Abstract
Our trial was performed to investigate the effect of fully fermented yeast Sacharomyces cerevisiae (Hilyses, ICC Company, Brazil) on the growth performance and immune response of Oreochromis niloticus. In this study, a total of 270 O. niloticus (50.7 ± 0.8 g) were randomly divided into 3 groups in triplicates. The control group was fed on the basal diet while the other two groups were fed on a basal diet supplemented with 0.2% and 0.4% of Hilyses. The trial period extended for 2 months. At the end of the feeding trial, oxidant and antioxidant parameters (MDA, catalase, and glutathione reductase), some innate immunological parameters and immune-related gene expression were measured. Histological examination of liver, spleen, kidney, and intestine was performed. Further, fish groups were challenged against Gram-negative and Gram-positive bacteria; A. hydrophila and L. garvieae. The results revealed significant improvement (p < 0.05) in growth performance and feed utilization in Hilyses-treated groups versus the control group. Blood parameters and liver and kidney functions of Hilyses-supplemented groups were similar to those of the control group. The histological findings of treated groups showed normal tissue structure with multiple focal lymphoid aggregations in the spleen, kidney, and intestine. Both levels of Hilyses successfully enhanced phagocytic activity/index, lysozyme activity, and gene expression of TNF-α, and IL-1β. Fish group fed on 0.4% Hilyses exhibited the highest expression of IL-1β and the least mortality percentages post challenges. Thus, dietary supplementation of Hilyses could promote the growth performance and immunity and increase the resistance of O. niloticus against diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of functional feed additives such as probiotics, prebiotics, synbiotics, herbal extracts, immunostimulants, nucleotides, and acidifiers has great impact on the global production of the commercial aquaculture all over the world (Naylor et al. 2000; Oliva-Teles 2012; Moffitt and Cajas-Cano 2014; Kobayashi et al. 2015; Abu-Elala and Ragaa 2015). Apart from boosting aquafeeds and safeguarding general health of aquatic animals, they have been found to possess beneficial immunostimulants and anti-stress relieving properties (Dawood et al. 2017). Among these functional feed additives, Saccharomyces cerevisiae yeast has been widely used (Scholz et al. 1999; Abu-Elala et al. 2013). It has been incorporated in aqua-feed in two forms: living whole yeast (single-cell protein, SCP), which has been proposed as probiotic, and the processed form (yeast extract), which has been proposed as prebiotics and/or immunostimulants, (Abu-Elala et al. 2013; Abu-Elala et al. 2018).
Hilyses® (ICC Brazil Company) is pure S. cerevisiae yeast resulting from the sugarcane fermentation process. It undergoes rigorous autolysis and enzymatic hydrolysis for releasing of intracellular content and breaking the RNA chain into nucleotides and nucleosides. It is also composed of the yeast cell wall that has a high amount of bioactive compounds, β-glucans which are successfully enhancing the resistance of fish and crustacean against bacterial and viral infections by improving the response capacity of innate immune system (Scholz et al. 1999; Sakai 1999; Chang et al. 2003; Li and Gatlin 2004; Citarasu et al. 2006; Lin et al. 2011), and mannan-oligosaccharides (MOS) that are known for their pathogen agglutination capacity, preventing the bacterial colonization on the intestinal villi, and these agglutinated bacteria will be excreted with the indigestible food (Klis et al. 2006; Ortuno et al. 2007).
In addition, it is also composed of a high amount of short chain poly peptides, free amino acids especially the glutamic acid and free nucleotides which are known to improve the gut health and increase the food palatability. Nucleotides are the building block of cell’s RNA, involved in all cellular processes (Lehninger et al. 2005). They synthesized de novo in most tissues, but some tissues such as intestinal cells, hepatopancrease, hemolymph cells, and immune system have limited capacity for synthesis and need exogenous supply. The body requirements of exogenous nucleotides may increase under certain conditions, e.g., tissue injury, liver dysfunction, under disease or stress, and in fast-growth life stage. Previous studies have been recorded that dietary inclusion of NTs provides the immune system with substrates and cofactors necessary to work probably (Lin et al. 2009; Murthy et al. 2009), up-regulates several immune-related genes (Murthy et al. 2009; Cheng et al. 2011), and increases the resistance to infectious diseases and the efficacy of vaccination in salmonids (Burrells et al. 2001a, b) as well as compensates the shortage of nucleotides in food rich with plant protein (Zhang et al. 2012).
