Abstract
Food web response to species loss has been investigated in several ways in the previous years. In binary food webs, species go secondarily extinct if no resource item remains to be exploited. In this work, we considered that species can go extinct before the complete loss of their resources and we introduced thresholds of minimum energy requirement for species survival. According to this approach, extinction of a node occurs whenever an initial extinction event eliminates its incoming links so it is left with an overall energy intake lower than the threshold value. We tested the robustness of 18 real food webs by removing species from most to least connected and considering different scenarios defined by increasing the extinction threshold. Increasing energy requirement threshold negatively affects food web robustness. We found that a very small increase of the energy requirement substantially increases system fragility. In addition, above a certain value of energy requirement threshold we found no relationship between the robustness and the connectance of the web. Further, food webs with more species showed higher fragility with increasing energy threshold. This suggests that the shape of the robustness–complexity relationship of a food web depends on the sensitivity of consumers to loss of prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food webs describe “who eats whom” in ecosystems and they are a central topic of research in ecology (Jordan et al. 2002; MacArthur 1955; May 1972, 2006; Montoya et al. 2006; Pimm 1980; Bascompte et al. 2005; Allesina and Pascual 2008). After Albert et al. (2000) introduced the analogy of biological extinctions into computer networks, food web models, as network descriptions of ecological communities, have been extensively exploited to help understand secondary extinction (Dunne 2006), in the understanding that results could beneficially impact the science of conservation (Tylianakis et al. 2010).
Food web response to species loss has been extensively investigated in the last years. In particular, the bulk of the literature on the subject (Allesina and Bodini 2004; Allesina et al. 2009; Allesina and Pascual 2009; Bodini et al. 2009; Dunne et al. 2002, 2004; Dunne and Williams 2009; Estrada 2007; Gilbert 2009; Solé and Montoya 2001) frames the problem in a topological perspective, in which secondary extinctions occur when a species in the food web remains without connections to the rest of the system following an initial (primary) extinction event. In silico removal experiments conducted on food web structures to explore patterns of secondary extinctions showed a low sensitivity of food webs to random removal of nodes (error resistance). However, when certain key nodes go extinct, many species are at risk of extinction (attack proneness; Allesina and Bodini 2004; Dunne et al. 2002, 2004; Dunne and Williams 2009).
Selecting species to be removed in these experiments automatically defined key species in the network. Although recently more realistic tactics for species removal have been proposed (Srinivasan et al. 2007), two main criteria have guided this selection, namely species connectivity (Dunne et al. 2002, 2004; Solé and Montoya 2001), and a criterion that considers the ‘effective communication’ between species in relation to specific functions they perform such as energy delivery (Allesina and Bodini 2004; Allesina et al. 2006; Estrada 2007). In general, food web models do not take into account changes in trophic interactions (“rewiring”) following species loss. On the other hand, adaptive trophic behaviour has been proposed as a key explanatory mechanism underlying the structure and properties of complex natural food webs, such as resilience and resistance of ecosystems to anthropogenic and other perturbations (Valdovinos et al. 2010). Staniczenko et al. (2010) analysed food web robustness including adaptive trophic behaviour in the form of topological “rewiring” and found that robustness to primary species removal increased noticeably.
Many of the studies cited above analysed binary (i.e. qualitative) food webs, that is food web that consider only the presence or absence of trophic interactions. In binary food webs, interaction strength, i.e. the amount of energy passing from resource species to their consumers, is not described, and thus no quantitative descriptor (i.e., strength) is associated to the links. However, link strength was proved to be a key factor in food web dynamics. The distribution of weak and strong interactions may affect food web stability (McCann et al. 1998; Neutel et al. 2002; Teng and McCann 2004; O’Gormann and Emmerson 2009), and patterns of secondary extinctions seem to be strongly influenced by the specific configuration of weak and strong links in food webs (Eklöf and Ebenman 2006; Fowler 2009).
