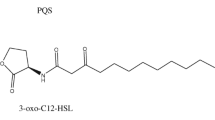
Abstract
The main goal of this study was to evaluate the inhibition of Pseudomonas aeruginosa virulence factors and Quorum Sensing during exposure to carvacrol. P. aeruginosa (ATCC 10154) was exposed to carvacrol determining changes in biofilm development, motility, acyl-homoserine lactones (AHL) synthesis and relative expression of lasI/lasR. Docking analysis was used to determinate interactions between carvacrol with LasI and LasR proteins. P. aeruginosa produced 60% lower AHLs when exposed to carvacrol (1.9 mM) compared to control, without affecting cellular viability, indicating a reduction on the LasI synthase activity. AHL-C12, C6, and C4 were detected and related to biofilm development, motility, and pyocyanin production, respectively. The presence of carvacrol reduced the expression of lasR, without affecting lasI gen. Moreover, computational docking showed interactions of carvacrol with amino acids in the active site pocket of LasI (−5.6 kcal mol−1) and within the binding pocket of LasR (−6.7 kcal mol−1) of P. aeruginosa. These results demonstrated that virulence of P. aeruginosa was reduced by carvacrol, by inhibiting LasI activity with the concomitant reduction on the expression of lasR, biofilm and swarming motility. This study provides relevant information about the effect of carvacrol against quorum sensing to inhibit virulence factors of P. aeruginosa at enzymatic and gene levels. These findings can contribute to the development of natural anti-QS products, which can affect pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is a Gram-negative pathogenic bacterium responsible for pneumonia and cystic fibrosis, diseases caused by biofilm development (Olsen 2015). These bacteria use intercellular communication, also called Quorum Sensing (QS), to regulate virulence and to acquire protection from environmental stress (Sharma et al. 2014). This process is coordinated through signalling molecules, known as acyl-homoserine lactones (AHL) that increase with cell density (Nazzaro et al. 2013). These molecules are produced by LasI synthase, reaching a threshold concentration sensed by the LasR protein triggering the activation or repression of specific genes related to virulence factors (Bottomley et al. 2007; Reading and Sperandio 2006). For this reason, this pathway is considered a potential target to control virulence of P. aeruginosa.
Strategies to interfere biofilm formation are been demanded and proposed considering that conventional antibiotics and disinfectants were designed to act against planktonic cells and their action against biofilm embedded bacteria is less efficacious (Olson et al. 2002). The use of plant compounds has shown interesting results to advance in this problem; e.g. α-pinene, linalool and limonene had been tested to reduce QS activity and biofilm formation of pathogenic bacteria (Kerekes et al. 2013). Moreover, other terpenes like carvacrol, citronellol, cinnamaldehyde and eugenol affected biofilm formation and swimming motility of Pseudomonas fluorescens, E. coli and Pectobacterium carotovorum producing more susceptible biofilms to eradication (Gutierrez-Pacheco et al. 2018; Niu and Gilbert 2004). Similarly, gingerol decreased the production of AHLs by P. aeruginosa, affecting relative expression of lasI/lasR and reducing pyocyanin production and exoprotease activity (Kim et al. 2015). Despite that anti-QS and -virulence activity of plant compounds has been extensively studied, still there is a lack of information about their specific mode of action against most bacteria, specifically against P. aeruginosa.
Joshi et al. (2016) proposed by computational docking that carvacrol could interact with the binding sites of ExpI and ExpR, QS proteins of P. carotovorum. In addition, this previous work showed that this compound down-regulated expression of genes expI/expR that are involved in the regulation of virulence factors (extracellular polymeric substances, exoenzymes, and biofilm formation) of this phytopathogenic bacterium. Previously, it was reported that carvacrol, inhibited pyocyanin production and biofilm development in P. aeruginosa (Tapia-Rodriguez et al. 2017) without studying its mode of action. With this in mind, this work explored changes on virulence and QS of P. aeruginosa exposed to carvacrol.
