Abstract
The quorum sensing network of Pseudomonas aeruginosa mediates the regulation of genes controlling biofilm formation and virulence factors. The rise of drug resistance to Pseudomonas aeruginosa infections has made quorum sensing–regulated biofilm formation in clinical settings a major issue. In the present study, LasR inhibitors identified in our previous study were evaluated for their antibiofilm and antiquorum sensing activities against P. aeruginosa PAO1. The compounds selected were (3-[2-(3,4-dimethoxyphenyl)-2-(1H-indol-3-yl)ethyl]-1-(2-fluorophenyl)urea) (C1), (3-(4-fluorophenyl)-2-[(3-methylquinoxalin-2-yl)methylsulfanyl]quinazolin-4-one) (C2) and (2-({4-[4-(2-methoxyphenyl)piperazin-1-yl]pyrimidin-2-yl}sulfanyl)-N-(2,4,6-trimethylphenyl)acetamide) (C3). The minimum inhibitory concentrations of C1 and C2 were 1000 μM, whereas that of C3 was 500 μM. At sub-MICs, the compounds showed potent antibiofilm activity without affecting the growth of P. aeruginosa PAO1. Electron microscopy confirmed the disruption of biofilm by the selected compounds. The antiquorum sensing activity of the compounds was revealed by the inhibition of violacein in Chromobacterium violaceum and the inhibition of swimming and swarming motilities in P. aeruginosa PAO1. Furthermore, the compounds also attenuated the production of quorum sensing–mediated virulence factors. The qRT-PCR revealed the downregulation of quorum sensing regulatory genes, namely lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR. The selected compounds also exhibited lower cytotoxicity against peripheral blood lymphocytes. Thus, this study could pave a way to explore these compounds for the development of therapeutic agent against Pseudomonas aeruginosa biofilm–related infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the biggest challenges faced by the healthcare sector and a major health concern worldwide is the rising incidence of microbial resistance to conventional antibiotics. Numerous resistant bacterial species have emerged due to the widespread microbial resistance brought about by the overuse of antibiotics (Rashiya et al. 2021). The bacteria develop such a resistant nature through biofilm formation, persister cells, exopolysaccharide (EPS) matrix protection, limited antibiotic penetration, slower growth rate, efflux pumps and horizontal gene transfer (Qin et al. 2022; Ciofu and Tolker-Nielsen 2019; Sharma et al. 2019). Among them, one essential resistance mechanism is biofilm development since the therapy for diseases caused by biofilm formation results in antibiotic resistance due to their prolonged exposure to antimicrobial agents (Rabin et al. 2015; Singh et al. 2017). The Gram-negative bacteria with the highest level of clinical concern are Pseudomonas aeruginosa. Their unscrupulous characteristics lead to various clinical complications in humans including pneumonia, bacteraemia, bronchiectasis and cystic fibrosis. It is the third most common pathogen contributing to nosocomial infections, accounting for about 57% of cases (Kostylev et al. 2019; Rashiya et al. 2021). They are highly resistant to various antibiotics including cephalosporins, carbapenems, aminoglycosides and fluoroquinolones (Pang et al. 2018).
P. aeruginosa produces multiple virulence factors responsible for disease progression through mechanisms including modification of immune response, enforcement of adhesion ability, evasion of phagocytosis and destruction of host tissues (Moghaddam et al. 2014). It infects people by expressing virulence traits, namely lipopolysaccharide, elastase, pyocyanin, cyanide, rhamnolipids, exotoxin, alginate, antimicrobial resistance and flagellar motility, which lead to the formation of biofilm (Parasuraman et al. 2020). The secreted virulence factors enable P. aeruginosa to establish a stable structural architecture called biofilm that implicates in its associated infections (Teerapo et al. 2019). Biofilms are conglomerate of microorganisms encased in an exopolymer matrix of DNAs, proteins, polysaccharides, lipids and other constituents. Bacteria within biofilms are protected from clearance by the immune system and therefore become a causative agent of chronic infections in humans (Ganesh and Rai 2017). Antibiotic resistance among bacteria in biofilms is higher than that of organisms in planktonic states due to differences in their physiology and phenotypic characteristics (Teerapo et al. 2019).
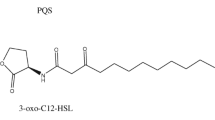
Quorum sensing (QS) is a communication system in P. aeruginosa regulated by signalling molecules known as autoinducers (AIs). The P. aeruginosa–associated quorum sensing system influences and coordinates primary physiological functions that contribute to pathogenicity by controlling the genes involved in the creation of biofilms and the generation of virulence factors (Kalia et al. 2019). Acyl homoserine lactone (AHL) molecules are coordinators of the QS networks with three established systems, namely las consisting of lasI and lasR, rhl consisting of rhlI and rhlR and pqs (Pseudomonas quinolone signal) systems that are activated in a cascade manner with lasI/R holding the highest position in the hierarchy (Kostylev et al. 2019; Zhong et al. 2020). When the autoinducer of the lasI/R system N-(3-oxododecanoyl)-L-homoserine lactone (3OC12-HSL) binds to LasR, it triggers the expression of downstream genes, such as lasI synthase, and produces 3OC12-HSL. The LasR-3OC12-HSL complex regulates the expression of rhlI, rhlR, pqsR and pqsABCDE genes. The other system rhlR, when binds to N-butanoyl-L-homoserine lactone (C4-HSL), drives up the expression of genes necessary for biofilm development and the production of virulence factors (O’Reilly et al. 2018). Finally, pqs utilises its autoinducer 2-heptyl-3-hydroxy-4-quinolone and controls the lasI/lasR system to induce rhlI/rhlR expression (Luo et al. 2017).
An established finding that P. aeruginosa regulate virulence traits by QS offered a new strategy for developing robust and novel drug targets (Defoirdt 2018; Deryabin et al. 2019). Many researchers have already reported that QS suppression effectively reduces P. aeruginosa pathogenicity and the development of biofilms (Scoffone et al. 2019). Quorum sensing inhibitors (QSIs) appear to have reduced risk of resistance in contrast to existing antibiotics since they decrease the pathogenicity of bacteria without affecting their growth pattern (Whiteley et al. 2017). Therefore, targeting QS-mediated gene expression would be one potential technique to be considered for treating infections and biofilm development in multi-drug-resistant (MDR) P. aeruginosa (Kalia et al. 2019). Generally, there are two ways to interfere with QS: enzyme degradation and small molecule binding. In the latter, AHL analogues were frequently used to bind to the QS receptor region. Recent research has suggested that P. aeruginosa QS suppression through small molecule leads has remarkably reduced the development of biofilms. Due to the hierarchical pattern of the QS system in P. aeruginosa, extensive studies are targeted towards LasR (Zhou et al. 2017).
The issue of implant-associated infections brought on by antibiotic-resistant P. aeruginosa biofilms has gained attention due to the rapid growth of implantable biomedical equipment (Arciola et al. 2018). Implantable and prosthetic devices may get infected during surgery or at any point of time. Infection rates in implants are impacted by elements such as variations in implant surface hydrophilicity, surface charge, surface energy and biomaterial composition. One of the reasons implantable devices become infected is that, in comparison to natural tissue, a biomedical device requires a 10,000 times lower bacterial load to colonise it (Roehling et al. 2017).
Infections brought on by bacterial contamination of implants and prosthetic medical devices can be fatal, resulting in device failure, persistent infections and high rates of death and morbidity. The treatments employed to treat these infections are ineffectual due to antibiotic-resistant strains like P. aeruginosa and high possibilities of re-infection on the new implant (Sohns et al. 2017). It was believed that altering the implant surface with antimicrobial surface coatings would effectively solve the issue of these implants associated bacterial infections. Hence, coating the implant surface with compounds that possess antimicrobial, antiquorum sensing and antivirulence properties would be a promising remedy to prevent bacterial colonisation (Esteves et al. 2022).
Many biofilm and QSIs have been so far identified, including berberine (Zhao et al. 2022), clove oil (Razdan et al. 2022), eugenol (Shariff et al. 2022), ginkgolic acid (Suo et al. 2022), hispidulin (Anju et al. 2022), carvacrol (Walczak et al. 2021), cuminaldehyde (Chatterjee et al. 2021), 6-methyl coumarin (Bajire et al. 2021), epigallocatechin-3-gallate (Hao et al. 2021), 1,8-cineole (Karuppiah et al. 2021), wogonin (Wang et al. 2021), butein (Zhong et al. 2020, b), naringenin (Hernando-Amado et al. 2020), andrograpanin (Majumdar et al. 2020), α-terpineol (Bose et al. 2020), salicylic acid (Ahmed et al. 2019), 5-hydroxymethylfurfural (Rajkumari et al. 2019), hordenine (Zhou et al. 2018), baicalin (Luo et al. 2017), resveratrol (Chen et al. 2017), 6-gingerol (Kim et al. 2015), meta-bromo-thiolactone (O'Loughlin et al. 2013), ellagic acid (Sarabhai et al. 2013), 7-fluoroindole (Lee et al. 2012) and ajoene (Jakobsen et al. 2012).
Despite numerous attempts have been made to identify a lead molecule with antibiofilm and antiquorum sensing activities, the spread of bacterial resistance continues at an alarming rate (Rasamiravaka et al. 2015). In our previous study, we identified three lead compounds against the LasR protein of P. aeruginosa by structure-based virtual screening (SBVS) approach (Vetrivel et al. 2021). Then, this study was carried out to validate the efficacy of the selected compounds for antibiofilm and antiquorum sensing properties using in vitro studies.