Most of the studies on secondary extinction that include interaction strength as key parameter adopted a dynamical approach (Borrvall et al. 2000; Eklöf and Ebenman 2006; Binzer et al. 2011; Curtsdotter et al. 2011) to overcome the limitations associated to the pure topological analyses. In fact, the topological approach precludes the possibility to assess top-down and indirect effects, allows for extinction only after complete resource loss and does not consider adaptive response of species. It is possible to improve the topological analyses taking into account the amount of energy associated to the links. For example, Allesina et al. (2006) analysed secondary extinction in food web through topological analysis considering interaction strength. They showed that when links with strength below a given threshold are removed and, consequently, species survival is guaranteed only by links stronger than the amount fixed by the threshold, the number of bottlenecks (i.e. node upon which other species obligatory depend for their energy requirements), as well as the risk of secondary extinction, increase.
A recent analysis performed on model food webs explored the effect on robustness of a secondary extinction criterion based on thresholds of minimum energy requirement for species survival, rather than merely the absence of a connection to at least one prey (Thierry et al. 2011). This work, in which species are randomly removed, showed that energetic extinction criteria assumed in extinction scenarios greatly affect the robustness of model food webs. Nonetheless, how energetic extinction criteria affect the robustness of real food webs is still a largely unexplored issue.
The main aim of this paper is to explore how patterns of secondary extinctions deviate from those in a pure topological approach when link magnitude is added to the model and assumptions about minimum level of energy required for species survival are introduced. To give a broader picture of what adding link magnitude to topological analysis implies, we investigated robustness–connectance and robustness–richness relationships and the difference in the number of secondary extinctions under different energy threshold requirements in 18 real food webs whose links have been estimated as energy flows.
Methods
Food web data set
We analysed the weighted food webs of 18 ecosystems of various size (i.e. number of species S; min size S = 23; max size S = 248). We chose only webs with species richness S > 23 to avoid bias due to small web size (Bersier and Sugihara 1997). Data from two web sites were used to reconstruct the webs. The site of the Across Trophic Level System Simulation project (http://www.cbl.umces.edu/~atlss/) provided detailed information for eight ecosystems (Everglades gramminoid marshes. Florida Bay, Mangrove ecosystems, Cypress wetlands, wet and dry season). The Ulanowicz’s web page (http://www.cbl.umces.edu/~ulan/ntwk/network.html) provided data for eight other networks (Upper, Middle and Lower Chesapeake Bay, Chesapeake Mesohaline ecosystem, Mondego estuary, St. Marks river, Lake Michigan and Narraganset bay).
Two ecosystems complete the list: the Caribbean reef food web described by Bascompte et al. (2005), and the network of the Lake Santo described in Bondavalli et al. (2006). We excluded non-living nodes from the networks. The 18 food webs are described in Table 1.
The selected food webs were previously investigated as ecological flow networks; that is, graphs of ecosystems in which the magnitude of trophic transfers from prey to predators is known. In particular, trophic exchanges were quantified as grams of carbon per square meter per year, describing biomass exchanges between resources and consumers. Carbon is a proxy for energy as primary producers capture solar energy and stores it in the form of carbon based molecules that form the standing stocks of the network components (Ulanowicz 1986).
The analysis of link distributions revealed that in the 18 food webs one sole resource may satisfy in average up to 70 % of the energy requested by a single node. In 7 out of the 18 selected food webs, this value is around 50 %. In general, the fraction of biomass carried by the strongest incoming link is higher than 40 %, on average. This result means that energy distribution across incoming links to all nodes is not randomly distributed, but few stronger interactions are responsible for delivering most of the energy to nodes.
The results of this analysis are shown in the Electronic supplementary material.