Materials and methods
Quantification of acyl-homoserine lactones
Twelve milliliters of P. aeruginosa (ATCC 10154) 1 × 106 CFU mL−1 cultures in LB broth were exposed to different concentrations of carvacrol (0–7.9 mM) and incubated for 48 h at 37 °C. This experiment was carried out three times, and there were three replicates per assay. To obtain cell-free supernatants, cultures were centrifuged at 2348 g (4 °C) for 5 min in an Allegra 64R centrifuge (Hettich, Tuttlingen, Germany). Twelve mL of centrifuged sample diluted with 25% ACN was applied to a solid phase extraction cartridge (previously conditioned with methanol and water) and then was washed with ACN-water 40/60 (v/v), then the solutes were eluted with iPrOH-hexane 85/15 (v/v). The eluted was dried under a nitrogen stream for 30 min and dissolved in water containing 30% of ACN. Quantitation of AHLs was performed with a Waters UPLC (Milford, MA, USA) equipped with a 2996 PDA detector and a BEH C18 column (1.7 μm, 100 mm × 3 mm). The column thermostat was set to 60 °C and the auto-sampler temperature to 27 °C. The flow rate was 0.8 mL min−1 and the injection volume was 5 μL, injected by use of a full loop. A linear gradient was applied with starting with water containing 20% ACN as mobile phase; the ACN content was increased to 100% in 5 min. The detection wavelength was set to 197–400 nm (1.2 nm width) and the scan rate was 20 Hz. Results were expressed as μM of AHL detected.
Biofilm formation of P. aeruginosa
The effect of carvacrol during biofilm formation was evaluated enumerating attached cells on stainless steel 304 coupons (1 × 1 × 0.1 mm) using the protocol of Hui and Dykes (2012) with modifications. For the assay, 6 mL of LB broth with carvacrol (1.9 mM) were inoculated with 1 × 106 CFU mL−1 of P. aeruginosa at 37 °C at different incubation times (6, 12, 24 and 48 h). Then, coupons were removed and washed with sterile saline solution and sonicated for 5 min in 3 mL of sterile saline solution. These adhered cells were quantified using serial dilutions plating in LB agar incubated at 37 °C for 24 h. These experiments were performed by triplicate expressing results as Log CFU cm−2. Additionally, the same experiment was performed using glass coverslips to allow fluorescence microscopy analysis at 6, 12, 24 and 48 h of incubation at 37 °C. Every biofilm sample was stained with 0.1% Syto9, fixed for 30 min in a dark-room, washed and dried to observe at 400x magnification using a fluorescence inverted microscope Axio Vert.1 (Carl-Zeiss, NY, USA). Moreover, the percentage of covered area was calculated using ImageJ software version 2016 (KCL, London, UK), measuring the percentage of stained area; these experiments were done in triplicate.
P. aeruginosa motility
Swarming motility of P. aeruginosa exposed to carvacrol was determined using the methodology proposed by Cheng et al. (2016). For the assay, 20 μL of P. aeruginosa (1 × 106 CFU mL−1) cultures in 6 mL of LB broth exposed to carvacrol (0–7.9 mM) were inoculated in LB semisolid plates (0.5% agar) incubated at 37 °C for 24 h. Then, the motility spread was measured after 24 h, expressing the results in mm; this test was carried out in triplicate.
Changes on the protein profile of P. aeruginosa
Six milliliters of P. aeruginosa (1 × 106 CFU mL−1) cultures exposed 48 h to carvacrol (0–3.9 mM) were centrifuged for 10 min (11,600 g at 4 °C; Heraeus, Cavenago Brianza, Italy) and washed in 0.05 mM Tris-HCl. Cells pellet were re-suspended in 300 μL of Tris-HCl (0.05 mM) with SDS (2%). Samples were sonicated for 3 min and mixed for 5 min with glass beads (0.15–0.2 mm diameter, Sigma) at 100 °C. Glass beads were removed by centrifugation (11,600 g at 4 °C) for 15 min. Protein profiles were analysed using a Lab-on-a-chip electrophoresis (Experion Pro260 Kit, Bio-Rad, California, USA), following manufacturer instructions. Finally, the protein content was measured with an Experion analyzer using a 10 mW semiconductor laser at 630 nm (Nazzaro et al. 2009). All these experiments were performed in triplicate.