Materials and methods
Selected compounds
Three LasR inhibitors, namely (3-[2-(3,4-dimethoxyphenyl)-2-(1H-indol-3-yl)ethyl]-1-(2-fluorophenyl)urea), (3-(4-fluorophenyl)-2-[(3-methylquinoxalin-2-yl)methylsulfanyl]quinazolin-4-one) and (2-({4-[4-(2-methoxyphenyl)piperazin-1-yl]pyrimidin-2-yl}sulfanyl)-N-(2,4,6-trimethylphenyl)acetamide), were chosen from the Schrödinger small molecule database in our previous study by a molecular docking–based virtual screening process (Vetrivel et al. 2021), and they were given the designations C1, C2 and C3, accordingly. The three selected compounds are synthetic small molecules purchased from Mcule (https://mcule.com/), Hungary, and Life Chemicals (https://lifechemicals.com/), Canada.
Microbial strain and culture conditions
The wild-type bacterial strain Pseudomonas aeruginosa PAO1 (MTCC 2453) was acquired from the Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. The strain was grown on Nutrient Agar plates and kept at 4 °C for storage. Stock cultures of the strain were preserved in 50% glycerol at − 20 °C. A single colony of bacteria was cultured in Luria–Bertani (LB) broth before each experiment for 16–18 h at 37 °C. The overnight grown culture was then diluted at a ratio of 1:100 in LB broth and calibrated to an optical density (OD) of 0.5 at 600 nm which corresponds to 1 × 105 colony-forming units (CFU/ml).
Determination of minimum inhibitory concentration of selected compounds
The minimum inhibitory concentration (MIC) of the selected compounds against Pseudomonas aeruginosa PAO1 was identified using micro broth dilution (Sarkar et al. 2018). In brief, twofold serial dilution of the compounds whose concentrations ranged from 1.95 to 4000 μM was performed in LB broth. About 100 μl of overnight P. aeruginosa PAO1 culture (diluted 1:100) was added to all wells of a 96-well microtitre plate consisting of serially diluted samples. The microtitre plate was kept for 18–24 h at 37 °C. The MIC was described as the lowest concentration of the compound that entirely prevented the ability of the organism to grow visibly. A concentration range below the MIC referred to as sub-minimum inhibitory concentrations (sub-MICs) was taken for further experimental analysis.
Biofilm inhibition assay
The assay for biofilm inhibition was conducted using the crystal violet staining protocol mentioned by Lee et al. (2011). The overnight P. aeruginosa PAO1 culture (diluted 1:100) was treated with selected compounds in a 96-well microtitre plate and incubated for 24 h at 37 °C. Prior to the biofilm assay, the growth of the bacteria in the presence of the compounds was assessed by measuring their OD at 600 nm using Bio-Rad iMark Microplate Absorbance Reader. Thereafter, the plates were washed with phosphate-buffered saline (PBS), pH 7.4, to completely eliminate the free-floating planktonic cells. The remaining biofilm cells were stained at room temperature for 20 min using a 0.1% crystal violet solution. The excess stain was drained out, and the wells were rinsed with PBS and resuspended in 95% ethanol. The cells suspended in ethanol were measured at 570 nm to quantify the biofilm.
Effect of selected compounds on preformed matured biofilm
The ability of the selected compounds to destroy the preformed matured biofilm was measured using an overnight culture of P. aeruginosa PAO1 (diluted 1:100) in LB broth contained in a microtitre plate and incubated at 37 °C for 24 h. Post incubation, planktonic cells were removed and LB broth was used to wash the wells. To each well, fresh LB broth containing the compounds was added. After 24 h of incubation, the remaining biofilm cells were measured by crystal violet staining procedure as described in the section “Biofilm inhibition assay”.
Evaluation of bacterial cell viability-MTT reduction assay
The MTT assay method reported earlier by Saising et al. (2012) was employed to determine the metabolic activity of viable biofilm cells. P. aeruginosa PAO1 biofilms were established and treated with selected compounds as previously stated in the crystal violet staining assay (section “Biofilm inhibition assay”). After incubation, the media from the wells were aspirated and the attached biofilm was rinsed with 0.01 mol l−1 of PBS. To each of the wells, 200 μl of freshly prepared media with 0.5 mg ml−1 of MTT reagent was added and incubated at 37 °C for 2 h in the dark. After incubation, the media was aspirated, followed by 200 μl of dimethyl sulfoxide (DMSO) was added to solubilise the formazan crystals formed. The optical density of the solution was recorded at 570 nm.
Biofilm detachment assay
Biofilm detachment assay was performed in accordance with the protocol suggested by Davies et al. (1998). Biofilms were allowed to grow on microtitre plates as previously mentioned and exposed to selected compounds for 24 h at 37 °C. After incubation, 4 μl of 10% sodium dodecyl sulfate (SDS) was added to each of the wells and incubated for 30 min. The detached bacterial cells were measured at an OD of 600 nm. The loosely adhered cells were removed, and the wells were rinsed with PBS. The leftover attached biofilm cells were measured by crystal violet staining procedure as described in the section “Biofilm inhibition assay”.
EPS inhibition assay
The amount of EPS produced by P. aeruginosa PAO1 in treated and untreated conditions was evaluated by the phenol–sulphuric acid method as reported by Rasamiravaka et al. (2015). In brief, the diluted overnight culture of P. aeruginosa PAO1 was grown with and without selected compounds at 37 °C for 24 h. The cells were centrifuged for 15 min at 10,000 rpm. The obtained pellets were collected and resuspended in high-salt buffer containing 10 mM KPO4, pH 7.0, 5 mM NaCl and 2.5 mM MgSO4 and again centrifuged for 30 min at 10,000 rpm. Post centrifugation, the supernatant fraction was incubated overnight with three volumes of chilled 100% ethanol for EPS precipitation at 4 °C. A mixture of cold phenol and concentrated sulphuric acid (H2SO4) was added to the precipitated EPS, and the OD was read at 490 nm.
Alginate inhibition assay
Production of alginate was assayed in accordance with the procedure previously reported by Owlia et al. (2007). In brief, 70 μl of compounds treated with P. aeruginosa PAO1 culture supernatants was added to a mixture containing boric acid/H2SO4 (4:1) (600 μl) and vortexed. To this mixture, carbazole (0.2%) solution (20 μl) was added. The contents were mixed well and kept at 55 °C for 30 min. The OD of the solution was recorded at 530 nm.
Estimation of biofilm total protein concentration
The amount of protein extracted from the biofilm was estimated according to the procedure mentioned by Das et al. (2016). To determine the concentration of total extractable protein, diluted overnight P. aeruginosa PAO1 culture was incubated for 48 h with and without selected compounds at sub-MICs. After incubation for 48 h, the planktonic cells were drained, and the attached biofilm cells were gently rinsed with PBS and boiled with 0.5 N NaOH (5 ml) for 30 min. The mixture was subjected to centrifugation (10,000 rpm) for 5 min. The obtained supernatant fraction was used to determine the protein concentration by Lowry’s method (Lowry et al. 1951).
Field emission scanning electron microscopy analysis
The changes in biofilm architecture and morphology of cells on treatment with selected compounds were observed by field emission scanning electron microscopy (FESEM) following the procedure reported by Singh et al. (2017) with minor modifications. The biofilms were allowed to grow on glass slides immersed in LB broths containing selected compounds for 48 h at 37 °C. Post incubation, glass slides with biofilms were incubated with glutaraldehyde (2.5%) at 4 °C overnight. After fixation with glutaraldehyde, gradient ethanol series (10–95%) was used to dehydrate the samples for 10 min. The dried slides were gold sputter coated and examined using FESEM (Sigma Carl Zeiss, Jena, Germany).
Violacein inhibition assay
The antiquorum sensing ability of the selected compounds was observed using an agar disc diffusion assay reported by Chu et al. (2013) with few modifications. The overnight cultured quorum sensing reporter strain Chromobacterium violaceum (MTCC 2656) was diluted in LB broth to attain OD600 = 0.132. LB agar plates were prepared, and 100 μl of diluted suspension was streaked onto the plates. Sterile discs with sub-MICs of selected compounds were kept on the agar plates and then incubated (30 °C) for 24 h. A colourless zone surrounding the disc indicates inhibition of violacein pigment production.
Swimming and swarming motility assays
The method described by Packiavathy et al. (2014) was used to assess the swimming and swarming motilities of P. aeruginosa PAO1. Diluted overnight cultures containing selected compounds were inoculated on the swimming agar medium plates containing 1% tryptone, 0.5% NaCl and 0.3% agar, respectively. In case of swarming motility, the culture was inoculated on the swarming agar medium plates made up of 1% peptone, 0.5% NaCl, 0.5% agar and 0.5% filter-sterilised glucose. The inoculated plates were examined for swimming and swarming motilities after 24 h of incubation at 37 °C.
Assays for virulence factors
Diluted P. aeruginosa PAO1 (OD600 = 0.5) overnight culture was added into LB broth containing sub-MICs of selected compounds and kept for 24 h at 37 °C. After incubation, bacterial cells were taken for centrifugation (10,000 × g) at 4 °C for 8 min. The obtained supernatants were filtered using a 0.22-μm syringe filter. The various biochemical assays to study the effectiveness of selected compounds on virulence factors, namely pyocyanin, rhamnolipid, protease, alkaline protease and lipase, were subsequently carried out using this filter-sterilised filtrate.