Extinction scenario
In the binary scenario, a species is considered extinct when it loses all its incoming links and remains without resource to exploit. In a more realistic approach, a species can go extinct when the level of incoming energy is reduced below a certain quantity or fraction. It may be assumed that there is a given threshold below which species persistence is not possible. For example, consider a species i that requires to survive at least 30 % of its original incoming energy (i.e. the incoming energy before any species has been removed); if following a node removal connections to the former species are lost so that it can get only 20 % of its original incoming energy, the species would go extinct. According to this principle, we defined an extinction scenario in which each consumer’s incoming energy is recalculated after any single node removal and it is then compared to a parameter called energy threshold (v) that defines the minimum level of energy necessary for species’ survival. These thresholds can be viewed as a way to tuning the susceptibility of a consumer to the loss of its resources. We indicate with e(i) the fraction of the original incoming energy to species i after node removal. Thus, we assume that a species i goes extinct after a node removal if e(i) ≤ v.
In a binary extinction scenario, the threshold is implicitly assumed equal to 0 and a species goes extinct when its energy inflow is zero (Allesina and Bodini 2004; Allesina et al. 2009; Dunne et al. 2002, 2004; Dunne and Williams 2009; Montoya et al. 2006; Solé and Montoya 2001). A value v = 0.1 means that a species goes to extinction if the energy intake is equal or lower than 10 % of the original incoming energy, that is when the 90 % of the inflow is lost. For v = 0.2 a species disappears if after node removal its energy inflow becomes equal or lower than 20 % of the original intake, that is when the 80 % of the initial inflow to it is lost. We repeated this procedure increasing v by 10 % at every step. Nodes were sequentially removed in order of decreasing connectance (Dunne et al. 2002, 2004; Solé and Montoya 2001) and of decreasing out-degree (number of outgoing links from a node, Solé and Montoya 2001). We call the first scenario “the most connected removal” and the second “the most outgoing removal”. In the case of ties (i.e. species with the same degree), we randomly defined the order. After each removal, we computed the “fraction removed”, i.e. the number of removed species divided by the total number of species, and the “fraction extinct”, i.e. the proportion of species that go extinct as primary plus secondary extinction. Robustness is measured as the proportion of species that has to be deleted to cause the extinction of 50 % of the species in the food web (R 50, Dunne et al. 2002, 2004; Curtsdotter et al. 2011).
In addition, after any removal event we compared the number of secondary extinction induced by the energy threshold value with the number of species that go secondarily extinct in the pure topological approach (v = 0). This “extinction gap” is obtained by calculating at each removal step the difference between the number of species predicted to undergo extinction in the binary scenario and the number extinct for each energy threshold scenario. The “extinction gap” describes along the removal sequence the level of additional secondary extinction induced by the increased consumer susceptibility defined by the threshold value. The maximum value for this parameter is the “maximum extinction gap”. For a certain energy threshold, it represents the maximum variation in secondary extinction with respect to the binary scenario; as such, it provides a measure of how much the binary approach underestimates secondary extinction. Further, we considered the percent of nodes removed at which the maximum extinction gap is emerged. An example of the extinction scenario with extinction gap curves for three values of energy threshold is showed in Fig. 1.
a Most connected removal extinction scenario as a function of three energy threshold in Cypress wet food web (S = 65). The fraction of vanishing nodes (nodes removed plus secondarily extinct, y axis) is plotted against the fraction of nodes that are removed (x axis). The bisector line means no secondary extinction scenario. The curves indicate secondary extinction rates as a function of energy thresholds; v = 0 (black line), v = 0.3 (blue line), v = 0.8 (green line). b Extinction gap for v = 0.3 and c Extinction gap for v = 0.8 of the extinction scenario represented in chart (a). Fraction of nodes removed (x axis) and extinction gap (y axis). The extinction gap curve denotes the additional fraction of nodes extinct along the removal sequence passing from binary (v = 0) to a certain energy threshold. This curve yields the maximum extinction gap, that is the maximum value of the function, and the fraction of removals at which maximum extinction gap appeared
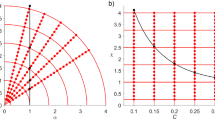
Finally, we used linear regressions to examine the relationship between robustness (R 50) at each level of energy threshold and four measures of food web complexity. We tested the relationship of robustness versus two binary parameters of networks complexity, namely species richness (S) and connectance (C = L/S 2). Then, we analysed the relationship between robustness and two quantitative measures of network complexity: quantitative unweighted linkage density (LD q ) and quantitative unweighted connectance (LD q /S) (Banašek-Richter et al. 2009; Bersier et al. 2002).
