method’ after determining the protein values of the ingredients (Table S2), calculating the proportion of each ingredient providing allowance for the premix. The vitamin-mineral mix (Kalvimin Forte) used as a premix contained 5,000,000 IU Vit. A, 2.0 g Vit. B2, 6.0 mg Vit. B12, 1,000,000 IU Vit. D3, 4.0 g calcium pantothenate, 800.0 g calcium, 150.0 g phosphorus, 27.5 g manganese, 1.0 g iodine, 7.5 g iron, 15.0 g zinc, and 2.0 g copper. The cod liver oil (SEACOD) of M/s Universal Medicare Ltd., Mumbai contained health-promoting factors Vit. A, Vit. D, EPA and DHA. S. cerevisiae MTCC 172 (from the Institute of Microbial Technology, Chandigarh, India) was grown for 48 h in 10 l nutrient broth (4.0 g yeast extract, 5.0 g casein digest, 50.0 g dextrose, 0.55 g monopotassium phosphate, 0.425 g potassium chloride, 0.125 g calcium chloride, 0.125 g magnesium sulfate, 0.0025 g ferric chloride, 0.0025 g manganese sulfate, 0.0022 g bromocresol green)Footnote 1 at 30 °C in shake-bottles. The biomass thus obtained was centrifuged at 5,000 rpm for 15 min at 4 °C, repeated thrice with sterile 1.0 % NaCl solution washing. The pelleted cells were added at the rate of 0.50, 0.75 and 1.00 % to the feeds L1, L2 and L3 respectively (the control feed LC had no probiotic supplementation). To have a better understanding, the morphological characteristics of both the lyophilized and the live yeast cells were viewed under the scanning electron microscope at the Institute of Minerals and Materials Technology (IMMT), Bhubaneswar. The dough was prepared and the feeds were pelleted through a manually-operated pelletizer, dried to <10 % moisture in a thermostatic oven at 37 °C, and stored at room temperature in airtight jars. The proximates of the feeds were analyzed, the values of which are detailed in Table S2 and Fig. S1.
Growth and Dietary Performance
The experimental setup consisted of fifteen 200 l capacity rectangular aquaria (each treatment in triplicates). Each aquarium was aerated continuously and stocked with 15 nos. of rohu fry. Water quality (such as the temperature, pH, dissolve oxygen, total alkalinity, and total ammonium) was monitored at weekly intervals following standard methods [14]. Fishes were fed once in the morning (between 8.00 and 8.30 AM) and again in the afternoon (between 4.00 and 4.30 PM) daily, at 4.0 % of body weight in two equal installments [15]. The leftover feed and the fecal matter were siphoned out an hour after the feeding. 50 % of the water was replenished daily with conditioned (aged) water to ensure acceptable water quality. Three fishes were weighed fortnightly using an electronic weighing balance, and the feed quantity was adjusted accordingly based on the realized average weight. The dietary performance was evaluated based on the nutritional indices like live weight gain (LWG), average daily growth (ADG), feed conversion ratio (FCR), specific growth rate (SGR), and protein efficiency ratio (PER). Two fishes from each treatment were sacrificed (euthanized) through overdose anaesthetization (30 mg/l; three minutes) by MS222 (Sigma Chemicals, India) with the provision of continuous aeration and pH adjustment to 7.5 with bicarbonate, and stored at −20 °C until further analyses.
Proximates Analyses
Proximates of ingredients, feeds and body carcass were analyzed by standard methods [16]. The moisture contents were determined by drying the samples in hot-air oven at 110 °C for 24 h. The crude protein contents were estimated by micro-Kjeldahl method, and the crude lipid contents by the soxhlet extraction method. Samples were incinerated at 500 ± 50 °C for 10 h in a muffle furnace to estimate the ash contents. Defattened samples were estimated for the crude fiber after lipid extraction.
Biochemical Analyses
The DNA and RNA contents were analyzed from a 200.0 mg hepatopancreatic sample obtained from three test individuals, homogenized with 0.25 M sucrose solution, repeatedly centrifuged with 500 µl 5 % trichloroacetic acid (TCA), followed by dissolution in 500 µl of 0.6 and 0.3 N perchloric acid (PCA), and 0.3 M KOH, following standard procedure. The supernatant was used for RNA estimation (at 270 and 290 nm) spectrophotometrically. Then, the pellet was heated at 70 °C for 40–60 min with 1.5 ml cool 0.6 N PCA, and cold centrifuged with 400 µl of 0.6 N PCA at 5,000 rpm for 10 min. The DNA in this supernatant was estimated at 270 and 290 nm. The negative blanks were prepared by 0.3 and 0.6 N PCA, respectively.