However, the cell wall fraction is rich in bioactive and immunostimulant compounds like β-glucans and mannan oligosaccharides. Hence, it seems likely that the whole yeast biomass, after rupturing of cell walls, may be the most attractive feed ingredient, by combining the properties as a source of nutrients and bioactive components. There is little information concerning the potential of fully fermented S. cerevisiae on tilapia culture. Therefore, this feeding trial was conducted to determine the effects of Hilyses® (ICC Brazil), fully fermented yeast, rich with nucleotides, β-glucans, mannanoligosaccharides and essential amino acids on the growth performance, antioxidant, immune response, and disease resistance in cultured O. niloticus during grow-out stage.
Material and methods
Fish and experimental diet
The experiment was carried out in Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Cairo University, Egypt. A total of (270) Nile tilapia with an average weight 50.7 ± 0.8 g, were obtained from a commercial supplier Kafr el-Shiekh, Egypt. Fish randomly divided into 3 groups with three replicates of 30 fish per replicate, representing three nutritional groups. Fish fed on a basal diet represented the control group, while the other two experimental groups were fed on basal diet supplemented with the tested additive (Hilyses Brazil Company) at 0.2% and 0.4%, respectively. Hilyses is a commercial fermented yeast product rich with 63 g/kg nucleotides, 235 g/kg β-glucan, and 142 g/kg MOS (ICC company, Brazil). All diets were formulated from practical ingredients to be iso-nitrogenous (280 g kg−1) and iso-energetic (4100 kcal kg−1 gross energy) to satisfy the nutrient requirements of O. niloticus (NRC 2011) (Table 1). The diets were prepared by individually weighing each component and thoroughly mixing the minerals, vitamins (premix), and additives with corn. Hilyses was mixed thoroughly in the stated quantities with a small amount of feed. Dry ingredients were homogenized in a horizontal drum mixer; oil and water were added subsequently to the mixer and then portioned into smaller batches. Gelatin dissolved into hot water was added as a binder to each batch. The resulting dough was pressed through a single-screw meat grinder with 3-mm die and dried in an oven at 50 °C for 12 h. Dried strands were broken into 3-mm pellets and kept frozen at − 20 °C until feed.
The experimental fish were fed the diets at a rate of 3% of total body weight according to NRC 2011, two times/day (8:00 am and 4:00 pm) for 60 days and weighed biweekly to adjust the daily requirements. Each 50-L tank was supplied with dechlorinated water and was equipped with air supply, heater with thermostat, and LED light. Tanks were monitored daily for temperature (26 ± 1 °C), dissolved oxygen ˃ 6 mg/L and every 2 days for ammonia 0.12–0.23 mg/l, and pH 7.2 ± 0.5. The present study was performed in compliance with the Guide for the Care and Use of Laboratory Animals approved by the Institutional Animal Use and Care Committee (IACUC), Cairo University, Giza, Egypt.
Growth performance and feed utilization
The body weight of fish in each group was recorded on an individual basis every 2 weeks to adjust the daily requirements of food. After 60 days, the growth and feed utilization parameters, including body weight gain, total feed intake, feed conversion ratio, specific growth rate, and protein efficacy ratio, were calculated (Abu-Elala et al. 2018).
Haemato-immunological tests
At the termination of the feeding trial, fish (5 fish/replicate) were euthanized with MS-222 10 mgL-1. Heparinized blood samples were used to determine RBCs, WBCs count, haemoglobin concentration, and phagocytic activity test (Abu-Elala and Ragaa 2015). The non-heparinized blood samples were centrifuged at 3000 rpm for 15 min to separate serum. Sera were used for determination of total protein, albumin, and globulin, serum transaminases ALT and AST (Reitman and Frankel 1957), creatinine (Husdan and Rapoport 1968), blood urea nitrogen (BUN) (Fawcett and Scott 1960), and lysozyme activity test (Abu-Elala and Ragaa 2015).
Evaluation of oxidant and antioxidant biomarkers
For evaluation of oxidative damage, 10% of liver tissue were homogenized in cold phosphate buffer saline (pH 7.4) using a homogenizer. Then the homogenates were centrifuged at 4000 rpm for 15 min. The supernatants were used for measuring of total protein of tissue homogenate (Bradford 1976), malondialdehyde (MDA) (Albro et al. 1986), Catalase (Aebi 1984), and glutathione reduced (GSH) (Goldberg and Spooner 1983).