Results
Food web robustness (R 50) decreases as energy threshold increases with both removal criteria. As consumer susceptibility increases, a smaller number of nodes have to be removed to trigger the extinction of 50 % of the species in the network. The shape of R 50 as a function of the energy threshold is reported for each food web in Fig. 2. The full set of the R 50 outcomes is in Table 1 in the Electronic supplementary material. In general, removing species according to their outgoing connectance yields more secondary extinctions with respect to the extinction scenario in which species are removed in decreasing order of general connectance (Fig. 2).
R 50 (y axis) as a function of energy threshold (x axis) for each food web. Most connected removal (blue line); most outgoing removal (green line). Networks robustness (R 50) decreases when energy threshold is increased and this pattern holds for both removal criteria. The larger secondary extinction produced by the removal of the most general resources is indicated by the fact that the green curve (removal according to outgoing connectance) is always below the blue curve (removal based on general connectance). Food web keys are in Table 1
We investigated how the maximum extinction gap changes as a function of the energy threshold (Fig. 3). The curves show that in every web a larger fraction of species would go extinct passing from v = 0 (binary scenario) to v = 0.1 and v = 0.2. For most of the selected food webs, an energy threshold of 0.1 is enough to induce a very large additional secondary extinction with respect to the v = 0 secondary extinction. For example, in the Everglades dry network (S = 63) with the most outgoing removal scenario the maximum extinction gap is about 60 % in the transition from v = 0 to v = 0.1 (panel j in Fig. 3). In the Florida Bay wet (S = 123, panel q in Fig. 3), by imposing a threshold v = 0.1 the secondary extinction would increase more than 40 % with respect to the one obtained using v = 0. For the Florida Bay wet food web, this means that about 50 more species would go extinct. This rapid increase in terms of secondary extinction occurs for both removal criteria, but the maximum extinction gap is generally higher when species are removed focusing on their outgoing connectance, as shown in Fig. 3, where the green curves lie above the blue lines.
Maximum extinction gap (y axis) as a function of energy threshold (x axis) for each food web. Most connected removal (blue line); most outgoing removal (green line). The Maximum extinction gap represents for each energy threshold the highest discrepancy between secondary extinction events for the scenarios discussed in this paper with respect to those obtained in a binary scenario. In every web, a large fraction of species would go extinct passing from v = 0 (binary scenario) to v = 0.1 and v = 0.2. In the majority of the selected food webs assuming v = 0.1 is enough to induce a 30 % increase in secondary extinctions with respect to the binary scenario. Food web keys are in Table 1
As shown in Fig. 4, the maximum extinction gap generally occurs after 20–40 % of the species are removed. In some food webs, increasing the threshold from v = 0 to v = 0.2 causes the maximum extinction gap to arise when less than 20 % of the species are removed.
Fraction removed to max extinction gap (y axis) as a function of energy threshold (x axis) for each food web. Most connected removal (blue line); most outgoing removal (green line). The maximum extinction gap generally occurs after 20–40 % of the species are removed. In some food webs increasing the threshold would cause the maximum extinction gap to arise when less than 20 % of the species is lost. Food web keys are in Table 1
We should expect a sharp increase in secondary extinction increasing the energy threshold while the species with higher out-degree are responsible to a greater energy provision to consumers.
To test this hypothesis, we plotted for each food web the “species outflow energy” (total species outflow) as a function of the “species out degree” (number of outgoing links). No clear relationship between the out-degree of a species and its outflow energy is found (see Fig. 13 in the Electronic supplementary material).