Pyocyanin assay
Briefly, the treated and untreated cell-free P. aeruginosa PAO1 supernatants were added to an equal volume of chloroform and mixed thoroughly for extraction of pyocyanin. The recovered pyocyanin pigment from the chloroform layer was dispensed into another tube, and re-extraction was done with 0.2 M HCl to obtain a solution of crimson to deep red colour. The inhibition of pyocyanin pigment was analysed by recording the OD of the solution at 520 nm (Essar et al. 1990).
Rhamnolipid assay
Rhamnolipid assay was carried out following the protocol reported by Kim et al. (2015). The cell-free culture supernatants (300 μl) of P. aeruginosa PAO1 were extracted using diethyl ether (600 μl). The organic phase was evaporated at 35 °C under reduced pressure, and deionised water (100 μl) was added. One hundred microlitres of the sample was mixed with orcinol solution containing 900 μl (0.19%) in 53% H2SO4. The contents were heated (80 °C) for 30 min and cooled (RT) for 15 min. The production of rhamnolipid was analysed from the absorbance measured at 421 nm.
Protease assay
The impact of compounds on protease secretion by P. aeruginosa PAO1 was evaluated using the skim milk agar plate method reported previously by Lee et al. (2012) with slight modifications. Skim milk agar plates were prepared using skim milk agar, and wells were punctured on the surface of the agar plates. The cell-free supernatant (25 μl) was added to the wells, and the plates were kept for 24 h at 35 °C. Protease activity was observed by the zone of clearance around the wells.
Alkaline protease assay
The activity of alkaline protease was determined using the supernatants of P. aeruginosa PAO1 (diluted 1:100) overnight cultures in the presence and absence of selected compounds. To 500 μl of the supernatant, assay buffer (1500 μl) containing 20 mM Tris–HCl and 1 mM CaCl2 (pH 8.0) with Hide-Remazol blue powder (50 mg) was added. Tubes were incubated with constant rotation for 1 h at 37 °C. Post 1 h, the tubes were kept on ice to halt the reaction and the contents were centrifuged (4000 × g) for 5 min. The absorbance of the solution was observed at 590 nm (Howe and Iglewski 1984).
Lipase assay
Lipase assay was performed using the reaction mixture containing 600 μl of the filtered supernatant, 600 μl of 10% Tween 20 in Tris buffer, 100 μl of 1 M CaCl2 and 1600 μl of double-distilled water. The mixture was incubated for 24 h with continuous agitation (200 rpm) at 37 °C. After incubation, the OD of the mixture was recorded at 400 nm for assessment of lipase activity (Alhajlan et al. 2013).
Gene expression study of QS genes by qRT-PCR
P. aeruginosa PAO1 biofilms were formed in LB broth with and without the selected compounds at their sub-MICs for 24 h at 37 °C. Total RNA extraction was carried out using an RNA isolation kit following the manufacturer’s instructions (TIANGEN Biotech Co., Ltd., Beijing, China). The primers used for lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR were synthesised by Eurofins Scientific (Eurofins, Europe) and are enlisted in Table S1. The reaction for reverse transcription was done with a PrimeScript RT Reagent Kit (TaKaRa, Tokyo, Japan) utilising the template (total RNA) for 15 min (reverse transcription) at 37 °C thrice and for 5 min (inactivation of reverse transcription) at 85 °C.
The qRT-PCR was performed following the instructions of the manufacturer using the SYBR® Premix Ex Taq™ II Kit (TaKaRa, Japan). Complementary DNA (cDNA) was used as the template for qRT-PCR, and the entire reaction system consisted of SYBR® Premix Ex Taq™ II (2 ×) (10 μl), forward primer (0.8 μl), reverse primer (0.8 μl), ROX reference dye (50 ×) (0.4 μl), cDNA template (2 μl) and double-distilled water (6 μl). The Rotor-Gene Q 2Plex real-time PCR system from Qiagen (Qiagen Sciences, Hilden, Germany) was used as the qRT-PCR experiment. The qRT-PCR reaction conditions were as follows: initial denaturation for 30 s at 95 °C, followed by subsequent denaturation (40 cycles) for 5 s at 95 °C, with annealing and extension at 60 °C for 30 s. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was employed as a reference positive control. The relative changes in gene expression were observed using the 2−ΔΔCT method.
Determination of cytotoxicity of selected compounds
Cytotoxicity of the compounds was studied using human peripheral blood lymphocyte (PBL) cells. The cells were maintained in RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS), 0.5% penicillin–streptomycin and phytohaemagglutinin. The medium containing PBL was centrifuged (5000 × g) for 10 min. The supernatant was removed, and the pellet was resuspended with fresh medium (1 ml). The cells were seeded at a density of 1 × 106 cells in a 96-well plate and treated with the selected compounds based on the MIC values (125–850 μM). The optimal dose was identified on incubating the cells with different concentrations of compounds for 24 h. Then, MTT reduction assay was carried out to measure the cytotoxicity as previously reported by Igarashi and Miyazawa (2001).
Statistical analysis
Each of the experiment was carried out in triplicates, and the results are provided as mean ± standard deviation (SD). One-way ANOVA was done to analyse statistical differences, with p values < 0.05 and < 0.01 defined as statistically significant.
Results
Determination of MIC and sub-MIC of selected compounds
The MIC of the selected compounds (C1–C3) against P. aeruginosa PAO1 was ascertained by the method of micro broth dilution. The MIC was found to be 1000 μM for C1 and C2, whereas 500 μM for C3. The ½ and ¼ MICs referred to as sub-MICs of the compounds were used to assess the antibiofilm and antiquorum sensing potencies in subsequent studies (Table S2).
The effect of the selected compounds at sub-MICs on P. aeruginosa PAO1 growth was initially analysed, which differentiated the antibiotic activity from the antiquorum sensing activity of the compounds. The bacteria were incubated in a 96-well microtitre plate with sub-MICs of the selected compounds for 24 h. Growth was evaluated by the cloudiness of the media measured at 600 nm. The results (Fig. 1) inferred that sub-MICs of the compounds had no significant impact on P. aeruginosa PAO1 growth. Therefore, sub-MICs of the compounds were used in subsequent studies.
Biofilm inhibition assay
Crystal violet staining assay was employed to assess the impact of the selected compounds on P. aeruginosa PAO1 biofilm development. A significant (p < 0.01) decline in biofilm formation was observed for all three compounds as shown in Fig. 2. The compounds could prevent the formation of biofilm to about more than 50%. The percentage of biofilm inhibition by C1 at ½ MIC and ¼ MIC was found to be 84.86% and 80.94%, respectively, while the percentage of inhibition by C2 at ½ MIC and ¼ MIC was 83.66% and 80.70%, followed by C3 at 83.06% and 81.81%, respectively.
Effect of selected compounds C1, C2 and C3 on the formation of biofilm by P. aeruginosa PAO1 assayed post 24 h of incubation through crystal violet staining by recording the absorbance at 570 nm. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. **p < 0.01: significance compared with control
Effect of selected compounds on preformed matured biofilm
The P. aeruginosa PAO1 biofilm eradication ability of the selected compounds was investigated by crystal violet staining assay method. The free-floating cells from the matured biofilm were discarded after 24 h of incubation, and the attached cells were incubated with fresh LB broth containing sub-MICs of compounds. The compounds showed a dose-dependent potential to destroy the preformed matured P. aeruginosa PAO1 biofilms (Fig. 3) where the biofilm disruption ability was higher for ½ MICs compared to ¼ MICs. The highest (67.32%) disruption ability was exhibited by C1 at ½ MIC and the lowest (46.37%) was by C2 at ¼ MIC, respectively.
Effect of selected compounds on the disruption of preformed matured biofilm of P. aeruginosa PAO1 determined by crystal violet staining assay after 24 h of incubation with selected compounds in LB broth. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars indicate the SD of triplicate values. *p < 0.05; **p < 0.01: significance compared with control
Evaluation of bacterial cell viability
MTT assay was used to validate bacterial cell viability after treatment with the compounds. Results of the MTT assay (Fig. 4) proved the crystal violet data that was previously obtained (Fig. 2) and revealed that the viability of bacterial cells was decreased compared to the control. This demonstrates that the percentage of metabolically active biofilm-forming cells decreased after treatment with ½ MIC of compounds C1, C2 and C3, whereas the percentage of viable biofilm cells was higher at a lower concentration of ¼ MIC.
Effect of selected compounds at sub-MIC doses on P. aeruginosa PAO1 cell viability. The viability of bacterial cell was evaluated by MTT assay, and the percentage of cell viability was indicated compared to control which was considered as 100%. Data represented as mean ± standard deviation (SD) of triplicate values, and error bars define the SD of triplicate value
Biofilm detachment assay
Detachment assay was carried out to determine the impact of the selected compounds on biofilm adherence to the substrate. The compounds exhibited an alteration in the capacity of biofilm cells to adhere to a surface. They showed a gradual reduction in bacterial attachment compared to the control (Fig. 5). A decrease in the absorbance at 570 nm (Fig. 5) on treatment with compounds suggested significant (p < 0.01) biofilm detachment.