Quantitative real-time polymerase chain reaction (qRT-PCR) of immune response genes (IL-1β and TNF-α)
Total RNA was extracted from liver tissue by GF-1 Total RNA Extraction Kit according to the manufacturer instructions. The concentration and purity of the total RNA samples were obtained using a Nanodrop ND-1000 spectrophotometer. c-DNA synthesis was obtained by using reverse transcriptase (Fermentas, EU). Real-time PCR (qPCR), PCR amplification and analyses were carried out according to Abu-Elala et al. (2018). The expression relative to the control was calculated using the equation 2−∆∆CT(Livak and Schmittgen 2001).
Histological examination
Tissue specimens (liver, kidney, spleen, and intestine) from different fish groups were taken and fixed in 10% neutral formalin for 2 h. Washing was done in tap water then in serial dilutions of alcohol (methyl, ethyl, and absolute ethyl) for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 °C in a hot-air oven for 24 h. Paraffin bees wax tissue blocks were prepared for sectioning at 4-μm thickness by sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by haematoxylin and eosin stain for examination through the light electric microscope (Bancroft and Gamble 2008).
Challenge test
At the end of the feeding trial, two challenge tests were carried out using virulent strains of Lactococcus garvieae (Abu-Elala et al. 2019) and Aeromonas hydrophila (Abu-Elala and Ragaa 2015) (ccession no. LCO12339). The strains were grown in trypticase soya broth (TSB, Difco, USA) incubated at 25 °C for 24 h. The challenge test was performed according to Abu-Elala et al. (2018).
Ten fish/group replicates were inoculated intra-peritoneal with 0.2 ml 3 × 108 CFU/ml L. garvieae and another group was inoculated intra-peritoneal with 0.2 ml 1.5 × 107 CFU/ml A. hydrophila. For each microbial challenge test, ten fish were injected with a sterile broth medium (control negative). The cumulative mortality% for each of the experimental groups was recorded up to the 7th day post challenge.
Statistical analysis
The obtained data were calculated and statistically analysed using SPSS software version 21 for Windows. The differences between groups were determined with variance analysis (one-way ANOVA) using the probability level of 0.05 for the rejection of the null hypothesis. Significant differences among means were determined by turkey multiple range tests. Data were expressed as means ± SEM.
Results
Growth performance and feed utilization
The influence of dietary supplementation of Hilyses on the growth performance and feed utilization of O. niloticus are summarized in Table 2. At the end of the feeding trial, fish groups fed on 0.2% and 0.4% Hilyses revealed significant (p ≤ 0.05) increase in the live body weight and specific growth rate with respect to the control group. The result of feed utilization showed a significant improvement in the FCR and PER in Hilyses-supplemented groups compared to control one. Both levels of Hilyses showed no difference in the growth performance parameters.
Haematological findings
Data of serum biochemical parameters at the end of the experiment for the different experimental groups were clarified in Table 3; results showed that Hilyses-supplemented groups had a non-significant difference (p ≤ 0.05) in levels of RBCs, WBCs, HB, GPT, GOT, creatinine, and BUN as compared to the control groups.
Oxidant and antioxidant biomarkers
MDA, an oxidant biomarker, had shown a non-significant difference (p ≤ 0.05) in all experimental groups. However, there was a significant increase in catalase and reduced glutathione levels in both Hilyses-supplemented groups versus the control group (Table 4).
Innate immunological parameters
Regarding the innate immunological parameters, fish groups fed on 0.2% and 0.4% Hilyses-supplemented diet showed a significant (p ≤ 0.05) increase in phagocytic activity, phagocytic index and lysozyme activity versus fish fed on a basal diet (Fig. 1).
Dietary inclusion of Hilyses® in O. niloticus diet showed significant increase in phagocytic activity (a), phagocytic index (c), and lysozyme activity (d). Vertical bars represented the mean ± SE. Asterisk indicates significant difference among the groups (p ≤ 0.05) according to one-way ANOVA followed by Tukey’s test. b Phagocytic cells of 0.4% Hilyses® supplemented group engulfed more than one Saccharomyces cerevisiae yeast cell (Giemsa stain × 1000)
Relative quantitative PCR of immune gene expression
Adding of 0.4% of Hilyses to fish diet elicited a significant increase in pro-inflammatory cytokines and immune-related gene (TNFα and IL1β) (p ≤ 0.5) compared to 0.2% supplemented groups, especially in the expression of IL1β (Fig. 2).