As for the complexity–stability relationship, we found: (1) a positive linear relation for both removal criteria between food web robustness (R 50) and binary connectance (C) when v < 0.3; (2) a negative linear relationship between robustness (R 50) and species richness (S) when v > 0.1 for most outgoing removal; (3) a positive linear model of the robustness versus Quantitative Linkage Density (LD q ) when v = 0; (4) a negative linear relation between robustness and Quantitative Linkage Density (LD q ) when v > 0.7 for most connected removal and v > 0.4 for most outgoing removal; a positive linear fit for v = 0.1 and v = 0.2 of robustness with Quantitative Connectance (LD q /S) for most outgoing removal. All linear fits with significance value and slope are shown in Table 2.
Discussion
In order to make more realistic the response of real food webs to species loss we introduced a new criterion according to which a species’ extinction may occur before all of its incoming links vanish. Our results show that secondary extinctions in food webs increase with the minimum level of energy necessary for species survival (i.e. consumers are more sensitive to energy intake decrease). These results are in agreement with the recent analysis by Thierry et al. (2011), which showed that the severity of the energy threshold negatively affects the robustness of food webs.
Thierry et al. (2011) tested the robustness of model food webs of small size (S = 40) to random removal with energy intake threshold as criterion of species extinction and by explicitly modelling food web rewiring (i.e. the process by which species in the food web can shift their diet on new prey in the case original items has been lost). They found that both mechanisms affect food web robustness; specifically, increasing the severity of energy threshold increases the risk of secondary extinction.
With respect to Thierry et al. (2011), we used real weighted food webs of various sizes instead of model food webs and we removed species according to their connectance level (general or outgoing). Despite these differences, we found a similar decrease in food web robustness when consumer susceptibility to resource shortage increases.
In several webs, removing species with the highest number of outgoing links caused higher damage in the network at every energy threshold (Fig. 2). This indicates the removal based on outgoing connectance as a more efficient strategy to intercept species responsible to energy delivery in the food web. Removing species according the out-degree is more efficient when more connected species (outgoing + ingoing) are not nodes with more outgoing interactions, as in the case of top predators with many resources (i.e. nodes with no outgoing and many ingoing links; Solé and Montoya 2001).
We observed a rapid increase in secondary extinction moving from v = 0 (binary) to v = 0.1 scenarios. This means that increasing the consumer’s susceptibility to resource availability by only 10 %, a relatively narrow fraction of species has to be removed to produce a large extra cascading extinction in comparison to the binary scenario.
A similar outcome was obtained by Allesina et al. (2006), who performed a secondary extinction analysis based on dominance relationships, that is relationships that identify species that are necessary for other species’ survival (i.e. species responsible for energy delivery in the network). Allesina et al. (2006) applied the dominator tree model in eight weighted food webs. They removed all links in the web that were below a given magnitude, and built the dominator tree associated to the remaining structure. A sudden increase in secondary extinction emerged after imposing link threshold equal to 15 %. In other words, a great number of species emerged as dominated by other nodes after the weaker links were removed. As noted by the authors, the dominator method to forecast secondary extinction removing weaker links from the web does not take into account the fact that an array of links of magnitude lower than the threshold could guarantee species’ survival. This oversimplification could have increased the number of secondary extinction events beyond what would be observed if the effective energy budget for each node could be computed. In our extinction scenarios, their oversimplification is overcome and the presence of a certain number of weak links can support a species. Nonetheless, we found a similar quick rise in secondary extinctions by increasing energy requirement threshold, and we can hypothesize that this pattern holds in general, when nodes that are crucial for energy delivery, either bottlenecks or hubs, are removed from the web.
The rapid increase in secondary extinction due to a minimum increase of the energy threshold can be explained by the fact that in binary food webs interactions strength can be viewed as uniformly distributed among the links, which thus equally contribute to the consumers’ needs. When the real magnitude of links is specified and an energy threshold criterion introduced, a certain fraction of weaker links that remain after a node is removed may not be sufficient to guarantee species' persistence. Since the distribution of link magnitude is skewed toward weaker links, as shown in the method section for the webs considered here and in many other papers (see Fowler 2009 and references there in), it is likely that species can be supported by many weak links which, on the other hand, can easily disappear for a small increase in the energy threshold, leading to a series of extinctions. Thus, the increased risk of secondary extinction by introducing energy threshold would be further increased by the skewness in consumer’s inflow.