The intestinal protease and α-amylase, and the hepatic GOT and GPT activities were determined as per standard methods. Small portions of the different regions of the alimentary canal were collected in ice-cooled petridishes, weighed, chopped, and a 5 % homogenate was prepared. The enzymes in the supernatant were centrifuged at 2,500 rpm for 15 min using cool 0.1 M phosphate buffer (pH 7.0) and assayed. The proteolytic activity was determined using Bovine albumin (BSA) as the substrate, and the amylase activity was quantified spectrophotometrically by measuring their increase in reducing power of a starch solution (amount of maltose liberated). The differences in the readings between the experimental and the 0th h tube indicated the enzymatic activities. The absorbance was estimated through the standard curves. The specific enzyme activities were expressed as mg of product liberated/h/mg substrate.
Blood Parameters
Blood samples were obtained from the caudal peduncle and/or the heart by cardiac puncture using disposable plastic syringes fitted with 26-gauge needle moistened with heparin, and expelled immediately into separate heparinized vials on ice [17]. Hematological parameters like total serum protein (TSP) and albumin were estimated following standard methods, and the equation: total serum protein = globulin content + albumin protein.
Determining Immunity Levels
The blood was collected from fish of each group on the final (60th) day by a 2-ml glass syringe rinsed with an anticoagulant (EDTA). A part of the collected blood was heparinized, and the rest was allowed to clot for serum samples. The samples were preserved at −20 °C for further analyses. The heparinized blood samples of each group were pooled in three aliquots immediately after collection. Part of the sample was analyzed for leucocratic value. The rest of the heparinized sample was utilized for phagocytic assay. Aliquots of the collected sera were kept for serum bactericidal activity study.
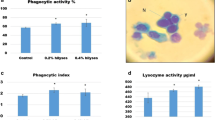
For immunological assay, 0.1 ml of freshly prepared NBT solution was added to an equal volume of the heparinized blood and 15 μl of stimulant solution in the incubating bottle. The bottles were serially-incubated at 37 and 26 °C for 10 min each. 50–70 μl of this blood was transferred onto a clean slide to make a thick smear. The slides were air-dried, stained with Wright’s stain, and observed under oil-immersion. The percentage of the positive (violet colored Formosan granules in the cytoplasm) cells provided clues about the nonspecific immunity status. The bactericidal activity of each group was measured as mean ± SEM of the bacterial count (×103 CFUs/ml), with the serum samples plated in duplicate.
Challenge Study
After the 60-day period, the fish were challenged with active Aeromonas hydrophila AH2 cultured and maintained in Aeromonas selective medium (M884, Hi-Media). Taking care not to manually handle them, the fry were immersed twice with a week interval in A. hydrophila AH2 suspension (diluted with sterile saline to obtain the desirable live cell concentrations), initially at 105 followed by 107 CFUs/ml population. The challenge tests with 10 fry per replicate were conducted in triplicates alongside a control with no pathogen challenge. Without any provision of diet what-so-ever during the trial, and the fish mortality in each individual tank was recorded, along with any sign of illness including erratic swimming. Samplings were done from the representative fish for the immunological assays as detailed above.
Statistical Analyses
Descriptive statistics was employed to verify the veracity of all the data, summarized as means, standard deviation (SD) and standard error of the mean, using the Windows-compatible SPSS 17. Correlation and regression tests were performed while evaluating the dietary performances, nutritional indices, enzymatic activities, RNA:DNA ratio, hematological, serological, immunological studies and challenge trials. Significant differences between the means of the treatments were tested by Duncan Multiple Range Test (DMRT). Two-factor without replication Analysis of Variance (ANOVA) was applied to detect the significant differences between the treatments in terms of growth and survival.
Results
The results as the means of at least five disconnected values (Mean ± SEM) are provided in the tables. The figures with different superscripted letter in the same column, wherever provided, are statistically significantly different (p ≤ 0.05). The proximate compositions of different feed ingredients are presented in Fig. S1, and the isocaloric and isonitrogenous formulations of each diet are presented in Table S1. The moisture contents of the mustard oil cake, rice polish and fish meal were 8.20, 13.21 and 7.64 % respectively, and the gross energy (kJ/g) values were 17.82, 9.91 and 16.40 respectively. The dry-matter based average percent crude protein and the crude lipid values were 39.80 and 9.07 respectively (Table S2). The scanning electron micrographs revealed the lyophilized whole yeast (Fig. S2a) as ellipsoidal structure, and the live yeast in the pellet (Fig. S2b) was smooth-walled, spherical, off-white color with an average diameter of 2.5 μm.