Relative expression of the lasR/lasI genes of Pseudomonas aeruginosa
RNA was extracted and cDNA was obtained from the treated and control bacteria for lasR (LasR-F 5’-TGCCGATTTTCTGGGAACC-3′, LasR-R 5’-CCGCCGAATATTTCCCATATG-3′), and lasI genes (LasI-F 5’-TCGACGAGATGGAAATCGATG-3′, LasI-R 5’-GCTCGATGCCGATCTTCAG-3′), and 16S-R (16S-F 5’-CAAAACTACTGAGCTAGAGTACG-3′, gene. 5’-TAAGATCTCAAGGATCCCAACGGCT-3′) was used as the constitutive (Cotar et al. 2010), in order to carry out quantification by real-time polymerase chain reaction (qPCR). The quantitative analysis was carried out using SYBR® Green Supermix (Bio-Rad, California, USA), all samples were analyzed in triplicate, each reaction included 50 ng of cDNA as template, 10 μL of SYBR Green qRT-PCR Master Mix, 1 μL of initiator LasR- F, or LasI-F 1 μL of LasR-R primer, or Las-R and 3 μL of water for a total volume of 20 μL per reaction. The PCR products were amplified in a Step-One ™ real-time PCR system (Applied Biosystems, California, USA). The amplification conditions were 40 cycles that sequentially included 95 °C for 5 min, 95 °C for 15 s, 60 °C for 1 min and 72 °C for 5 min. Results were expressed as relative levels of mRNA expression of the target genes lasR and lasI and normalized to expression levels of the constitutive 16S gene.
Molecular docking of proteins LasI and LasR of P. aeruginosa
Crystallographic structures of LasI (PDB 1RO5) and LasR (PDB 2UV0) proteins (Bottomley et al. 2007; Gould et al. 2004) were used as model receptors and carvacrol (PubChem 10,364) as ligand to compare with oxododecanoyl-homoserine-lactone (PubChem 3,246,941) interactions. The docking analysis was carried out using AutoDoc Vina application to obtain affinity energy (kcal mol−1) and root-mean-square deviations (RMSD) between proteins and carvacrol using UCSF Chimera version 11.2 software to identify amino acids involved with the obtained model interactions (Pettersen et al. 2004).
Statistical analysis
A completely randomized experimental design was applied to all assays. To evaluate changes in biofilm formation of P. aeruginosa exposed to carvacrol the experimental factors were the carvacrol dose (0–1.9 mM) and incubation time (0–48 h). For the effects on P. aeruginosa AHLs production (μM), motility (mm) and relative expression of lasR/lasI genes the experimental factor was the treatment with carvacrol (0–7.9 mM). An analysis of variance (ANOVA) was performed to determine significant differences, and a mean comparison using Tukey Kramer test was executed. Additionally, Pearson correlations were performed between the AHL content with motility and biofilm formation. The level of significance was set at P ≤ 0.05, using the software Number Cruncher Statistical Systems (NCSS) 2011.
Results
The production of C12-AHL (Fig. 1a) was different between control Pseudomonas and treated with carvacrol (P ≤ 0.05), showing a significant reduction of up to 60% using 1.9 mM of carvacrol, without affecting cell viability. Carvacrol at 1.9 mM reduced up to 20% the covered area of biofilms in glass surfaces (Fig. 1ba); meanwhile, with this treatment attached cells were reduced up to 1.5 Log CFU cm−2 during 48 h of biofilm formation on stainless steel (Fig. 1bb). Microscopic analyses showed that carvacrol decreased the covered area (Fig. 1c) by Pseudomonas biofilms on glass coverslips, indicating that this compound caused a negative impact on biofilm formation independently of the tested surface. The covered area and cell density in P. aeruginosa biofilms were correlated (r = 0.985; p = 0.0149) with C12-AHL content reductions.
a Production of 3-oxododecanoyl-homoserine lactones (C12-AHL) of P. aeruginosa exposed to carvacrol (0–7.9 mM). Different letters indicate statistical differences among treatments (P ≤ 0.05). b Covered area of P. aeruginosa biofilm development (a) in absence of carvacrol (control) and 1.9 mM; (b) Viable cells of P. aeruginosa within biofilm attached to stainless steel at 37 °C in presence of carvacrol (1.9 mM). c Epifluorescence microscopy of P. aeruginosa biofilm development on glass coverslips during incubation at 37 °C in absence (control; left panel) and presence of carvacrol at 1.9 mM (right panel)
The used doses of carvacrol (0.9–1.9 mM) affected (P ≤ 0.05) the transcription levels of lasR gene, while, lasI gene was not affected (Fig. 2). These results indicated a specific posttranslational effect of carvacrol against LasI. Similarly, C6-AHL production was reduced up to 80% with 1.9 mM of carvacrol and a total inhibition was found at higher doses (Fig. 3a). Also, a significant inhibition of swarming motility was achieved on P. aeruginosa exposed to different doses of carvacrol (0.9–7.9 mM) compared to untreated bacteria (Fig. 3b). On the other hand, a significant reduction of C4-AHL was observed at the highest tested concentration of carvacrol (Fig. 4); this reduction was statistically significant (P ≤ 0.05) and presented a strong correlation (r = 0.929; p = 0.0226) with the toxin pyocyanin. In the other hand, in Pseudomonas exposed to carvacrol most of the proteins (40–150 kDa) were attenuated compared to untreated cells (Fig. 5). These results could be attributed to the decrease of proteins related to virulence of P. aeruginosa, including protease (LasA) and elastase (LasB) with molecular weights among 45–50 kDa.