Detachment assay of the P. aeruginosa PAO1 biofilms grown adhering to a surface and subsequent treatment with selected compounds. Bacterial detachment was identified by measuring the OD at 570 nm. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. **p < 0.01: significance compared with control
EPS inhibition assay
P. aeruginosa PAO1 biofilm formation is closely linked to EPS production, contributing to its architecture and stability. The effect of selected compounds on EPS production was assessed using the phenol–sulphuric acid method. The compounds inhibited EPS production significantly (p > 0.01) with the maximum attenuation (69.93%) of EPS found at ½ MIC of C1 (Fig. 6).
Effect of selected compounds on exopolysaccharide (EPS) production by P. aeruginosa PAO1 quantified using the phenol–sulphuric acid method after 24 h of incubation. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. **p < 0.01: significance compared with control
Alginate inhibition assay
Alginate is one of the important constituents of the matrix material in P. aeruginosa PAO1 biofilms. Hence, the efficacy of selected compounds in impairing the production of alginate was evaluated. The compounds decreased the production of alginate when exposed to their dosage at sub-MICs. The highest inhibition of alginate production was observed at ½ MIC of C1 (63.57%) followed by C2 (60.14%), and C3 showed a lower effect (54.17%) compared to the other two compounds (Fig. 7).
Estimation of biofilm total protein concentration
The concentration of extractable protein in a biofilm is proportional to the number of bacteria present. The amount of extractable protein in P. aeruginosa PAO1 biofilms measured by Lowry’s method (Fig. 8) showed that treatment with ½ MIC of C1, C2 and C3 had significantly (p < 0.01) reduced the protein concentrations to 99.65 μg/ml, 112.43 μg/ml and 112.04 μg/ml compared to control (261.73 μg/ml). In the presence of ¼ MICs of compounds, the protein concentration in the biofilm was increased.
FESEM analysis
The morphological and structural changes of the biofilm in untreated and treated conditions were observed using FESEM (Fig. 9). The observations revealed that the control group appeared as a dense multi-layered EPS matrix with many colonies of varying sizes (Fig. 9a). In contrast, the treated groups showed a reduction in biofilm biomass and were found to be scattered in appearance (Fig. 9b–d). The results of FESEM analysis further confirm the efficacy of the selected compounds as potent disruptors of P. aeruginosa PAO1 biofilms.
Violacein inhibition assay
The antiquorum sensing activity was assessed by evaluating the effect of the selected compounds on QS-regulated violacein production in C. violaceum. The presence of a creamy white halo around the well against the purple lawn of bacteria indicates the antiquorum sensing activity whereas the translucent zone indicates the inhibition of the bacterial growth. The colourless zone of inhibition around the compound-treated wells was found to be opaque (Fig. S1) which indicated that halos are due to the quorum sensing inhibition and not the growth of bacteria. The diameter (mm) of zone of violacein pigment inhibition on treatment with ½ MIC and ¼ MIC of compounds is illustrated in Fig. 10. The zone diameter of violacein inhibition by the compounds ranged from 10.3 ± 0.04 to 15.08 ± 0.08 mm at ½ MICs whereas the zone diameter of violacein inhibition at ¼ MICs was found to be 11.66 ± 0.47 mm and 7.0 ± 0 mm for C1 and C2, respectively. It is noted that C3 at ¼ MIC did not exhibit any effect on violacein production (Fig. 10).
Effect of selected compounds on the motility behaviour of P. aeruginosa PAO1
The flagellum-driven swimming motility and swarming motility of P. aeruginosa PAO1 were affected by the selected compounds at their sub-MIC levels (Fig. 11). The swimming and swarming migration by P. aeruginosa PAO1 in the presence and absence of compounds noted after 24 h are given in Fig. S2 and Fig. S3. The maximum reduction in swimming motility diameter was recorded as 33 ± 1.0 mm by C1 at ½ MIC compared to the control (41.3 ± 0.57 mm). On the other hand, the findings of swarming motility (Fig. 11b) varied from those of swimming motility (Fig. 11a) where the selected compounds shrunk the zone of swarming motility to 4.8 ± 0.28 mm, 5.6 ± 0.57 mm and 6.5 ± 0.5 mm at ½ MIC of C1, C2 and C3, respectively, compared to the control (37.6 ± 0.57 mm).
Effect of selected compounds on the motility of P. aeruginosa PAO1 measured after incubation at 37 °C (24 h). a Swimming motility diameters of P. aeruginosa PAO1 with and without the compounds. b Swarming motility diameters of P. aeruginosa PAO1 in the presence and absence of compounds. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. *p < 0.05; **p < 0.01: significance compared with control
Effect of selected compounds on virulence factor production by P. aeruginosa PAO1
The impact of selected compounds on the production of QS-dependent virulence traits, namely pyocyanin, rhamnolipid, protease, alkaline protease and lipase, was determined to further confirm its antiquorum sensing ability. Pyocyanin is a greenish-blue phenazine pigment and contributes to the progression and pathogenesis of chronic infections in P. aeruginosa (Parai et al. 2018). All three compounds showed a significant decrease in pyocyanin production, with C1 showing the maximum (78.51%) at ½ MIC concentration (Fig. 12a).
Inhibitory effect of selected compounds on the QS-mediated secretion of virulence factors by P. aeruginosa PAO1. a Pyocyanin. b Rhamnolipid. c Protease. d Alkaline protease. e Lipase. Production levels of each virulence factor are quantified as a relative measure to that of the control. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. *p < 0.05; **p < 0.01: significance compared with control
Rhamnolipids are essential biosurfactants for biofilm architecture and swarming motility (Rashiya et al. 2021). The production of rhamnolipids was decreased at both ½ MIC and ¼ MIC upon treatment with C1, C2 and C3, respectively (Fig. 12b). Proteases hold a significant role in the formation of biofilm, motility and antibiotic resistance mechanisms in P. aeruginosa (Kida et al. 2013). The compounds inhibited protease activity, as evidenced by a decrease in the zone of clearance (Fig. 12c) on skim milk agar plates containing casein protein. The other factors, namely alkaline protease (Fig. 12d) and lipase (Fig. 12e), were also found to be significantly (p < 0.01) decreased on treatment with the selected compounds.
Gene expression study of QS-regulated genes of P. aeruginosa PAO1 by qRT-PCR
The mRNA expression levels of various QS-regulated genes of P. aeruginosa PAO1 in the presence of compounds were detected by qRT-PCR. The compounds were found to negatively affect the QS-controlled gene expressions such as lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR based on real-time PCR data (Fig. 13). In particular, C1 drastically reduced the expression of genes including lasI, lasR, rhlI, rhlR and lasB by greater than 85% compared to C2 and C3. All three compounds significantly (p < 0.01) decreased the expression of pqsA and pqsR genes by greater than 90% (Fig. 13). These results suggested that the selected compounds could significantly downregulate the genes of the QS regulatory networks of P. aeruginosa PAO1.
Relative gene expression of QS-controlled genes, namely lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR, of P. aeruginosa PAO1. Cultures were treated with selected compounds for 24 h, and their relative gene expression was quantified by qRT-PCR. Data represented as mean ± standard deviation (SD) of triplicate values. Error bars define the SD of triplicate values. *p < 0.05; **p < 0.01: significance compared with control
Cytotoxicity of selected compounds
The cytotoxicity of the selected compounds on mammalian cells (human PBL cells) was investigated by performing MTT reduction assay. The percentage of cytotoxicity was found to be less than 40% on exposure to different concentrations of compounds (Fig. 14). This suggested that the compounds show lower cytotoxicity against human peripheral blood lymphocyte cells.
Discussion
Extensive antibiotic usage in humans has led to the spread of pathogens with enhanced resistance worldwide. Conventional approaches to the development of new antibiotics have failed in the therapy for infections caused by strains that are resistant to antibiotics. As a result, novel approaches to prevent the emergence of drug resistance should be pursued (Sarkar et al. 2015). P. aeruginosa, a multi-drug-resistant pathogen, forms strong biofilms regulated by a communication network called quorum sensing. Biofilms of P. aeruginosa are highly resistant to antimicrobial treatments because of their strong exopolysaccharide matrix material (Ganesh and Rai 2017). Inhibition of QS in P. aeruginosa, which aids in biofilm formation, could be a potential alternative for treating its associated infections. This strategy could be exploited, as the quorum sensing inhibitors do not affect bacterial growth and could attenuate pathogenesis alone to prevent antibiotic resistance (Scoffone et al. 2019). Hence, this approach of QS interference might address MDR strains of P. aeruginosa and may be developed as anti-infective agents or as a combinatorial therapy along with available antibiotics (Zhong et al. 2020).