Real-time PCR of mRNA levels of TNF-α and IL-1β genes of the liver tissue of fish groups fed on basal diet, 0.2% and 0.4% Hilyses®. a Evaluation of TNF-α and 726 IL-1β genes expression in 0.2% Hiylses, 0.4% Hiylses compared with the control group. b Electrophoretic mobility of quantitative RT-PCR products of TNF-α, IL-1β, and GAPDH (internal control) genes on 2% agarose gel. Lane 1 = control; lane 2 = 0.2% Hiylses, and lane 3 = 0.4% Hiylses. The data were normalized to an endogenous reference, GAPDH and expressed as relative to control. Vertical bars represented the mean ± SE (n = 5). Asterisk indicates significant difference at (p ≤ 0.05)
Histological findings
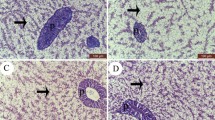
The control group showed normal tissue structures of liver, kidney, spleen, and intestine. Liver of fish groups fed on Hilyses at 0.2–0.4% inclusions showed greater vacuolization of the hepatocytes with no tissue alteration in the liver, indicating a well-nourished fish. In addition, kidneys of fish fed on a diet supplemented with 0.4% Hilyses, showed mild vacuolization in the tubular lining epithelium with a focal lymphoid cell aggregation between tubules. The spleen showed focal wide areas of melanin-pigmented cells with aggregations in the white pulps. Intestinal tissues showed mild hypertrophy in the lining mucosal epithelium with a wide cryptic area and focal lymphoid cell proliferation in the mucosa and submucosa (Fig. 3).
a Spleen of O. niloticus fed Hilyses showing wide focal areas of melanomacrophage aggregation in the white pulps (H&E × 200). b Intestine of Hilyses-treated group shows mild hypertrophy in the lining mucosal epithelium with wide area for the crypts and long intestinal villi. Focal lymphoid cells proliferation was detected in the mucosa and sub mucosa (H&E × 200). The two pictures were compared with tissues of the control group on the left side
Challenge test
Fish groups fed on Hilyses-supplemented diet exhibited significant immunological resistance against experimental infection with L. garvieae. Fish groups fed on 0.4% and 0.2% Hilyses-supplemented diet showed 30% and 47% mortalities, respectively, and 80% mortality in the control group (Figure 3a). The moribund fish showed nervous signs, eye opacity, and hemorrhagic unilateral or bilateral exophthalmia (Fig. 4b, c). Regarding, the experimental infection with A. hydrophila, the fish group fed on basal diet showed 97% mortality while groups fed on 0.2% and 0.4% Hilyses showed 53% and 43% mortalities, respectively (Fig 5a). Moribund fish showed external haemorrhages, skin ulcer, and abdominal distention (Fig 5b, c).
Discussion
Research on micronutrients and immunostimulants in aqua-feed is needed to provide insights concerning the interactions between nutrition and physiological responses and offer solutions that could reduce risks from infectious diseases (Li and Gatlin 2004).