The water quality during the 60-day feeding trial is presented in Table S3. The parameters analyzed neither followed any specific trend nor differed appreciably between the treatments, attributable to the continuous-flow controlled conditions and the isocaloric feeds used. The values for various parameters ranged from: pH 7.42 ± 0.216–7.60 ± 0.215, total alkalinity 126.28 ± 1.723–138.17 ± 1.211 ppm, dissolved oxygen 4.90 ± 0.334–5.31 ± 0.291 ppm, and the total ammonium 0.290 ± 0.020–0.372 ± 0.031 ppm. The average temperature was 32.000 ± 1.225 °C.
A maximum weight gain (Table S4) of 6.04 ± 0.013 g was obtained in L3-fed fish followed by L2 (5.54 ± 0.002 g), L1 (5.23 ± 0.026 g) and LC (4.73 ± 0.012 g). There were significant and consistent growth variations among the feeds, with a better (lower) FCR in L3-fed fish (2.37 ± 0.010). It was observed that the highest SGR of 2.14 ± 0.003 was obtained in L3-fed fish showing a better nutrient utilization. The same feed also registered a maximum PER of 1.41 ± 0.006 (Table S5), indicating that the protein was utilized better for growth and metabolism.
Comparing the initial and final carcass composition vis-à-vis various feeds revealed an increment in the final carcass protein and lipid contents (Table S5). The maximum percent carcass protein (68.95 ± 0.050) and lipid (17.95 ± 0.020) increase were recorded in L3-fed fish, while the lowest was in LC. It was observed that the protein and lipid levels increased with an increased probiotic supplementation, indicating that probiotic supplementation enhanced the carcass quality. This is further corroborated by the better gut enzyme activities (Table S6). Intestinal protease and α-amylase activities increased significantly with increasing probiotics concentration. Appreciably high protease and α-amylase activities (24.97 ± 0.016 and 11.02 ± 0.025, respectively) were recorded in L3, while they were the lowest (20.30 ± 0.068 and 10.05 ± 0.045, respectively) in the control. L3 also registered the maximum GPT (59.36 ± 0.096) and GOT (64.60 ± 0.258) values, whereas the lowest (14.16 ± 0.026 and 40.85 ± 0.163, respectively) were in the control (Table S6). Further, increasing from LC through L3 over the initial values (1.32 ± 0.013), the maximum RNA:DNA ratio (2.15 ± 0.016) was recorded in L3, the least (1.68 ± 0.024) being in LC (p ≤ 0.05; Fig. S3).
The hematology results before and after the challenge trial are presented in Table S7. The highest total erythrocyte count (TEC), total leukocyte count (TLC), hemoglobin (Hb) and hemoglobin concentration (Hct), and the lowest mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC) were observed in L3, whereas the lowest TEC, TLC, Hb, Hct and the highest MCV, MCH and MCHC were observed in the control. Hematological values showed a decrement after the challenge trial, although the discernible trends were similar. The effect of the different feeding treatments on the RNA:DNA and the albumin:globulin ratios are presented in Figs. S3 and S4, respectively. The maximum albumin:globulin ratio (1.462 ± 0.006) was recorded in the control and the lowest (1.215 ± 0.011) in L3, a reverse trend than the RNA:DNA ratio.
The results of the nonspecific immunity assay are presented in Table S8. Significant variations in the phagocytic ratio (42.00 ± 2.11), phagocytic index (2.51 ± 0.07) and leucocratic values (60.50 ± 0.35) were recorded in L3. The NBT positive cells were the maximum in L3 and the least in LC. A significantly low bactericidal activity (4.26 ± 0.06 × 103 CFUs/ml) was recorded in L3 compared to the control (7.86 ± 0.08 × 103 CFUs/ml). A maximum of 96.66 % survival was recorded in the challenge trial (L3), followed by 73.33 % in L2, 60 % in L1, and 30 % in LC (Table S9). The ANOVA between the treatments and the days revealed significant differences in the survival. Multiple correlation revealed that the ADG was positively correlated with the carcass CP (p ≤ 0.05), GPT (p ≤ 0.05), GOT (p ≤ 0.01), leucocrit value (p ≤ 0.05), NBT (p ≤ 0.01) and the survival (p ≤ 0.05), but negatively correlated with albumin:globulin ratio (p ≤ 0.05), bactericidal activity (p ≤ 0.01), and FCR (p ≤ 0.05). The enzymatic activities correlated positively with the different growth indices, viz., carcass CP and CL.
