Abstract
The small leucine rich proteoglycans (SLRPs), structurally consisting of protein cores and various glycosaminoglycan side chains, are grouped into five classes based on common structural and functional properties. Besides being an important structural component of extracellular matrix (ECM), SLRPs have been implicated in the complex network of signal transduction and host immune responses. The focus of this review is on SLRPs in host immunity. Because host immunity plays an important part in the pathogenesis of renal diseases, the role of SLRPs in this set of diseases will also be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small leucine rich proteoglycans (SLRPs), named after their relatively small size and the leucine rich repeats (LRR) in their structures, consist of two main structural components: protein cores and various glycosaminoglycan (GAG) side chains, which form decorin, biglycan, and lumican etc (Dellett et al. 2012; Schaefer and Iozzo 2008). The SLRP family currently has 17 members that are grouped into five distinct classes based on their conservation and homology at the protein and genomic levels, the number of the LRRs, the spacing of the N-terminal cysteine residues in the protein cores, and their chromosomal organization (Fig. 1) (Dellett et al. 2012; Schaefer and Iozzo 2008). The unique structural characteristics of GAG types provide some of the structural basis for the multitude of the biological functions of SLRPs (Theocharis et al. 2010). So far, biglycan and decorin from class I are among the best studied SLRPs in a variety of biological and pathological processes (Dellett et al. 2012; De Felice et al. 2003; Gubbiotti et al. 2015). Besides being an important component of ECM, SLRPs have been implicated in cell proliferation and migration (Dellett et al. 2012; Schaefer and Iozzo 2008; Kalamajski and Oldberg 2009; Keene et al. 2000; Kresse et al. 1997). Recent studies also demonstrate that SLRP family members are involved in different signaling pathways including TGF-β/Nodal/Smad2 pathway, BMP/Smad1 pathway, EGF pathway, MAPK/FGF pathway, TLR pathway, purinergic pathway and mTOR signaling pathway, indicating an essential role of this family in coordinating other important cellular processes such as fibrosis, autophagy and host immune responses etc (Dellett et al. 2012; Gubbiotti et al. 2015; Babelova et al. 2009; Schaefer et al. 2007; Goldoni et al. 2009; Kou et al. 2010; Tomoeda et al. 2008; Albig et al. 2007; Chen et al. 2011; Nikitovic et al. 2011; Rehn et al. 2006, 2008; Ohta et al. 2011, 2006; Kuriyama et al. 2006; Morris et al. 2007; Iozzo 2015; Schaefer et al. 2017). With more and more studies focused on this family in recent years, the category of the physiological functions and pathological roles of SLPRs are involving rapidly. Current review will specifically focus on the role of SLRPs in host immune responses. Because host immune responses play an important part in the pathogenesis of renal diseases, the role of SLRPs in this set of diseases will also be discussed.
Phylogenetic tree of the human small leucine-rich repeat proteoglycan (SLRP) family. The phylogenetic tree of the growing human SLRP protein family is generated by multiple sequence alignment using ClustalW2 from the European Bioinformatics Institute. Horizontal distances of the bars are proportional to the predicted evolutionary distance of the available human SLRP sequences
SLRPs and innate immunity
SLRPs and TLR 2/4 signaling pathway
The leucine-rich repeat motifs in the core protein of SLRPs and the structural similarity between SLRPs and the pathogen associated molecular patterns (PAMPs) suggest that SLRPs play a role in host immunity (Shao et al. 2012; Schaefer et al. 2002, 2005; Wu et al. 2007). Indeed, certain SLRPs such as biglycan were described as “analogous to PAMPs” in some studies due to their ability to induce innate immune response by their own without the need of PAMPs (Schaefer et al. 2005; Al Haj Zen et al. 2003).