The study demonstrated the potential of three synthetic compounds, namely (3-[2-(3,4-dimethoxyphenyl)-2-(1H-indol-3-yl)ethyl]-1-(2-fluorophenyl)urea), (3-(4-fluorophenyl)-2-[(3-methylquinoxalin-2-yl)methylsulfanyl]quinazolin-4-one) and (2-({4-[4-(2-methoxyphenyl)piperazin-1-yl]pyrimidin-2-yl}sulfanyl)-N-(2,4,6-trimethylphenyl)acetamide) (C1–C3), as antibiofilm and antiquorum sensing inhibitors. The compounds were retrieved as hits against the specific target protein LasR of P. aeruginosa through a molecular docking–based virtual screening approach used in our previous work (Vetrivel et al. 2021). These compounds were selected as they possessed the highest docking scores in the screening deck and did not form a significant hydrogen bond contact with the amino acid residue Trp60 in the active site which is required for the activation of LasR. Also, the lack of the ability of these compounds to form hydrogen bonds with crucial active site residues, namely Tyr56, Trp60, Asp73 and Ser129, will lead to the inactivation of LasR and attributed to its QS inhibitory activity (Vetrivel et al. 2021). Thus, the inactivation of the QS pathway would lead to the suppression of biofilm formation and virulence factor production in P. aeruginosa. The current investigation was done to ascertain whether the selected compounds could retard bacterial communication and bacterial attachment and reduce preformed biofilms and secretion of virulence factors at sub-MIC levels. Additionally, we looked at the impact of these compounds on the QS-controlled gene expression patterns.
The most important feature to be considered in developing any antimicrobial agent is its ability to target bacterial virulence rather than destroying the bacterial strain (Pattnaik et al. 2018). In this context, our results showed that the compounds possessed lower MICs (Table S2) and did not possess natural antimicrobial properties. The sub-minimum inhibitory concentrations (½ MIC and ¼ MIC) of the compounds were evaluated for their impact on bacterial growth. The results revealed that the compounds did not affect the growth pattern of P. aeruginosa PAO1 at its sub-MIC doses (Fig. 1). Yet, it possesses an inhibitory effect on both the earliest and matured levels of the biofilm cycle. As a result, the ability of the selected compounds to prevent the formation of P. aeruginosa PAO1 biofilms, as well as their anti-QS efficacy and the production of QS-associated virulence factors, was investigated at sub-MIC levels.
In general, P. aeruginosa utilises signalling molecules referred to as AHLs to synchronise the gene expression in forming biofilm, motility and secretion of virulence factors (Chan et al. 2015). The emergence of various topological changes including cell–cell communications, overproduction of EPS, extracellular fibrillar networks, pellicles, multiple layers and microcolony contributed to stable biofilm states (Rollefson et al. 2011). Therefore, during the beginning phase of biofilm development, growth of bacteria is rapid and surface adhesion occurs reversibly within a few hours (Sarkar et al. 2015).
The crystal violet staining assay revealed that all three compounds effectively prevented P. aeruginosa PAO1 from forming biofilms (Fig. 2). C1 exhibited the highest potency to inhibit biofilm formation at ½ MIC dose compared to C2 and C3. A similar study by Rierra et al. (2010) reported that inhibition of P. aeruginosa biofilms by cephalosporin CXA-101 occurred dose dependently. Disruption of preformed matured biofilm is an important characteristic as it triggers the dispersion of biofilms (Rendueles et al. 2013). Our results (Fig. 3) demonstrated that the compounds possessed the capacity to disrupt preformed matured biofilm of P. aeruginosa PAO1 with the highest eradication of 67.32% at ½ MIC level of C1 followed by C2 with 48.73%, and C3 with 61.05%. The activity of metabolically active biofilm cells was quantified by MTT reduction assay to validate the crystal violet data. Due to cell inactivation, the number of active biofilm-forming cells in P. aeruginosa PAO1 exposed to ½ MIC of C1, C2 and C3 was drastically reduced (Fig. 4). This finding suggested that as the number of biofilm-forming cells decreased, the compounds could further reduce the amount of biofilm formed.
The initial step in biofilm formation is the adherence of bacteria in the planktonic state to a tissue or an abiotic surface (Jorge et al. 2012). Our findings showed that the compounds could prevent bacterial attachment to the surface with a remarkable decrease in absorbance at 570 nm (Fig. 5). Hence, this antiadhesive activity of the compounds may further impact the development of biofilm by P. aeruginosa PAO1. The structured matrix consisting of exopolysaccharides, lipids, proteins and biofilm persistence are well correlated as they block the invasion of antimicrobial agents into cells by serving as a protective shield (Flemming and Wingender 2010; Zhou et al. 2017). In connection to various reports, it is also suggested that inhibition of EPS has a positive effect on antibiotic activity by enhancing drug penetration and thereby arresting progression of biofilm formation, disruption of biofilm structure and demolition of entrapped bacterial cells (Viju et al. 2013).
P. aeruginosa PAO1 biofilms showed a significant decrease in EPS production after exposure to sub-MICs of the selected compounds. The maximum inhibition of EPS to 69.93% was exhibited by C1 (Fig. 6). The rhl QS system regulated EPS, alginate and rhamnolipid production in P. aeruginosa PAO1 holds a predominant role in the development of biofilm and protects bacteria from adverse conditions inside hosts as well as aids in the attachment to host cells (Kalishwaralal et al. 2010). As shown in Figs. 6 and 7, the compounds inhibited EPS and alginate production at ½ MIC with the highest attenuation rate compared to ¼ MIC level. These findings agreed with Rasamiravaka et al. (2015), who stated that increasing the concentration of the QSI quercetin increased the inhibition of EPS and alginate in a concentration-dependent manner. A decrease in the production of EPS would likely seem to enhance the penetration capacity of antibiotics into the bacterial cells. Thus, these characteristics to inhibit or destroy biofilm formation, disruption, attachment and EPS production could portray these compounds as better antibiofilm agents. In addition, the reduced total protein concentration in P. aeruginosa PAO1 biofilms treated with compounds (Fig. 8) suggested that they may interfere the EPS matrix material made up of sugars and proteins.
SEM results (Fig. 9) further confirmed our in vitro studies and proved that the compounds could reduce biofilm by preventing and disrupting bacterial cell attachment to the surface. These results agreed with the previous report by Kim et al. (2015), who examined the same pattern of biofilm inhibition on treatment with the QSI 6-gingerol. Violacein is a purple pigment regulated by a LuxR homologue and CViR in Chromobacterium violaceum and responds to AHL molecule (Singh et al. 2017). The selected compounds exhibited an inhibitory effect on violacein pigment production at sub-MIC (Fig. 10 and Fig. S3). Ravichandran et al. (2018) have observed that the QS mechanism in C. violaceum was inhibited by QSIs, namely catechin 7-xyloside, sappanol and butein and represented wide-spectrum anti-infective activity. According to Pattnaik et al. (2018), Diaporthe phaseolorum SSP12 fungal extract which was successful in destroying P. aeruginosa biofilms inhibited the production of violacein in C. violaceum in a concentration-dependent way.
P. aeruginosa biofilm formation is invariably mediated by colonisation and attachment, which are initiated via swimming and swarming motilities (Luo et al. 2017). Earlier reports have suggested that downregulation of motility and attachment decreases the severity in pathogenesis and would directly impact biofilm development in P. aeruginosa (Gutierrez et al. 2013; El-Mowafy et al. 2014). The experimental evidence obtained in this study revealed that the compounds did not markedly reduce swimming motility (Fig. 11a). In contrast, it showed a more significant impact on the swarming motility of P. aeruginosa PAO1 at sub-MIC levels compared to the control (Fig. 11b). These results agreed with the report by Luo et al. (2017) who evidenced that the QSI baicalin derived from Scutellaria baicalensis had a minimal effect on swimming motility but had a significant impact on swarming motility that eventually demonstrated the QSI’s capacity to disrupt type IV pili and flagella function.
The pathogenicity of P. aeruginosa–mediated infections is widely dependent on the virulence factors. Hence, the effect of QSIs at sub-MIC levels on the QS-controlled secretion of virulence factors by P. aeruginosa PAO1 was studied. Pyocyanin is a secondary redox metabolite and a phenazine derivative that mediates chelates iron uptake and various cellular functions that influence the expression of virulence (Stehling et al. 2008). Pyocyanin production by P. aeruginosa PAO1 was significantly (p < 0.01) reduced when treated with sub-MIC of C1 (78.51%), C2 (77.53%) and C3 (68.85%) (Fig. 12a). Our findings agreed with those of Ouyang et al. (2016), who discovered that P. aeruginosa PAO1 produces less pyocyanin in the presence of QSI quercetin at a concentration of 16 μg/ml.
Vandeputte et al. (2011) stated that when exposed to 4 mM naringenin, virulence factors like pyocyanin and elastase were reduced. Furthermore, the other indicators of QS, namely rhamnolipid, protease, alkaline protease and lipase, were also analysed (Fig. 12b–d). Rhamnolipids are amphiphilic biosurfactants that facilitate motility in P. aeruginosa and are linked with dispersion of matured biofilm (O’May and Tufenkji 2011). They also serve as protective shields in the innate immune system (Jensen et al. 2007). In such context, rhamnolipid production was significantly affected in the presence of C1, C2 and C3 (Fig. 12b) with the highest inhibition of 73.97% exhibited by C1 (½ MIC). Kim et al. (2015) proved that rhamnolipid production was decreased in P. aeruginosa PAO1 by around 36–60% on treatment with the QSI 6-gingerol at a concentration of 0.1–100 μM. Inhibition of rhamnolipids is crucial as it aids in creating and maintaining fluid channels around the biofilm base which help in oxygen and water flow (Rashiya et al. 2021).