The relationship between skewness in interaction strength and network robustness would require further scrutiny although other papers suggested that the risk of secondary extinction would increase when the distribution of links magnitude becomes skewed (Borrvall et al. 2000).
A very interesting pattern that emerged in our analyses is the lack of a positive relationship between food web robustness (R 50) and classical parameters of food web complexity, i.e. connectance and species richness when energy threshold is above a certain value (i.e. above v = 0.2). As shown in previous analyses (Dunne et al. 2002, 2004; Gilbert 2009; Dunne and Williams 2009), we found that food web robustness increases with connectance when the criterion of species extinction is binary (i.e. v = 0 scenario in Table 2). This positive relationship still holds for energy thresholds equal to 0.1–0.2. However, when v is above 0.2 any connection between R 50 and connectance is lost. In other words, when we increase consumer susceptibility, secondary extinction is not dampened by the number of interactions present in the network.
This finding differs from binary predictions in which food webs display increasing robustness with connectance (Dunne et al. 2002, 2004; Dunne and Williams 2009) and it is somehow in agreement with the negative relationship between connectance and robustness discovered for food web models when energy thresholds are more severe than most lenient binary scenario (Thierry et al. 2011). Thierry et al. (2011) showed that more connected webs would be less robust than less connected webs when more severe energy thresholds are assumed for consumers and species are deleted at random. A possible explanation may be that in more connected webs species’ removal would cause many species to lose part of their energy intake. When the threshold is lenient, losing energy is unimportant and each link plays a role for species' survival. On the contrary, when energy threshold is high enough the loss of even a minimum amount of resource can drive a consumer to extinction. Such a lethal perturbation could propagate more rapidly in more linked system than in less connected networks, inducing the absence of the connectance–robustness relationship formerly showed in binary analyses. Montoya and Sole (2003) showed that, for binary webs, secondary extinctions following the loss of highly connected prey may increase with the size of the ecosystem because degree distributions (i.e. the distribution of trophic interactions among species) become more skewed in larger and more linked networks. In this sense, such strictly topological and our weighted plus energy threshold perspectives show here provide similar results.
The reversal of the positive relationship between linkage density and the robustness of a web when energy threshold is increased is evident observing the linear fits of the R 50 with quantitative linkage density (LD q ). When v = 0, the networks robustness follows the quantitative linkage density for both removal criteria. For middle values of the energy threshold, no relationship is found. When energy threshold is more severe, we attested to the reversal of the relation and web robustness is lower for that network with higher quantitative linkage density. Therefore, the stabilizing role of connectance found in binary models seems not to be preserved in weighted food webs with various removal criteria.
It is worth noting that the new pattern for which connectance and robustness are not positively related is consistent with analyses on secondary extinctions that also included population dynamics that showed that species loss caused a proportionally greater risk of extinction in densely connected webs compared to sparsely connected structures (Eklöf and Ebenman 2006).
In addition, by deleting species in decreasing order of outgoing connectance level, we found a negative linear fit between robustness and species richness when assuming more severe consumer susceptibility (i.e. v > 0.2 in Table 2). On the contrary, in binary investigations, no relationship between robustness and the size of real food webs (Dunne et al. 2002, 2004) was found, whereas in model food webs species richness positively affect robustness (Dunne and Williams 2009). The lower robustness observed in our analyses for larger networks can be explained by the partitioning of biomass inflows, which become more skewed (i.e. few strong and many weak flows, yielding a long-tailed distribution) as species richness increases (Banašek-Richter et al. 2009). This would indicate larger webs as richer of weak trophic interactions than smaller webs. If secondary extinction depends on binary assumption, this increasing skewness would not affect food web robustness because links are considered only as presence–absence of trophic interaction. When a more severe energy requirement is imposed, food webs possessing a higher fraction of weak links may undergo broader extinction events.