Dietary inclusion of 0.2 and 0.4% Hilyses showed significant increases in growth and feed utilization of O. niloticus treated groups. A similar result was observed in rainbow trout (Oncorhynchus mykiss) fed on fermented yeast (5 g kg−1) for 50 days (Sheikhzadeh et al. 2012). This may be due to a number of chemicals and bioactive compounds present in Hilyses like free amino acids (glutamic acid), NTs and MOS. They have been known to increase the food palatability, improve the digestibility and keep the gut health. Previous literatures reported that NTs have a significant role in the stimulation, proliferation, and maturation of gastrointestinal tract and have growth-promoting effects in juvenile red drum (Scianops ocellatus), Asian carp (Catla catla), barramundi (Lates calcarifer), Cobia (Rachycentron canadum), grouper (Epinephelus malabaricus), white shrimp, black tiger shrimp, rainbow trout, and Atlantic salmon (Carver and Walker 1995; Burrells et al. 2001b; Li and Gatlin 2004; Lin et al. 2009; Li et al. 2007a, b; Dimitroglou et al. 2010; Sauer et al. 2011; Cheng et al. 2011; Huu et al. 2012; Ringo et al. 2012; Peng et al. 2013). In consent with our results, Staykov et al. (2007), Torrecillas et al. (2007), Grisdale-Helland et al. (2008), and Dimitroglou et al. (2010) recorded that dietary MOS increases the weight gain in rainbow trout, sea bass, Atlantic salmon, and gilthead sea bream and affects the gut health by reducing pathogen adsorption and eliminating them out of the gut (Newman 2007). Almost all studies on glucans approved their immune-stimulatory effect, while the growth-enhancing effect was disputable. Misra et al. (2006) and Huu et al. (2016) reported the growth promoting effect of dietary glucans in Labeo rohita fingerlings and Pampano fish. However, Sang and Fotedar (2010) observed no significant effect on the growth of sea bass. To date, the exact mechanism of how dietary β-glucans work to enhance growth is unknown. One of the speculations is that local intestinal inflammatory response produced by dietary glucans could stimulate the proliferation of beneficial gut flora and get rid of the pathogenic ones (Aramli et al. 2015). Moreover, the degradation of β-glucan in the digestive glands by glucanases to produce energy permits the use of more proteins for growth (Lopez et al. 2003).
Although haematological parameters were claimed to provide vital information for monitoring fish health and nutritional status, the present study showed no significant differences in haematological parameters with Hilyses supplementation at 0.2 and 0.4%. Yet, the results of serum biochemical parameters indicated that dietary supplementation of Hilyses has enhanced growth performance without apparent impairment of fish health status. Similar findings were recorded by Heidarieh et al. (2013).
Our results revealed that dietary inclusion of Hilyses for 60 days increased phagocytic activity and index, lysozyme activity, and peroxidases and up-regulated the immune-related genes (TNF-α and IL-1β). Furthermore, O. niloticus fed on 0.4% Hilyses showed lower cumulative mortality after challenging with L. garvieae and A. hydrophila compared to 0.2% Hilyses and control groups. Similar results have been reported by Choudhury et al. (2005) who stated that 0.4% dietary yeast inclusion improved blood innate immune capacity and RPS after challenge with A. hydrophila in rohu (Labeo rohita) juveniles. These may be due to the high amount of β-glucan, nucleotides and mannan-oligosaccharides in Hilyses-treated diet. These bioactive immunostimulant compounds have been previously investigated in several literatures alone or in combination (Gu et al.2011; Abu-Elala et al. 2018).
The most powerful immunostimulant compounds in this product are β-glucans which are known to enhance the survivability of fish, most likely via stimulation of both innate and adaptive immune reactions (Welker et al. 2007; Sealey et al. 2008; Rodríguez et al. 2009; Pionnier et al. 2013; Przybylska-Diaz et al. 2013; Ringø et al. 2018). Dietary supplementation of glucans were reported to activate macrophages in trout and exhibited the highest killing ability of Aeromonas salmonicida (Jørgensen et al. 1993), lower the attachment of sea lice to fish and reduce mortalities caused by infectious salmon anaemia virus, viral hemorrhagic septicemia, and Piscirickettsia salmonis (Burrells et al. 2001a; Beaulaurier et al. 2012). Moreover, they also increase the cytokines, complement, lysozyme production, and antibody formation (Raa 1992; Løvoll et al. 2007). The effect of glucans on gene expression