Proteases are hydrolytic enzymes that counteract the effects of the host defence system and aid in the damage of host tissues (Andrejko et al. 2013). P. aeruginosa produces various extracellular proteases, namely protease IV, alkaline protease, elastase A (LasA protease) and elastase B (LasB elastase) (Le Berre et al. 2008). The impact of selected compounds to degrade casein protein in skim milk agar plates determines its effect on the protease activity of P. aeruginosa PAO1. The results (Fig. 12c) revealed a smaller zone of protease clearance around the wells treated with compounds. Inhibition of protease production suggested that the compounds could further retard biofilm formation by P. aeruginosa. Elastases are prototypes that promote bacterial invasion by degrading infected tissues (Hoge et al. 2010). Together, lasA protease and lasB elastase are essential for the expansion of infections by rupturing the interstitial tissues in hosts (Stehling et al. 2008).
Alkaline protease is an exoprotease that helps in the maturation of lasA protease. However, it is less effective than the post-lysine cleaving enzyme and elastase. It is a metalloprotease that actively participates in the hydrolysis of important biological proteins like matrix metalloproteinases, complement factors, cytokines, human IFN-γ and TNF-α (Aybey and Demirkan 2016). In our study, we noticed a considerable decline in alkaline protease secretion by P. aeruginosa PAO1 exposed to sub-MICs of compounds (Fig. 12d) with the highest inhibition shown by C1 (70.12%) compared to control. As the las QS system controls the production of alkaline protease, a decrease in the production of alkaline protease might be due to the inhibitory effect of the compounds on las system. The compounds were also found to be effective in inhibiting lipase production by P. aeruginosa PAO1 with the highest inhibition of 78.46% exhibited by C1 (Fig. 12e).
The P. aeruginosa regulatory QS system (las and rhl) plays a crucial role in controlling the synthesis of these virulence components and their activity. When cell density reaches a threshold, an increased number of AHLs binds to lasR and activates the expression of essential regulatory genes of QS in P. aeruginosa PAO1. Additionally, several pieces of evidence have also reported that the las system is majorly attributed to biofilm formation and regulates rhl expression. Furthermore, the cells that significantly expressed las genes were found during the initial phase of biofilm formation and were stable throughout the infection (Furiga et al. 2016; Teerapo et al. 2019). Therefore, qRT-PCR was done to corroborate the results of gene expression analysis of QS-regulated genes with that of the virulence factor production in P. aeruginosa PAO1 investigated previously through various biochemical assays.
lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR are the major QS genes that are linked to biofilm formation and virulence factor production in P. aeruginosa. Downregulating these genes would possibly shut down the entire QS network of P. aeruginosa and prevent the spread of biofilm development. According to qRT-PCR results, the expression of target QS genes, namely lasI, lasR, rhlI, rhlR, lasB, pqsA and pqsR, was downregulated compared to untreated control. Downregulation of the aforesaid downstream QS genes supports our above observations with respect to the antivirulence activity of the compounds (Fig. 13). Generally, lasIR circuit controls the genes, namely toxA (exotoxin A), lasA (protease) and lasB (elastase); rhlIR system regulates genes rhlAB (rhamnolipids), lecA (lectins) and aprA (alkaline protease); and pqs controls phzABCDEFG and phzM (pyocyanin) genes (Jakobsen et al. 2013). In particular, lasR regulates the expression of genes necessary for elastase and protease activity, along with the genes of the rhl QS system (Singh et al. 2017). The rhl system aids in the expression of genes required for the production of pyocyanin and rhamnolipid. Aside from that, the rhl system is also necessary for the maintenance of non-colonised channels surrounded by macrocolonies in structured biofilms and promotes mushroom-shaped structures in later stages of the biofilm cycle. On the other side, the rhlR and pqsR genes on binding to their specific signalling molecules initiate the formation of biofilm in P. aeruginosa by mediating the synthesis of eDNA release, swarming motility, pyocyanin and rhamnolipid (Chatterjee et al. 2017).
From Fig. 13, a significant reduction in the expression of lasI and lasR on binding of QSIs would have led to the decrease in the production of virulence factors, namely protease, elastase, rhamnolipids, alkaline protease and pyocyanin in P. aeruginosa along with downregulation of the rhl QS system. The downregulation of genes of the rhl system, namely rhlI and rhlR, might have suppressed the production of pyocyanin and rhamnolipid. Downregulation of lasR influenced the gene necessary for elastase activity (lasB), whose expression was also found to be decreased and could lead to inhibition of elastase production in P. aeruginosa. A substantial decrease in the expression of pqsA and pqsR genes (Fig. 13) further enhanced the capacity of these compounds to eradicate P. aeruginosa biofilms along with the key regulators (las and rhl) of QS circuitry. These findings concord with the study of Zhong et al. (2020) who stated that QSIs of LasR, namely catechin 7-xyloside, sappanol and butein, downregulated the expression of lasI, lasR, rhlI and rhlR genes. This type of reduced gene expression not only lowers the virulence factor production but also impacts the synthesis of QS signalling molecules and the formation of biofilm since these functions are interlinked in the QS network of P. aeruginosa. Thus, it would be suggested that interference with such critical systems rather than directly killing bacteria would decrease the problem of developing resistance which is, currently, an issue of concern.
Finally, the cytotoxicity of the compounds against eukaryotic cells may represent a barrier that eventually limits their utilisation in clinical fields (Kang et al. 2014). The compounds showed low toxicity against human peripheral blood lymphocyte (PBL) cells (Fig. 14). Thus, these findings confront the application of the three selected compounds as leads towards the development of potent therapeutic agent with antibiofilm, antiquorum sensing and antivirulence properties.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Ahmed S, Rudden M, Smyth TJ, Dooley JSG, Marchant R, Banat IM (2019) Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol 103:3521–3535. https://doi.org/10.1007/s00253-019-09618-0
Alhajlan M, Alhariri M, Omri A (2013) Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother 57:2694–2704. https://doi.org/10.1128/AAC.00235-13
Andrejko M, Zdybicka-Barabas A, Janczarek M, Cytryńska M (2013) Three Pseudomonas aeruginosa strains with different protease profiles. Acta Biochim Pol 60:83–90. https://doi.org/10.18388/abp.2013_1955
Anju VT, Busi S, Mohan MS, Ranganathan S, Ampasala DR, Kumavath R, Dyavaiah M (2022) In vivo, in vitro and molecular docking studies reveal the anti-virulence property of hispidulin against Pseudomonas aeruginosa through the modulation of quorum sensing. Int. Biodeterior. Biodegradation. 174. https://doi.org/10.1016/j.ibiod.2022.105487
Arciola CR, Campoccia D, Montanaro L (2018) Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16. https://doi.org/10.1038/s41579-018-0019-y
Aybey A, Demirkan E (2016) Inhibition of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa by human serum paraoxonase. J Med Microbiol 65:105–113. https://doi.org/10.1099/jmm.0.000206
Bajire SK, Jain S, Johnson RP, Shastry RP (2021) 6-Methylcoumarin attenuates quorum sensing and biofilm formation in Pseudomonas aeruginosa PAO1 and its applications on solid surface coatings with polyurethane. Appl Microbiol Biotechnol 105:8647–8661. https://doi.org/10.1007/s00253-021-11637-9
Baysse C, Cullinane M, Dénervaud V, Burrowes E, Dow JM, Morrissey JP, Tam L, Trevors JT, O’Gara F (2005) Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiol 151:2529–2542. https://doi.org/10.1099/mic.0.28185-0
Bose SK, Nirbhavane P, Batra M, Chhibber S, Harjai K (2020) Nanolipoidal α-terpineol modulates quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Nanomedicine 15:1743–1760. https://doi.org/10.2217/nnm-2020-0134
Chan K-G, Liu Y-C, Chang C-Y (2015) Inhibiting N-acyl-homoserine lactone synthesis and quenching Pseudomonas quinolone quorum sensing to attenuate virulence. Front Microbiol 6:1173. https://doi.org/10.3389/fmicb.2015.01173
Chatterjee M, D’Morris S, Paul V, Warrier S, Vasudevan AK, Vanuopadath M, Nair SS, Paul-Prasanth B, Mohan CP, Biswas R (2017) Mechanistic understanding of phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl Microbiol Biotechnol 101:8223–8236. https://doi.org/10.1007/s00253-017-8546-4
Chatterjee S, Paul P, Chakraborty P, Das S, Sarker RK, Sarkar S, Das A, Tribedi P (2021) Cuminaldehyde exhibits potential antibiofilm activity against Pseudomonas aeruginosa involving reactive oxygen species (ROS) accumulation: a way forward towards sustainable biofilm management. 3 Biotech. 11:485–496. https://doi.org/10.1007/s13205-021-03013-1