Discussion
Probiotics have demonstrated several useful roles in aquaculture [1, 3, 18–20], also from India [21] and China [22] perspectives, including competitive exclusion of pathogenic bacteria [23–25], improvement of water quality [1, 2, 26], enhancement of the immune response of the host species [26–30], and improved nutrition assimilability of the host by producing supplemental digestive enzymes [31]. Thus, probiotics help reduce the use of antimicrobial chemical compounds while improving the appetite and/or growth performance of this farmed aquaculture species. Probiotics are demonstrably beneficial in rearing of fish juvenile [28, 31], and shellfish [32, 33]. Navarrete and Ramirez [3] have comprehensively discussed the inclusion of yeast in fish feed. Yeast reportedly modulates enzyme activities and gene expression [34]. Wang [31] recorded significant growth in carp larvae involving non-yeast based probiotic diets. Young common carp responded well registering better growth and survival [36].
MS222, also known as finquel, tricaine, tricaine methanesulfonate (TMS), 3-aminobenzoic acidethyl ester methanesulfonate and metacaine, overdose for anaesthetization is dependent on the water temperature and hardness, the fish species, its size and density. Induction (anaesthetization) and recovery times inversely correlate with the body weight, this being more pronounced in small fish. In general, anesthetic doses are usually between 25 and 100 mg/l and excessively long exposures at 50 mg/l or more may induce mortalities. A lethal dose of 400–500 mg/l is generally used for euthanasia of salmonids.
Probiotics improved the gut enzymatic activity and stimulated digestion resulting in better feed utilization and growth. Nutritional and health benefits to the juveniles is attributable to the detoxification of potentially harmful compounds in feeds, denaturation of potentially indigestible component in the diet by hydrolytic enzymes like amylases and proteases, production of vitamins like biotin and B12, production of antagonistic compounds and stimulation of the host immunity [18, 23, 30]. Given that probiotics are microbial cells, it is appropriate to consider the whole cells as well as the products hitherto regarded as immunostimulants for their safety standards [35]. Many studies have utilized the whole microbial cell components as immunostimulants against pathogens [27–30]. Immunostimulants augment the 2nd (phagocytosis) and 3rd (antibody production) levels of host defense mechanism, and also boost the chemiluminescent response and superoxide anion production. Probiotic feeding reportedly stimulated the cellular rather than the humoral immunity, by increasing the number of erythrocytes, macrophages, lymphocytes and also enhancing the lysozyme activity, and thus, behaving as a simili to oral vaccines.
Blood, as a fluid connective tissue, is a recommended pathophysiological reflector. Thus, the hematological parameters are important in diagnosing the health status. In the present study, it was observed that L3-fed fish showed a superior hematological status compared to other diets, indicating not only the positive impact of the feed but also demonstrating the reliability of blood parameters as physiological reflection [30]. Although all the feeds were isonitrogenous, L3 diet performed better in terms of the weight gain percentage and the specific growth rate. A better carcass composition and lesser nitrogen excretion in L3 are attributable to the optimal probiotic concentration. Therefore, a probiotic concentration of 1.0 % in juvenile fish feed might be recommended for an optimal dietary utilization, and overall wellbeing.
The RNA:DNA is a dependable indicator of the growth trend, and a higher RNA:DNA ratio indicates better growth. In the present study, this ratio was the greatest in L3 diet which also demonstrated the highest dietary utilization and growth. The same feed also reflected relatively high GOT and GPT levels, attributable to a better dietary protein utilization. Most di- and oligo-peptides of protein catabolism undergo transamination by the cytosol and the mitochondrial transaminases, the activity being induced by proteinaceous diets. The present study demonstrated a positive correlation between the probiotic concentration, and the GOT and the GPT levels.
The results support the use of yeast for growth, nutrient utilization, and also as a good immunostimulant in L. rohita juveniles. Yeast ingestion seemed to enhance the non-specific immune response, the activation mechanism involved being attributable to the yeast cell wall carbohydrates. Whole yeast added to the feed provided better disease-resistance by stimulating phagocytic function, immunoglobulin production and increased protection as evidenced from the pathogen challenge. Yeast supplementation reportedly improved the immune response in IMC [8]. Evidence is growing that the previously considered as nutritionally nonessential nucleic acids from yeast sources are essential for mammalian immunity. Thus, not only the sugars but also the nucleic acids, particularly the yeast RNA, might act as immune activators. Ortumo et al. [7] reported a similar study using lyophilized S. cerevisiae in gilt-head seabream.