a Production of hexanoyl-homoserine lactones (C6-AHL) of P. aeruginosa exposed to different concentrations of carvacrol (0–7.9 mM). Different letters indicate statistical differences with control (P ≤ 0.05). b Swarming motility of P. aeruginosa exposed to carvacrol and incubated at 37 °C. Values are expressed as the mean ± standard deviation (SD) of three samples. Different literals indicate significant differences (P ≤ 0.05)
Computational docking modelling reflected that carvacrol showed affinity to interact with LasI and LasR proteins. However, it has to be remembered that at gene level only a decrement on the expression of lasR was caused by carvacrol and a direct effect inhibiting activity of LasI was detected by a lower C12-AHL production. With this in mind, figure 1 and 2 (supplementary material) showed the possible interactions between LasI synthase, identifying two binding pockets with variable dimensions, the first related to the entrance of the acyl carrier protein and the second to the S-Adenosyl methionine and active site. This active site is a three-dimensional structure V-shaped cleft of β4-sheet and β5-sheet, surrounded by Leu102, Val143, Phe105, Ser103, Arg104, Thr142, Val148, Thr144, Trp33, Phe27, Thr145, Arg30, and Met79. This arrangement showed an electrostatic affinity (score: −5.6 kcal mol−1, RDSM: 2.6) of (OH−) between carvacrol hydroxyl group that could interact through Van der Waals forces with amino group (NH3+) of the amino acid residue Arg104 located within LasI active site. In addition, interactions between carvacrol and the binding site of C12-AHL within the receptor protein LasR showed affinity (score: −6.7 kcal mol−1, RSMD: 2.5) mainly mediated by the hydroxyl group (OH−) of carvacrol and the amino group (NH3+) of Asp73. Therefore, if carvacrol occupies the LasR binding site pocket, where C12-AHL hydrocarbon chain interacts, this stops the expression of virulence factors related genes in P. aeruginosa.
Discussion
This study reports a potential anti-virulence mode of action of carvacrol against P. aeruginosa as a post-translational inhibition against LasI activity and the concomitant reduction on the production of lasR gene expression, AHLs, and related virulence factors. LasI activity mediates the production of C12-AHL, molecule that at certain concentration is sensed by receptor LasR, forming a LasR-C12-AHL complex able to interact with DNA expressing different virulence factors responsible of biofilm development. In addition, in normal circumstances, this complex also triggers the expression of the RhII synthase, responsible of the production of C6-AHL, C4-AHL and the expression of rhIR. RhIR protein forms complexes with C6 and/or C4-AHL, responsible of motility and pyocyanin toxin expression, important virulence factors of P. aeruginosa. The action of carvacrol caused a reduction in AHLs, possibly affecting LasI activity, down-regulating the cascade of signals, pathways and expression of virulence of P. aeruginosa. However, even when lasR expression was affected, also the LasR sensing activity could be affected by interaction with carvacrol as showed by the docking analysis. A previous study published by Joshi et al. (2016) reported that carvacrol caused a reduction in the relative expression of esaI and esaR genes, which codifies for AHL synthase and receptor protein, respectively, decreasing the virulence of the phytopathogenic bacteria P. carotovorum. In addition, carvacrol showed efficacy to decrease EPS production in P. carotovorum; however, this still needs to be determined for P. aeruginosa.
Some studies have reported the effectivity of essential oils against P. aeruginosa virulence; for example, it has been shown that menthol and clove oil inhibited pyocyanin production and reduced biofilm formation (Husain et al. 2013, 2015). Moreover, Myszka et al. (2016), reported a reduction of C6-AHL and C8-AHL content in Pseudomonas flourescens exposed to thyme oil, tymol and carvacrol, showing a decrement in motility, biofilm formation and relative expression of flgA gene. However, this report did not include changes on the QS system as a target as was done in the present study. It has to be mentioned that a more complete picture about the proposed anti-virulence mode of action of carvacrol needs to be completed by over-expressing and purifying LasI protein to study the changes in the kynetic parameters of the enzymatic inhibition and in situ studies about the efficacy of carvacrol to delay infection of Pseudomonas. In addition, further studies evaluating the interaction carvacrol-LasR is needed at crystal levels.