Chen T, Sheng J, Fu Y, Li M, Wang J, Jia A-Q (2017) 1H NMR-based global metabolic studies of Pseudomonas aeruginosa upon exposure of the quorum sensing inhibitor resveratrol. J Proteome Res 16:824–830. https://doi.org/10.1021/acs.jproteome.6b00800
Chu W, Zhou S, Jiang Y, Zhu W, Zhuang X, Fu J (2013) Effect of traditional Chinese herbal medicine with antiquorum sensing activity on Pseudomonas aeruginosa. Evid.-based Complement. Altern. Med. 648257. https://doi.org/10.1155/2013/648257
Ciofu O, Tolker-Nielsen T (2019) Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front. Microbiol. 10. https://doi.org/10.3389/fmicb.2019.00913
Das MC, Sandhu P, Gupta P, Rudrapaul P, De UC, Tribedi P, Akhter Y, Bhattacharjee S (2016) Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: a combinatorial study with azithromycin and gentamicin. Sci Rep 6:23347. https://doi.org/10.1038/srep23347
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Sci 280:295–298. https://doi.org/10.1126/science.280.5361.295
Defoirdt T (2018) Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol 26:313–328. https://doi.org/10.1016/j.tim.2017.10.005
Deryabin D, Galadzhieva A, Kosyan D, Duskaev G (2019) Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: modes of action. Int J Mol Sci 20:5588. https://doi.org/10.3390/ijms20225588
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shabaan MI (2014) Aspirin is an effective inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog 74:25–32. https://doi.org/10.1016/j.micpath.2014.07.008
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. https://doi.org/10.1128/jb.172.2.884-900.1990
Esteves GM, Esteves J, Resende M, Mendes L, Azevedo AS (2022) Antimicrobial and antibiofilm coating of dental implants-past and new perspectives. Antibiotics. 11. https://doi.org/10.3390/antibiotics11020235
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633. https://doi.org/10.1038/nrmicro2415
Fothergill JL, Neill DR, Loman N, Winstanley C, Kadioglu A (2014) Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat Commun 5:4780. https://doi.org/10.1038/ncomms5780
Furiga A, Lajoie B, El Hage S, Baziard G, Roques C (2016) Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by combining the drugs with a new quorum-sensing inhibitor. Antimicrob Agents Chemother 60:1676–1686. https://doi.org/10.1128/AAC.02533-15
Ganesh PS, Rai VR (2017) Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J Tradit Complement Med 8:170–177. https://doi.org/10.1016/j.jtcme.2017.05.008
Gutierrez M, Choi MH, Tian B, Xu J, Rho JK, Kim MO, Cho Y-H, Yoon SC (2013) Simultaneous inhibition of rhamnolipid and polyhydroxyalkanoic acid synthesis and biofilm formation in Pseudomonas aeruginosa by 2-bromoalkanoic acids: effect of inhibitor alkyl-chain-length. PLoS ONE 8:e73986. https://doi.org/10.1371/journal.pone.0073986
Hao S, Yang D, Zhao L, Shi F, Ye G, Fu H, Lin J, Guo H, He R, Li J, Chen H, Khan MF, Li Y, Tang H (2021) EGCG-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Int J Mol Sci 22:4946–4964. https://doi.org/10.3390/ijms22094946
Hernando-Amado S, Alcalde-Rico M, Gil-Gil T, Valverde JR, Martínez JL (2020) Naringenin inhibition of the Pseudomonas aeruginosa quorum sensing response is based on its time-dependent competition with N-(3-oxo-dodecanoyl)-L-homoserine lactone for LasR binding. Front. Mol. Biosci. 7. https://doi.org/10.3389/fmolb.2020.00025
Hoge R, Pelzer A, Rosenau F, Wilhelm S (2010) Weapons of a pathogen: proteases and their role in virulence of Pseudomonas aeruginosa. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. 2:383–395. Edited by A. Mendez-Vilas. Microbiology book series, Formatex Research Center.
Howe TR, Iglewski BH (1984) Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun 43:1058–1063. https://doi.org/10.1128/iai.43.3.1058-1063.1984
Igarashi M, Miyazawa T (2001) The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim Biophys Acta Mol Cell Biol Lipids 1530:162–171. https://doi.org/10.1016/s1388-1981(00)00180-3
Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen TB, Friedrich K, Uthe F, Jensen PØ, Moser C, Nielsen KF, Eberl L, Larsen TO, Tanner D, Høiby N, Bjarnsholt T, Givskov M (2012) Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother 56:2314–2325. https://doi.org/10.1128/aac.05919-11
Jakobsen TH, Bjarnsholt T, Jensen PØ, Givskov M, Høiby N (2013) Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol 8:901–921. https://doi.org/10.2217/fmb.13.57
Jensen PØ, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Høiby N (2007) Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiol 153:1329–1338. https://doi.org/10.1099/mic.0.2006/003863-0
Jorge P, Lourenço A, Pereira MO (2012) New trends in peptide-based anti-biofilm strategies: a review of recent achievements and bioinformatic approaches. Biofouling 28:1033–1061. https://doi.org/10.1080/08927014.2012.728210
Kalia VC, Patel SKS, Kang YC, Lee J-K (2019) Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv 37:68–90. https://doi.org/10.1016/j.biotechadv.2018.11.006
Kalishwaralal K, BarathManiKanth S, Pandian SRK, Deepak V, Gurunathan S (2010) Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B: Biointerfaces 79:340–344. https://doi.org/10.1016/j.colsurfb.2010.04.014
Kang SJ, Park SJ, Mishing-Ochir T, Lee B-J (2014) Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther 12:1477–1486. https://doi.org/10.1586/14787210.2014.976613
Karuppiah V, Thirunanasambandham R, Thangaraj G (2021) Anti-quorum sensing and antibiofilm potential of 1,8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J Microbiol Biotechnol 37. https://doi.org/10.1007/s11274-021-03029-y
Kida Y, Taira J, Yamamoto T, Higashimoto Y, Kuwano K (2013) EprS, an autotransporter protein of Pseudomonas aeruginosa, possessing serine protease activity induces inflammatory responses through protease-activated receptors. Cell Microbiol 15:1168–1181. https://doi.org/10.1111/cmi.12106
Kim H-S, Lee S-H, Byun Y, Park H-D (2015) 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 5:8656. https://doi.org/10.1038/srep08656
Kostylev M, Kim DY, Smalley NE, Salukhe I, Greenberg EP, Dandekar AA (2019) Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci U S A 116:7027–7032. https://doi.org/10.1073/pnas.1819796116
Le Berre R, Nguyen S, Nowak E, Kipnis E, Pierre M, Ader F, Courcol R, Guery BP, Faure K, Pyopneumagen Group (2008) Quorum-sensing activity and related virulence factor expression in clinically pathogenic isolates of Pseudomonas aeruginosa. Clin Microbiol Infect 14:337–343. https://doi.org/10.1111/j.1469-0691.2007.01925.x
Lee J-H, Cho MH, Lee J (2011) 3-indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 13:62–73. https://doi.org/10.1111/j.1462-2920.2010.02308.x
Lee JH, Kim YG, Cho MH, Kim JA, Lee J (2012) 7-Fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol Lett 329:36–44. https://doi.org/10.1111/j.1574-6968.2012.02500.x
Liang H, Li L, Dong Z, Surette MG, Duan K (2008) The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production. J Bacteriol 190:6217–6227. https://doi.org/10.1128/JB.00428-08
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Luo J, Dong B, Wang K, Cai S, Liu T, Cheng X, Lei D, Chen Y, Li Y, Kong J, Chen Y (2017) Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 12:e0176883. https://doi.org/10.1371/journal.pone.0176883
Majumdar M, Dubey A, Goswami R, Misra TK, Roy DN (2020) In vitro and in silico studies on the structural and biochemical insight of anti-biofilm activity of andrograpanin from Andrographis paniculata against Pseudomonas aeruginosa. World J Microbiol Biotechnol 36:143–160. https://doi.org/10.1007/s11274-020-02919-x
Moghaddam MM, Khodi S, Mirhosseini A (2014) Quorum sensing in bacteria and a glance on Pseudomonas aeruginosa. Clin Microbiol 3:156. https://doi.org/10.4172/2327-5073.1000156
O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL (2013) A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A 110:17981–17986. https://doi.org/10.1073/pnas.1316981110
O’May C, Tufenkji N (2011) The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol 77:3061–3067. https://doi.org/10.1128/aem.02677-10
O’Reilly MC, Dong SH, Rossi FM, Karlen KM, Kumar RS, Nair SK, Blackwell HE (2018) Structural and biochemical studies of non-native agonists of the LasR quorum-sensing receptor reveal an L3 loop “out” conformation for LasR. Cell Chem Biol 25:1128–1139. https://doi.org/10.1016/j.chembiol.2018.06.007
Ouyang J, Sun F, Feng W, Sun Y, Qiu X, Xiong L, Liu Y, Chen Y (2016) Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J Appl Microbiol 120:966–974. https://doi.org/10.1111/jam.13073
Owlia P, Rasooli I, Saderi H, Aliahmadi M (2007) Retardation of biofilm formation with reduced productivity of alginate as a result of Pseudomonas aeruginosa exposure to Matricaria chamomilla essential oil. Pharmacogn Mag 3:83–89
Packiavathy IASV, Priya S, Pandian SK, Ravi AV (2014) Inhibition of biofilm development of uropathogens by curcumin-an anti-quorum sensing agent from Curcuma longa. Food Chem 148:453–460. https://doi.org/10.1016/j.foodchem.2012.08.002