is rapid and doesn’t need long exposure; 45-min submersions in glucan each week caused enhanced gene expression of IL-1β, TNF-α, IL-6, IL-10, and TGF-β, sometimes even after first submersion (Verlhac et al. 1996). Carp treated with glucans for 15 days, followed by injection with Grass carp haemorrhage virus, showed elevated MX gene expression during early stages of infection (Kim et al. 2009). Also, SOD and CAT activities were higher in a fish group pre-treated with glucan than that infected and not pre-treated. Van der Marel et al. (2012) stated that common carp exposed to glucans revealed upregulation of β-defensive and mucin genes. On the other hand, dietary nucleotides have been reported to increase phagocytic activity of murine peritoneal macrophages, enhance T-cell-dependent antibody production, increase cytokines and interleukins, and reduce plasma cortisol and glucose levels, and increase resistance against diseases in various fish species (Sakai et al. 2001; Choudhury et al. 2005; Jha et al. 2007; Cheng et al. 2011). Several literatures reported that combination of glucans and mannan-oligosacchrides showed amelioration in immune activity and increases the immune-related gene expression in Nile tilapia (Selim and Reda 2015; Dawood et al. 2017; Abu-Elala et al. 2018). To conclude, dietary inclusion of fully fermented yeast S. cerevisiae after rupturing of the cell wall provides the fish with various nutrients and bioactive compounds, which improve the growth performance, immune response and increase the resistance of Nile tilapia against motile aeromonas septicaemia and lactococcosis.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Abu-Elala NM, Ragaa NM (2015) Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus. J Adv Res 6(4):621–629
Abu-Elala N, Marzouk M, Moustafa M (2013) Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int J Vet Sci Med 1:21–29
Abu-Elala NM, Younis NA, AbuBakr HO, Ragaa NM, Borges LL, Bonato MA (2018) Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egypt J Aquat Res 44(4):333–341
Abu-Elala NM, Samir A, Wasfy M, Elsayed M (2019) Efficacy of injectable and immersion vaccines against streptococcal infections in broodstock and offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 88:293–300
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Albro PW, Corbelt JT, Schroeder JL (1986) Application of the thiobarbiturate assay to the measurement of lipid peroxidation products in microsomes. Chem Biol Interact 86:185–194
Aramli MS, Kamangar B, Nazari RM (2015) Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol 47:606–610
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, Edinburgh
Beaulaurier J, Bickford N, Gregg JL, Grady CA, Gannam AL, Winton JR, Hershberger PK (2012) Susceptibility of Pacific herring to viral hemorrhagic septicemia is influenced by diet. J Aquat Anim Health 24(1):43–48
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burrells C, Williams PD, Forno PF (2001a) Dietary nucleotides: a novelsupplement in fish feeds 1. Effects on resistance to disease in salmonoids. Aquaculture 199:159–169
Burrells C, Williams PD, Southgate PJ, Wadsworth SL (2001b) Dietary nucleotides: a novel supplement in fish feeds 2. Effects on vaccination, saltwater transfer, growth rates and physiology of Atlantic salmon (Salmo salar L.). Aquaculture 199:171–184
Carver JD, Walker WA (1995) The role of nucleotides in human nutrition. J Nutr Biochem 6:58–72
Chang CF, Su MS, Chen HY, Liao IC (2003) Dietary β-1, 3-glucan effectively improves immunity and survival of Penaeus monodon challenged with white spot syndrome virus. Fish Shellfish immunol 15(4):297–310
Cheng Z, Buentello A, Gatlin DM (2011) Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish Shellfish Immunol 30:143
Choudhury D, Pal AK, Sahu NP, Kumar S, Das SS, Mukherjee SC (2005) Dietary yeast RNA supplementation reduces mortality by Aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shell fish Immunol 19:281–291
Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeusmonodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21(4):372–384
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S, Basuini MF, Hossain MS et al (2017) Dietary Supplementation of ß-glucan improve growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquacult Nutr 23:148–159
Dimitroglou A, Merrifield DL, Spring P, Sweetman J, Moate R, Davies SJ (2010) Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture. 300(1):182–188
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Goldberg DM , Spooner RJ (1983). In methods of enzymatic analysis. In: Bergmeyen HV (ed) 3rd edn. vol. 3, pp 258–265