In this research, extinction sequences were created by removing nodes from most to least connected. Removing nodes randomly or in an increasing order of connectance (that is from least to most connected nodes) was used in another research whose results are presently under review (Bellingeri and Vincenzi, in review). The outcomes are quite different from those discussed here. For example a random sequence of removal seems to produce a slower, linear increase of the number of secondary losses when increasing consumer susceptibility.
The analyses presented in this work suggest that knowing species–consumer susceptibility can be important to forecast secondary extinction and contribute to understand robustness–complexity relationship in food webs. If the amount of energy that a species can gather is the most severe factor that determines its survival (Binzer et al. 2011) to study species susceptibility to resource loss should be widely encouraged (Brose 2011; Thierry et al. 2011).
It is necessary to remark that in our analysis all species are assumed to be equally susceptible to energy intake decrease (i.e. all species have the same energy threshold), but species in real communities certainly show different levels of vulnerability to resource loss (Bodini et al. 2009; Ebenman 2011; Binzer et al. 2011). A straightforward extension of our extinction scenario would be introducing variability in species susceptibility. However, this information requires deeper scrutiny of species habits and such a detailed database remains far from being available.
Finally, a very interesting question is to investigate how the addition of dynamic, rewiring, top-down cascades and other indirect effects will modify secondary extinction patterns presented in this paper.
References
Albert R, Jeong H, Barabasi A (2000) Error and attack tolerance of complex networks. Nature 406:378–382
Allesina S, Bodini A (2004) Who dominates whom in the ecosystem? Energy flow bottlenecks and cascading extinctions. J Theor Biol 230:351–358
Allesina S, Pascual M (2008) Network structure, predator–prey motifs, and stability in large food webs. Theor Ecol 1:55–64
Allesina S, Pascual M (2009) Googling food webs: can an eigenvector measure species' importance? PLoS Comp Biol 5(9):e1000494
Allesina S, Bodini A, Bondavalli C (2006) Secondary extinctions in ecological networks: bottlenecks unveiled. Ecol Model 194:150–161
Allesina S, Bodini A, Pascual M (2009) Functional links and robustness in food web. Phil Trans Roy Soc B 364:1701–1709
Banašek-Richter C, Bersier LF, Cattin MF, Merz Y, Baltensperger R, Gabriel JP, de Ruiter P, Tavares-Cromar A, Ulanowicz RE, Winemiller K, Naisbit RE (2009) Complexity in quantitative food webs. Ecology 90:1470–1477
Bascompte J, Melián CJ, Sala E (2005) Interaction strength combinations and the overfishing of a marine food web. PNAS 102:5443–5447
Bersier LF, Sugihara G (1997) Scaling regions for food web properties. PNAS 94:1247–1251
Bersier LF, Banasek-Richter C, Cattin MF (2002) Quantitative descriptors of food-web matrices. Ecology 83:2394–2407
Binzer A, Brose U, Curtsdotter A, Eklöf A, Rall BC, Riede JO, de Castro F (2011) The susceptibility of species to primary extinctions in model communities. Basic Appl Ecol 12:590–599
Bodini A, Bellingeri M, Bondavalli C, Allesina S (2009) Using food web dominator trees to catch secondary extinctions in action. Phil Trans R Soc B 364:1725–1731
Bondavalli C, Bodini A, Rossetti G, Allesina S (2006) Detecting stress at the whole ecosystem level. The case of a mountain lake: Lake Santo (Italy). Ecosystems 9:768–787
Borrvall C, Ebenman B, Jonsson T (2000) Biodiversity lessens the risk of cascading extinction in model food webs. Ecol Lett 3:131–136
Brose U (2011) Extinctions in complex, size-structured communities. Basic Appl Ecol 12:557–561