Pang Z, Raudonis R, Glick BR, Lin T-J, Cheng Z (2018) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192. https://doi.org/10.1016/j.biotechadv.2018.11.013
Parai D, Banerjee M, Dey P, Chakraborty A, Islam E, Mukherjee SK (2018) Effect of reserpine on Pseudomonas aeruginosa quorum sensing mediated virulence factors and biofilm formation. Biofouling 34:320–334. https://doi.org/10.1080/08927014.2018.1437910
Parasuraman P, Devadatha B, Sarma VV, Ranganathan S, Ampasala DR, Reddy D, Kumavath R, Kim I-W, Patel SKS, Kalia VC, Lee J-K, Siddhardha B (2020) Inhibition of microbial quorum sensing mediated virulence factors by Pestalotiopsis sydowiana. J Microbiol Biotechnol 30:571–582. https://doi.org/10.4014/jmb.1907.07030
Pattnaik SS, Ranganathan S, Ampasala DR, Syed A, Ameen F, Busi S (2018) Attenuation of quorum sensing regulated virulence and biofilm development in Pseudomonas aeruginosa PAO1 by Diaporthe phaseolorum SSP12. Microb Pathog 118:177–189. https://doi.org/10.1016/j.micpath.2018.03.031
Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M (2022) Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 7. https://doi.org/10.1038/s41392-022-01056-1
Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO (2015) Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem 7:493–512. https://doi.org/10.4155/fmc.15.6
Rajkumari J, Borkotoky S, Reddy D, Mohanty SK, Kumavath R, Murali A, Suchiang K, Busi S (2019) Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: insights from in vitro, in vivo and in silico studies. Microbiol Res 226:19–26. https://doi.org/10.1016/j.micres.2019.05.001
Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 759348. https://doi.org/10.1155/2015/759348
Rashiya N, Padmini N, Ajilda AAK, Prabakaran P, Durgadevi R, Ravi AV, Ghosh S, Sivakumar N, Selvakumar G (2021) Inhibition of biofilm formation and quorum sensing mediated virulence in Pseudomonas aeruginosa by marine sponge symbiont Brevibacterium casei strain Alu 1. Microb. Pathog. 150:104693. https://doi.org/10.1016/j.micpath.2020.104693
Ravichandran V, Zhong L, Wang H, Yu G, Zhang Y, Li A (2018) Virtual screening and biomolecular interactions of CViR-based quorum sensing inhibitors against Chromobacterium violaceum. Front Cell Infect Microbiol 8:292. https://doi.org/10.3389/fcimb.2018.00292P
Razdan K, Gondil VS, Chhibber S, Singh KK, Sinha VR (2022) Levofloxacin loaded clove essential oil nanoscale emulsion as an efficient system against Pseudomonas aeruginosa biofilm. J. Drug Deliv. Sci. Technol. 68. https://doi.org/10.1016/j.jddst.2021.103039
Rendueles O, Kalpan JB, Ghigo J-M (2013) Antibiofilm polysaccharides. Environ Microbiol 15:334–346. https://doi.org/10.1111/j.1462-2920.2012.02810.x
Rierra E, Maciá MD, Mena A, Mulet X, Pérez JL, Ge Y, Oliver A (2010) Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J Antimicrob Chemother 65:1399–1404. https://doi.org/10.1093/jac/dkq143
Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, Kniha H, Gahlert M (2017) In vitro biofilm formation on titanium and zirconia implant surfaces. J. Periodontol. 88. https://doi.org/10.1902/jop.2016.160245
Rollefson JB, Stephen CS, Tien M, Bond DR (2011) Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriol 193:1023–1033. https://doi.org/10.1128/jb.01092-10
Saising J, Dube L, Ziebandt A-K, Voravuthikunchai SP, Nega M, Götz F (2012) Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob Agents Chem 56:5804–5810. https://doi.org/10.1128/AAC.01296-12
Sarabhai S, Sharma P, Capalash N (2013) Ellagic acid derivatives from Terminalia chebula Retz downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS ONE 8:e53441. https://doi.org/10.1371/journal.pone.0053441
Sarkar R, Mondal C, Bera R, Chakraborty S, Barik R, Roy P, Kumar A, Yadav KK, Choudhury J, Chaudhary SK, Samanta SK, Karmakar S, Das S, Mukherjee PK, Mukherjee J, Sen T (2015) Antimicrobial properties of Kalanchoe blossfeldiana: a focus on drug resistance with particular reference to quorum sensing-mediated bacterial biofilm formation. J Pharm Pharmacol 67:951–962. https://doi.org/10.1111/jphp.12397
Sarkar R, Mittal N, Sorensen J, Sen T (2018) A comparison of the bioactivity of usnic acid versus methylphloroacetophenone. Nat Prod Commun 13:1673–1676. https://doi.org/10.1177/1934578X1801301224
Scoffone VC, Trespidi G, Chiarelli LR, Barbieri G, Buroni S (2019) Quorum sensing as antivirulence target in cystic fibrosis pathogens. Int J Mol Sci 20:1838. https://doi.org/10.3390/ijms20081838
Shariff M, Chatterjee M, Morris SD, Paul V, Vasudevan AK, Mohan CG, Paul-Prasanth B, Biswas R (2022) Enhanced inhibition of Pseudomonas aeruginosa virulence factor production and biofilm development by sublethal concentrations of eugenol and phenyllactic acid. Lett Appl Microbiol 75:1336–1345. https://doi.org/10.1111/lam.13803
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 8. https://doi.org/10.1186/s13756-019-0533-3
Singh VK, Mishra A, Jha B (2017) Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum-sensing controlled virulence factor production in Pseudomonas aeruginosa. Front Cell Infect Microbiol 7:337. https://doi.org/10.3389/fcimb.2017.00337
Sohns JM, Bavendiek U, Ross TL, Bengel FM (2017) Targeting cardiovascular implant infection: multimodality and molecular imaging. Circ. Cardiovasc. Imaging. 10. https://doi.org/10.1161/CIRCIMAGING.117.005376
Stehling EG, da Silveira WD, da Silva LD (2008) Study of biological characteristics of Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis and from patients with extra-pulmonary infections. Braz J Infect Dis 12:86–88. https://doi.org/10.1590/s1413-86702008000100018
Suo A, Hua Z, Wu C, Fan G, Li T, Cong K (2022) Effects of ginkgolic acid (C15:1) on biofilm formation, pathogenic factor production and quorum sensing of Pseudomonas aeruginosa. Microb. Pathog. 173. https://doi.org/10.1016/j.micpath.2022.105813
Teerapo K, Roytrakul S, Sistayanarain A, Kunthalert D (2019) A scorpion venom peptide derivatives BmKn-22 with potent antibiofilm activity against Pseudomonas aeruginosa. PLoS ONE 14:e0218479. https://doi.org/10.1371/journal.pone.0218479
Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, Rajaonson S, Diallo B, Mol A, Baucher M, El Jaziri M (2011) The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiol 157:2120–2132. https://doi.org/10.1099/mic.0.049338-0
Vetrivel A, Natchimuthu S, Subramanian V, Murugesan R (2021) High-throughput virtual screening for a new class of antagonist targeting LasR of Pseudomonas aeruginosa. ACS Omega 6:18314–18324. https://doi.org/10.1021/acsomega.1c02191
Viju N, Satheesh S, Vincent SGP (2013) Antibiofilm activity of coconut (Cocos nucifera Linn.) husk fibre extract. Saudi J Biol Sci 20:85–91. https://doi.org/10.1016/j.sjbs.2012.11.002
Walczak M, Michalska-Sionkowska M, Olkiewicz D, Tarnawska P, Warżyńska O (2021) Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Mol 26:2723–2734. https://doi.org/10.3390/molecules26092723
Wang S, Feng Y, Han X, Cai X, Yang L, Liu C, Shen L (2021) Inhibition of virulence factors and biofilm formation by wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting pqs quorum-sensing system. Int J Mol Sci 22:12699. https://doi.org/10.3390/ijms222312699
Whiteley M, Diggle SP, Greenberg EP (2017) Progress in and promise of bacterial quorum sensing research. Nature 551:313–320. https://doi.org/10.1038/nature24624
Zhao Z, Guo M, Xu X, Hu Y, Liu D, Wang C, Liu X, Li Y (2022) In vitro synergistic inhibitory activity of natural alkaloid berberine combined with azithromycin against alginate production by Pseudomonas aeruginosa PAO. Med. Cell. Longev, Oxid. https://doi.org/10.1155/2022/3858500
Zhong L, Ravichandran V, Zhang N, Wang H, Bian X, Zhang Y, Li A (2020) Attenuation of Pseudomonas aeruginosa quorum sensing by natural products: virtual screening, evaluation and biomolecular interactions. Int J Mol Sci 21:2190. https://doi.org/10.3390/ijms21062190
Zhou J, Bi S, Chen H, Chen T, Yang R, Li M, Fu Y, Jia A-Q (2017) Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Front Microbiol 8:769. https://doi.org/10.3389/fmicb.2017.00769
Zhou J-W, Luo H-Z, Jiang H, Jian T-K, Chen Z-Q, Jia A-Q (2018) Hordenine: a novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. J Agric Food Chem 66:1620–1628. https://doi.org/10.1021/acs.jafc.7b05035
Author information
Authors and Affiliations
Contributions
Rajeswari Murugesan conceived and designed the study. Material preparation, data collection and analysis were performed by Aishwarya Vetrivel, Monica Ramasamy and Soundhariya Madheswaran. The results of the study were interpreted and approved by Rajeswari Murugesan. The first draft of the manuscript was written by Aishwarya Vetrivel. Preethi Vetrivel, Kavitha Dhandapani and Santhi Natchimuthu corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vetrivel, A., Vetrivel, P., Dhandapani, K. et al. Inhibition of biofilm formation, quorum sensing and virulence factor production in Pseudomonas aeruginosa PAO1 by selected LasR inhibitors. Int Microbiol 26, 851–868 (2023). https://doi.org/10.1007/s10123-023-00338-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00338-0