The results, thus, provide evidence that the whole cell S. cerevisiae supplementation to the formulated IMC feed exhibited better growth and dietary utilization, and enhanced nutrient quality, immune response and survival. The positive results of the challenge trial indicate the development of a wide-spectrum disease resistance owing to the ubiquitous presence and the opportunistic pathogenesis of Aeromonas. An optimal dosage of 1.0 g/kg feed, and the administration time in the feed, may be critical to validate the performance. Further study on the biochemistry, the intra-peritoneal attachment and the mode of action of the probiotic, either alone or in combination with a prebiotic, in aquaculture is suggested.
Notes
All the values are provided on per liter basis; the final pH was adjusted to 5.5.
References
Zhang X, Shu M, Wang Y, Fu L, Li W, Deng B, Liang Q, Shen W (2014) Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J Microbiol Biotechnol 30:2523–2531. doi:10.1007/s11274-014-1677-1
Mishra S, Pattnaik P, Mohanty S, Ayyappan S (2001) Probiotics: possible applications in aquaculture. Fish Chimes 21:31–37
Navarrete P, Tovar-Ramírez D (2014) Use of yeasts as probiotics in fish aquaculture. In: Hernandez-Vergara MP, Perez-Rostro CI (ed) Sustainable aquaculture techniques. INTECH Open Science, pp 135–172. doi:10.5772/57196
Takada Y, Nishino Y, Ito C, Watanabe H, Kanzaki K, Tachibana T, Azuma M (2014) Isolation and characterization of baker’s yeast capable of strongly activating a macrophage. FEMS Yeast Res 14:261–269. doi:10.1111/1567-1364.12098
Perez-Sanchez T, Ruiz-Zarzuela I, de Blas I, Balcazar JL (2013) Probiotics in aquaculture: a current assessment. Rev Aquac. doi:10.1111/raq.1203
Rozita K, Shila S, Mahdy C (2013) Effect whole and cell wall of Saccharomyces cerevisiae in immunity factors on rainbow trout (Oncorhynchus mykiss). American-Eurasian J Agric Environ Sci 13:633–638. doi:10.5829/idosi.aejaes.2013.13.05.224
Ortuno J, Cuesta A, Rodriguez A, Esteban MA, Meseguer J (2002) Oral administration of yeast Saccharomyces cerevisiae enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet Immunol Immunopathol 85:41–50. doi:10.1016/S0165-2427(01)00406-8
Mari S, Samy L, Jagruthi C, Anbazahan SM, Yogeshwari G, Thirumurugan R, Arockiaraj J, Mariappan P, Balasundaram C, Harikrishnan R (2014) Protective effect of chitin and chitosan enriched diets on immunity and disease resistance in Cirrhina mrigala against Aphanomyces invadans. Fish Shellfish Immunol 39:378–385. doi:10.1016/j.fsi.2014.05.027
Pooramini M, Kamali A, Hajimoradloo A, Alizadeh M, Ghorbani R, Hatami R, Haghparast S (2014) The effects of different concentrations of probiotic Saccharomyces cerevisiae on growth performance and survival rate of rainbow trout (Oncorhynchus mykiss), fry and resistance against salinity. Afr J Biotechnol 13:1160–1168. doi:10.5897/AJB2013.12214
Josue SP, Knauth P, Zaira L, Orfil GR, Ester MRM, Victor GA, Monique L, Rosa AUB (2014) Differences in the amount of β-glucan and mannan in strains of Saccharomyces cerevisiae and Meyerozyma guilliermondii isolated from agave must used in tequila production. Microbiol Res Int 2:1–8, ISSN: 2354-2128
Sych G, Frost P, Irnazarow I (2013) Influence of β-glucan (Macrogard®) on innate immunity of carp fry. Bull Vet Inst Pulawy 57:219–223. doi:10.2478/bvip-2013-0039
De BC, Meena DK, Behera BK, Das P, Mohapatra PD, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40:921–971. doi:10.1007/s10695-013-9897-0
El-Zaeem SY, Amer TN, El-Tawil NE (2014) Evaluation of the productive performance characteristics of red tilapia (Oreochromis sp.) injected with shark DNA into skeletal muscles and maintained diets containing different levels of probiotic and amino yeast. Afr J Biotechnol 11:7286–7293. doi:10.1016/j.ijmm.2009.08.005
APHA (2005) Standard methods for the examination of water and wastewater. 21st Ed. Am Public Health Assoc, Washington DC, www.standardmethods.org, ISBN:0875530478