Curtsdotter A, Binzer A, Brose A, Eklöf A, Riede J, de Castro F et al (2011) Robustness to secondary extinctions: comparing trait-based sequential deletions in static and dynamic food-webs. Basic Appl Ecol 12:571–580
Dunne JA (2006) The network structure of food webs. Ecological Networks: Linking Structure to Dynamics in Food Webs. In Pascual M, Dunne JA (eds.), Oxford University Press, New York
Dunne J, Williams R (2009) Cascading extinctions and community collapse in model food webs. Phil Trans R Soc B 364:1711–1723
Dunne J, Williams R, Martinez N (2002) Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol Lett 5:558–567
Dunne J, Williams R, Martinez N (2004) Network structure and robustness of marine food webs. Mar Ecol Prog Ser 273:291–302
Ebenman B (2011) Response of ecosystems to realistic extinction sequences. J Anim Ecol 80:307–309
Eklöf A, Ebenman B (2006) Species loss and secondary extinctions in simple and complex model communities. J Anim Ecol 75:239–246
Estrada E (2007) Food webs robustness to biodiversity loss: the roles of connectance, expansibility and degree distribution. J Theor Biol 244:296–307
Fowler M (2009) Extinction cascade and the distribution of species interactions. Oikos 119:864–873
Gilbert AJ (2009) Connectance indicates the robustness of food webs when subjected to species loss. Ecol Indic 9:72–80
Jordan F, Scheuring I, Vida G (2002) Species positions and extinction dynamics in simple food webs. J Theor Biol 215:441–448
MacArthur RH (1955) Fluctuation of animal populations and a measure of community stability. Ecology 36:533–536
May RM (1972) Will a large complex system be stable? Nature 238:413–414
May RM (2006) Network structure and the biology of populations. Trends Ecol Evol 21:394–399
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798
Montoya J, Sole R (2003) Topological properties of food webs: from real data to community assembly models. Oikos 102:614–622
Montoya J, Pimm S, Sole R (2006) Ecological networks and their fragility. Nature 442:259–264
Neutel AM, Heesterbeek JAP, De Ruiter PC (2002) Stability in real food webs: weak links in long loops. Science 296:1120–1123
O’Gormann EJ, Emmerson MC (2009) Perturbation to trophic interactions and the stability of complex food webs. PNAS 32:13393–13398
Pimm SL (1980) Food web design and the effect of species deletion. Oikos 35:139–149
Solé RV, Montoya JM (2001) Complexity and fragility in ecological networks. Proc R Soc Lond B 268:2039–2045
Srinivasan U, Dunne J, Harte J, Martinez N (2007) Response of complex food webs to realistic extinction sequences. Ecology 88:671–682
Staniczenko PPA, Lewis OT, Jones NS, Reed-Tsochas F (2010) Structural dynamics and robustness of food webs. Ecol Lett 13:891–899
Teng J, McCann KS (2004) Dynamics of compartmented and reticulate food web in relation to energetic flows. Am Nat 164:85–100
Thierry A, Beckerman AP, Warren PH, Williams RJ, Cole AJ, Petchey OL (2011) Adaptive foraging and the rewiring of size structured food webs following extinctions. Basic Appl Ecol 12:562–570
Tylianakis JM, Laliberté E, Nielsen A, Bascompte J (2010) Conservation of species interaction networks. Biol Conserv 143:2270–2279
Ulanowicz RE (1986) Growth and development: ecosystems. Phenomenology. Springer, NY, p 203
Valdovinos FS, Ramos-Jiliberto R, Garay-Narvaez L, Urbani P, Dunne JA (2010) Consequences of adaptive behaviour for the structure and dynamics of food webs Ecol Lett 13:1546–1559
Acknowledgements
Thanks to Francesco Scotognella and Emanuela Tenca for the helpful discussions and comments on earlier versions of this paper. We thank Simone Vincenzi and two anonymous reviewers for revisions that greatly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 475 kb)
Rights and permissions
About this article
Cite this article
Bellingeri, M., Bodini, A. Threshold extinction in food webs. Theor Ecol 6, 143–152 (2013). https://doi.org/10.1007/s12080-012-0166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-012-0166-0