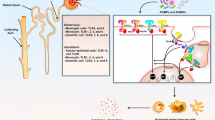
The best studied signaling pathway in innate immune responses activated by SLRPs is mediated by TLR4/2. As endogenous ligands for TLR 4 and TLR 2, decorin and biglycan stimulate macrophages to produce TNF α, IL-12, and MIP 2 (Schaefer et al. 2005; Moreth et al. 2012). In addition, biglycan itself can activate macrophages and activated macrophages will synthesize and secrete biglycan (Schaefer and Iozzo 2008; Schaefer et al. 2005), indicating a self-amplifying loop involving biglycan exists in the pathway leading to macrophage activation. Biglycan was also reported involved in the activation of NLRP3 inflammasome via the cooperativity of TLR2/TLR4 and P2X receptors leading to the secretion of mature IL-1 β both in a model of non-infectious inflammatory renal injury and in LPS induced sepsis (Babelova et al. 2009), indicating that biglycan may act as a danger associated molecular pattern (DAMP) that is proteolytically released from the ECM upon tissue stress or injury and then turn on host innate and adaptive immune responses (Kalamajski and Oldberg 2009; Schaefer et al. 2005; Nastase et al. 2012; Moreth et al. 2010; Popovic et al. 2011; Schaefer and Iozzo 2012). Recent studies further indicate that through TLR 2/4-NADPH oxidases (NOX) 1/4 -ROS and TLR 4-TRIF/MyD88-NOX 2 axes, biglycan fine-tunes IL-1 β production and maintains immune homeostasis (Schaefer et al. 2017). Interestingly, also through TLR2, biglycan induced the expression of hypoxia-inducible factor 2α (HIF-2α) resulting in increased erythropoietin production in the liver and kidney of a liver-specific, biglycan transgenic mouse model and subsequent enhanced production of erythrocytes (Frey et al. 2017), which potentially is related to inflammation and tumor progression. Signaling pathways through TLR2/4 by biglycan and decorin are summarized in Fig. 2.
Decorin and biglycan participate in multiple signaling pathways of innate and adaptive immune responses. As endogenous ligands for TLR 4 and TLR 2, decorin and biglycan stimulate macrophages to produce TNF α, IL-12, and MIP 2. In addition, biglycan itself can activate macrophages and activated macrophages will synthesize and secrete biglycan. Via the cooperativity of TLR2/TLR4 and P2X receptor biglycan is involved in the activation of NLRP3 inflammasome leading to the secretion of mature IL-1 β. IL-1 β production is also fine-tuned by biglycan through TLR 2/4-NOX 1/4 -ROS and TLR 4-TRIF/MyD88-NOX 2 axes. Also through TLR2 biglycan induces HIF-2α expression resulting in increased erythropoietin production and subsequent enhanced production of erythrocytes. Recent studies indicated that TGF-β-miR 21- PDCD 4-IL 10 signaling pathway participated in the post-transcriptional inhibition of IL-10 expression by biglycan. By inducing the secretion of RANTES, MCP-1, and MIP-1 α biglycan chemoattracted T cells to the sites of inflammation through TLR 2/4 signaling. TLR, toll-like receptor; TNF α, tumor necrosis factor α; MIP, macrophage inflammatory protein; NLRP3, NLR family, pyrin domain containing 3; NOX, NADPH oxidases; ROS, reactive oxygen species; TRIF: TIR-domain-containing adaptor-inducing interferon; MyD: myeloid differentiation factor; RANTES: regulated upon activation, normal T cell expressed and secreted; HIF-2 α: hypoxia-inducible factor 2α; TGF, transforming growth factor; miR, micro-RNA; PDCD: programmed cell death protein; MCP-1, monocyte chemoattractant protein-1
Also through TLR4 pathway, another SLRP family member---lumican was found protective against Gram negative bacterial infection, as indicated that Lum-/- peritoneal macrophages lost the capacity to phagocytoze non-opsonized Gram negative E. coli and P. aeruginosa in vitro (Shao et al. 2012; Wu et al. 2007). Interestingly, Chakravarti’s group showed some different results when they challenged the Lum-/- mice with LPS. They found that the Lum-/- mice were hyporesponsive to LPS-induced septic shock with poor induction of pro-inflammatory cytokines in the serum and a survival benefit was observed in the scenario of LPS challenge (Al Haj Zen et al. 2003). In the same study the authors also reported that challenging Lum-/- mice with live S. typhimurium didn’t lead to any difference in the production of TNF α in the serum of these animals compared with WT animals (Wu et al. 2007). These findings indicate that activation of innate immune responses in vivo is a complicated process and interaction between host and the whole bacterial versus LPS alone may be different. Notably, lumican deficiency didn’t affect the response of macrophages to other PAMPs suggesting the role of lumican in TLR-4 signaling pathway is specific (Al Haj Zen et al. 2003). However, not all inflammatory responses will cause tissue damage, and some recent data show that DAMPs that ligate TLR 2/4 drive renal regeneration (Anders and Schaefer 2014).