Nandeesha MC, Sentilkumar V, Antony Jesu Prabhu P (2013) Feed management of major carps in India, with special reference to practices adopted in Tamil Nadu. In: Hasan MR, New MB (eds) On-farm feeding and feed management in aquaculture. FAO Fisheries and Aquaculture Technical Paper No. 583, Rome, FAO, E-ISBN:978-92-5-107979-9, pp 433–462
AOAC (2000) Official methods of analysis. 17th Ed. Association of Official Analytical Chemists, Gaithersburg, Maryland, USA, ISBN:0935584676/9780935584677
Lavanya S, Ramesh M, Kavitha C, Malarvizhi A (2011) Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 82:977–985. doi:10.1016/j.chemosphere.2010.10.071
Oelschlaeger TA (2010) Mechanisms of probiotic actions: a review. Int J Med Microbiol 300:57–62. doi:10.1016/j.ijmm.2009.08.005
Wang Y, Li J, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4. doi:10.1016/j.aquaculture.2008.06.002
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14. doi:10.1016/j.aquaculture.2007.11.019
Sahu MK, Swarnakumar NS, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Indian J Microbiol 48:299–308. doi:10.1007/s12088-008-0024-3
Qi Z, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China—current state, problems and prospect. Aquaculture 290:15–21. doi:10.1016/j.aquaculture.2009.02.012
Balcazar JL, Vendrell D, Blas I, Ruiz-Zarzuela I, Girones O, Muzquiz JL (2007) In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet Microbiol 122:373–380. doi:10.1016/j.vetmic.2007.01.023
Denev S, Staykov Y, Moutafchieva R, Beev G (2009) Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture, 1:1–29 Int Aquat Res, ISSN: 2008-4935
Aly SM, Abd-El-Rahman AM, John G, Mohamed MF (2008) Characterization of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture 277:1–6. doi:10.1016/j.aquaculture.2008.02.021
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36:501–509. doi:10.1007/s10695-009-9320-z
Gomez GD, Balcazar JL (2008) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154. doi:10.1111/j.1574-695X.2007.00343.x
Harikrishnan R, Balasundaramb C, Heo MS (2010) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29:868–874. doi:10.1016/j.fsi.2010.07.031
Kim DH, Austin B (2006) Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol 21:513–524. doi:10.1016/j.fsi.2006.02.007
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14. doi:10.1016/j.fsi.2010.02.017
Wang Y (2011) Use of probiotics Bacillus coagulans, Rhodopseudomonas palustris and Lactobacillus acidophilus as growth promoters in grass carp (Ctenopharyngodon idella) fingerlings. Aquac Nutr 17:372–378. doi:10.1111/j.1365-2095.2010.00771.x
Zhou X, Wang Y, Li W (2009) Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 287:349–353. doi:10.1016/j.aquaculture.2008.10.046
Wang Y, Gu Q (2010) Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Mar Biol Res 6:327–332. doi:10.1080/17451000903300893
Tovar-Ramírez D, Mazurais D, Gatesoupe JF, Quazuguel P, Cahu CL, Zambonino-Infante JL (2010) Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 300:142–147. doi:10.1016/j.aquaculture.2009.12.015
Zhou X, Wang Y (2012) Probiotics in aquaculture–benefits to the health, technological applications and safety. In: Carvalho ED, David GS, Silva RJ (eds) Health and environment in aquaculture. InTech, ISBN: 978-953-51-0497-1, pp 1–13. doi: 10.5772/29037
Wang Y, Xu Z (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 127:283–292. doi:10.1016/j.anifeedsci.2005.09.003
Acknowledgments
In terms of materials and facilities, the support and cooperation received from the CIFA, Bhubaneswar for the various wet-lab analyses, the IMMT, Bhubaneswar for the scanning electron microscopy facility, and the IMTECH, Chandigarh for the pathogenic strain used in the study are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Partha Bandyopadhyay and Snehasish Mishra: Co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bandyopadhyay, P., Mishra, S., Sarkar, B. et al. Dietary Saccharomyces cerevisiae Boosts Growth and Immunity of IMC Labeo rohita (Ham.) Juveniles. Indian J Microbiol 55, 81–87 (2015). https://doi.org/10.1007/s12088-014-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-014-0500-x