Grisdale-Helland B, Helland SJ, Gatlin DM (2008) The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture. 283(1):163–167
Gu M, Ma H, Mai K, Zhang W, Bai N, Wang X (2011) Effects of dietary β-glucan, mannan oligosaccharide and their combinations on growth performance, immunity and resistance against Vibrio splendidus of sea cucumber, Apostichopus japonicus. Fish Shellfish Immunol 31(2):303–309
Heidarieh M, Mirvaghefi AR, Akbari M, Sheikhzadeh N, Kamyabi-Moghaddam Z, Askari H, Shahbazfar AA (2013) Evaluations of Hilyses™, fermented Saccharomyces cerevisiae, on rainbow trout (Oncorhynchus mykiss) growth performance, enzymatic activities and gastrointestinal structure. Aquac Nutr 19(3):343–348
Husdan H, Rapoport A (1968) Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem 14:222–238
Huu HD, Tabrett S, Hoffmann K, Köppel P, Lucas JS, Barnes AC (2012) Dietary nucleotides are semi-essential nutrients for optimal growth of black tiger shrimp (Penaeus monodon). Aquaculture 366(367):115–121
Huu HD, Sang HM, Thuy NTT (2016) Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol 54:402–410
Jha AK, Pal A, Sahu N, Kumar S, Mukherjee S (2007) Haemato-immunological responses to dietary yeast RNA, ω-3 fatty acid and β-carotene in Catla catla juveniles. Fish Shellfish Immunol. 23:917–927
Jørgensen JB, Sharp GJ, Secombes CJ, Robertsen B (1993) Effect of a yeast-cell-wall glucan on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immunol 3(4):267–277
Kim YS, Ke F, Zhang QY (2009) Effect of β-glucan on activity of antioxidant enzymes and Mx gene expression in virus infected grass carp. Fish Shellfish Immunol 27(2):336–340
Klis FM, Boorsma A, De Groot PWJ (2006) Cell wall construction in Saccharomyces cerevisiae. Yeast 23:185–202
Kobayashi M, Msangi S, Batka M, Vannuccini S, Dey MM, Anderson JL (2015) Fish to 2030: the role and opportunity for aquaculture. Aquac Econ Manag 19(3):282–300
Lehninger AL, Nelson DL, Cox MM (2005) Principles of Biochemistry, 4th edn. W.H. Freeman, New York
Li P, Gatlin DM III (2004) Dietary brewers yeast and the prebiotic Grobiotic AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops 9 M. saxatilis) to Streptococcus iniae infection. Aquaculture 231:445–456
Li P, Gatlin DM III, Neill WH (2007a) Dietary supplementation of a purifiednucleotide mixture transiently enhanced growth and feed utilization ofjuvenile red drum, Sciaenops ocellatus. J World Aquacult Soc 38:281–286
Li P, Lawrence A, Castille FL, Gatlin DM (2007b) Preliminary evaluation of a purified nucleotide mixture as dietary supplement for Pacific white shrimp (Litopenaeus vannamei). Aquacult Res 38:887–890
Lin H, Wang H, Shiau SY (2009) Dietary nucleotide supplementation enhances growth and immune responses of grouper, Epinephelus malabaricus. Aquacult Nutr 15:117–122
Lin S, Pan Y, Luo L, Luo L (2011) Effects of dietary β-1, 3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol 31(6):788–794
Livak KJ, Schmittgen HD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lopez NCG, Gaxiola G, Taboada G, Valenzuela M, Pascual C, Sánchez A, Rosas C (2003) Physiological, nutritional, and immunological role of dietary β-1,3-glucan and ascorbic acid 2-monophosphate in Litopenaeus vannamei juveniles. Aquaculture 224:223–243
Løvoll M, Fischer U, Mathisen GS, Bøgwald J, Ototake M, Dalmo RA (2007) The C3 subtypes are differentially regulated after immunostimulation in rainbow trout, but head kidney macrophages do not contribute to C3 transcription. Vet Immunol Immunopathol 117(3):284–295
Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 255:82–94
Moffitt CM, Cajas-Cano L (2014) Blue growth: the 2014 FAO state of world fisheries and aquaculture. Fisheries 39(11):552–553
Murthy HS, Li P, Lawrence AL, Gatlin DM (2009) Dietary b-glucan and nucleotide effects on growth, survival and immune responses of Pacific white shrimp, Litopenaeus vannamei. J Appl Aquacult 21:160–168
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Newman K (2007) Form follows function in picking MOS product. Feedstuffs 27
NRC (2011) Nutrient requirements of fish. National Academies Press, Washington, DC
Oliva-Teles A (2012) Nutrition and health of aquaculture fish. J Fish Dis 35(2):83–108
Ortuno A, Quesada M, Lopez-Claessens S, Castella J et al (2007) The role of wild boar (Sus scrofa) in the eco-epidemiology of R. slovaca in northeastern Spain. Vector Borne Zoonotic Dis 7:59–64
Peng M, Xu W, Ai Q, Mai K, Liufu Z, Zhang K (2013) Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybeam meal (Scophtalmus maximus L.). Aquaculture 392(395):51–58