P. aeruginosa virulence was reduced when exposed to carvacrol; it decreased autoinducers levels and the relative expression of lasR gene in P. aeruginosa. Specifically, this effect was associated with a possible inhibition of LasI activity affecting AHL production. These findings improve our understanding about the possible mode of action of carvacrol and that different target points can be addressed to reduce the virulence of this pathogen. These could be useful for the development of inhibitors directed towards the affectation of QS machinery reducing pathogenicity.
References
Bottomley MJ, Muraglia E, Bazzo R, Carfì A (2007) Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem 282(18):13592–13600
Cheng F, Ma A, Zhuang X, He X, Zhuang G (2016) N-(3-oxo-hexanoyl)-homoserine lactone has a critical contribution to the quorum-sensing-dependent regulation in phytopathogen Pseudomonas syringae pv. Tabaci 11528. FEMS Microbiol Lett 363(23):fnw265
Cotar AI, Chifiriuc MC, Dinu S, Pelinescu D, Banu O, Lazãr V (2010) Quantitative real-time PCR study of the influence of probiotic culture soluble fraction on the expression of Pseudomonas aeruginosa quorum sensing genes. Romanian Archives of Microbiology and Immunology 69(4):213–223
Gould TA, Schweizer HP, Churchill ME (2004) Structure of the Pseudomonas aeruginosa acyl-homoserinelactone synthase LasI. Mol Microbiol 53(4):1135–1146
Gutierrez-Pacheco M, Gonzalez-Aguilar G, Martinez-Tellez M, Lizardi-Mendoza J, Madera-Santana T, Bernal-Mercado A, Vazquez-Armenta F, Ayala-Zavala J (2018) Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 89:210–218
Hui YW, Dykes GA (2012) Modulation of cell surface hydrophobicity and attachment of bacteria to abiotic surfaces and shrimp by Malaysian herb extracts. J Food Prot 75(8):1507–1511
Husain FM, Ahmad I, Asif M, Tahseen Q (2013) Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J Biosci 38(5):835–844
Husain FM, Ahmad I, Khan MS, Ahmad E, Tahseen Q, Khan MS, Alshabib NA (2015) Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of gram-negative bacteria. Front Microbiol 6
Joshi JR, Khazanov N, Senderowitz H, Burdman S, Lipsky A, Yedidia I (2016) Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci Rep 6
Kerekes EB, Deák É, Takó M, Tserennadmid R, Petkovits T, Vágvölgyi C, Krisch J (2013) Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J Appl Microbiol 115(4):933–942
Kim H-S, Lee S-H, Byun Y, Park H-D (2015) 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 5:8656
Myszka K, Schmidt MT, Majcher M, Juzwa W, Olkowicz M, Czaczyk K (2016) Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int Biodeterior Biodegrad 114:252–259
Nazzaro F, Fratianni F, Coppola R, Sada A, Orlando P (2009) Fermentative ability of alginate-prebiotic encapsulated lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J Funct Foods 1(3):319–323
Nazzaro F, Fratianni F, Coppola R (2013) Quorum sensing and phytochemicals. Int J Mol Sci 14(6):12607–12619
Niu C, Gilbert E (2004) Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol 70(12):6951–6956
Olsen I (2015) Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 34(5):877–886
Olson ME, Ceri H, Morck DW, Buret AG, Read RR (2002) Biofilm bacteria: formation and comparative susceptibility to antibiotics. Canadian Journal of Veterinary Research = Revue Canadienne de Recherche Veterinaire 66(2):86–92
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Reading NC, Sperandio V (2006) Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett 254(1):1–11
Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R (2014) Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals 42(1):1–7
Tapia-Rodriguez MR, Hernandez-Mendoza A, Gonzalez-Aguilar GA, Martinez-Tellez MA, Martins CM, Ayala-Zavala JF (2017) Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 75:255–261
Acknowledgments
Mexican council for science and technology CONACYT (Project number CB-2013-01-222691). Thanks to Emmanuel Aispuro from the Plant Physiology Laboratory (CIAD) for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to be declared.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tapia-Rodriguez, M.R., Bernal-Mercado, A.T., Gutierrez-Pacheco, M.M. et al. Virulence of Pseudomonas aeruginosa exposed to carvacrol: alterations of the Quorum sensing at enzymatic and gene levels. J. Cell Commun. Signal. 13, 531–537 (2019). https://doi.org/10.1007/s12079-019-00516-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-019-00516-8