Pionnier N, Falco A, Miest J, Frost P, Irnazarow I, Shrive A, Hoole D (2013) Dietary β-glucan stimulate complement and C-reactive protein acute phase responses in common carp (Cyprinus carpio) during an Aeromonas salmonicida infection. Fish Shellfish Immunol 34(3):819–831
Przybylska-Diaz DA, Schmidt JG, Vera-Jimenez NI, Steinhagen D, Nielsen ME (2013) β-glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.). Fish Shellfish Immunol 35(3):998–1006
Raa, J (1992). The use of immunostimulants to increase resistance of aquatic organism to microbial infections. Dis Asian Aquacult 39–50
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Ringo E, Olsen RE, Vecino JLG, Wadsworth S, Song SK (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Marine Sci Res Dev 1:104
Ringø E, Faggio C, Chitmanat C, Doan H, Mai NT, Jaturasitha S, Hoseinifar SH (2018) Effects of corncob derived xylooligosaccharide on innate immune response, disease resistance, and growth performance in Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 495(1):786–793
Rodríguez I, Chamorro R, Novoa B, Figueras A (2009) β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol 27(2):369–373
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172(1):63–92
Sakai M, Taniguchi K, Mamoto K, Ogawa H, Tabata M (2001) Immunostimulant effects of nucleotide isolated from yeast RNA on carp, Cyprinus carpio L. J Fish Dis 24:433–438
Sang HM, Fotedar R (2010) Effects of dietary β–1, 3–glucan on the growth, survival, physiological and immune response of marron, Cherax tenuimanus (smith, 1912). Fish Shellfish Immunol 28(5):957–960
Sauer N, Mosenthin R, Bauer E (2011) The role of dietary nucleotides in single stomached animals. Nutr Res Rev 24:46–59
Scholz U, Diaz GG, Ricque D, Suarez LC, Albores FV, Latchford J (1999) Enhancement of vibriosis resistance in juvenile Penaeus vannamei by supplementation of diets with different yeast products. Aquaculture 176(3):271–283
Sealey WM et al (2008) Evaluation of the ability of barley genotypes containing different amounts of β-glucan to alter growth and disease resistance of rainbow trout Oncorhynchus mykiss. Anim Feed Sci Technol 141(1):115–128
Selim KM, Reda RM (2015) Beta-glucans and mannan oligosaccharides enhance growth and immunity in Nile Tilapia. N Am J Aquac 77(1):22–30
Sheikhzadeh N, Heidarieh M, Pashaki AK, Nofouzi K, Farshbafi MA, Akbari M (2012) Hilyses®, fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 32(6):1083–1087
Staykov Y, Spring P, Denev S, Sweetman J (2007) Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquac Int 15(2):153–161
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Izquierdo MS (2007) Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol 23(5):969–981
Van der Marel M, Adamek M, Gonzalez SF, Frost P, Rombout JH, Wiegertjes GF, Steinhagen D (2012) Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol 32(3):494–501
Verlhac V, Gabaudan J, Obach A, Schüep W, Hole R (1996) Influence of dietary glucan and vitamin C on non-specific and specific immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 143(2):123–133
Welker TL et al (2007) Immune response and resistance to stress and Edwardsiella ictaluri challenge in channel catfish, Ictalurus punctatus, fed diets containing commercial whole-cell yeast or yeast subcomponents. J World Aquacult Soc 38(1):24–35
Zhang J, Liu Y, Tian L, Yang H, Liang G, Xu D (2012) Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 3:1027–1032
Author information
Authors and Affiliations
Contributions
NMA-E and NAY contributed to the design of the experimental study, growth performance, haematological, immune response evaluation, and challenge test. HOAB determined the oxidant and antioxidant parameters and molecular and gene expression study. NMR contributed to the experimental diet formulation. LLB and MAB supply the study with Hilyses® product. All authors read and revised the manuscript.
Corresponding author
Ethics declarations
Ethics
The research was conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals approved by the Institutional Animal Use and Care Committee (IACUC), Cairo University, Giza, Egypt. All authors have reviewed the manuscript and approved its submission for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abu-Elala, N.M., Younis, N.A., AbuBakr, H.O. et al. Influence of dietary fermented Saccharomyces cerevisiae on growth performance, oxidative stress parameters, and immune response of cultured Oreochromis niloticus. Fish Physiol Biochem 46, 533–545 (2020). https://doi.org/10.1007/s10695-019-00711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00711-9