SLRPs and cytokine/chemokine production
SLRP-induced activation of innate immune responses leads to the production of cytokines and chemokines. In macrophages and dendritic cells soluble biglycan induces the expression of CXCL 13, a major chemoattractant for B cells, especially B1 cells, and an important biomarker for the disease activity of systemic lupus erythematosus (SLE), suggesting a role of biglycan in the pathogenesis of autoimmune diseases involving T cell independent and dependent autoantibody production (Nastase et al. 2012; Moreth et al. 2010). Consistent with this finding, biglycan deficient mice were found hypo-responsive to both LPS and zymosan-induced sepsis due to a mitigated inflammatory response that resulted in less end-organ damage.
Lumican also can induce the secretion of pro-inflammtory cytokines that recruit macrophages and neutrophils to the sites of injury (Albig et al. 2007; Vij et al. 2005; Carlson et al. 2007), and lumican deficiency leads to a marked reduction of neutrophil infiltration that impairs wound healing and resolution of inflammatory diseases (Frey et al. 2013; Hayashi et al. 2010; Lohr et al. 2012). When Lum-/- mice were challenged with P. aeruginosa, these mice failed to clear the bacterium from lungs and other tissues, and showed a dramatic increase in mortality.
As lumican and biglycan, decorin facilitates the transcription and translation of pro- inflammatory and most anti-inflammatory cytokines in the absence and presence of LPS. However, IL-10 is an exception: in the absence of LPS both transcription and translation of IL-10 is increased while in the presence of LPS IL-10 mRNA transcription is upregulated but protein translation is suppressed by decorin (Moreth et al. 2012; Merline et al. 2011), indicating the underlying mechanism of this process is far more complicated than just TLR 2/4 signaling. Indeed, recent studies indicated that TGF-β-miR 21-programmed cell death 4 (PDCD 4)-IL 10 signaling pathway also participated in the post-transcriptional inhibition of IL 10 expression (Fig. 2) (Nastase et al. 2018). In human gingival fibroblasts, decorin was found regulating the production of metalloproteinase (MMP)-1, -2, and -3, tissue inhibitors of metalloproteinase (TIMP) -2, and certain cytokines like TGF-β, IL-1β, IL-4 and TNF-α, suggesting decorin was also a part of tissue remodeling process (Al Haj Zen et al. 2003).
SLRPs as a bridge linking innate and adaptive immunity
Besides their direct involvements in innate immunity, SLRPs also act as a bridge linking innate and adaptive immune responses together. Through TLR 2/4 signaling biglycan regulates T cell activities such as chemoattracting T cells to the sites of inflammation by inducing the secretion of regulated upon activation, normal T cell expressed and secreted (RANTES), MCP-1, and MIP-1 α (Fig. 2). In addition by signaling through both TLRs and their adaptor proteins myeloid differentiation factor 88 (MyD 88) and TRIF β (TIR-domain-containing adaptor-inducing interferon β) biglycan facilitates MHC-I and MHC-II restricted T cell cross-priming (Moreth et al. 2012; Nastase et al. 2012; Moreth et al. 2010; Popovic et al. 2011; Kikuchi et al. 2000; Kitaya and Yasuo 2009; Sjoberg et al. 2009). Over-expression of soluble biglycan has markedly enhanced the systemic and renal outcome of SLE by TLR 2/4 dependent chemoattraction of macrophages and T- and B-lymphocytes (Moreth et al. 2010; Frey et al. 2013).
Implications of SLRPs in renal diseases
Studies have indicated that some SLRPs including decorin, biglycan, and lumican have distinct expression patterns in normal and diseased human kidneys (Schaefer et al. 2000, 2002; Schaefer 2011; Stokes et al. 2001, 2000), suggesting that SLRPs may take part in the pathogenesis of renal diseases. In the normal kidney, decorin and lumican mainly express in peritubular mesenchymal cells in tubulointerstitium with trace amount expression in the mesangial cells in glomerulus, while biglycan mainly expresses in peritubular mesenchymal cells and distal tubules in tubulointerstitium and endothelials in glomerulus with trace amount expression in mesangial cells and epithelial cells in glomerulus (Schaefer et al. 2000). In experimental renal injury, the expression of decorin, biglycan and lumican has been localized to glomerulosclerosis lesions and tubulointerstitial fibrosis (Schaefer et al. 1998; Okuda et al. 1990; Diamond et al. 1997; Silverstein et al. 2003). In end stage glomerulosclerosis, these SLRPs strongly accumulated in Bowman’s capsule and in areas of fibrous organization of the urinary space, which became progressively more pronounced with the extent of fibrosis, indicating the involvement of these SLRPs in renal diseases (Babelova et al. 2009; Kitaya and Yasuo 2009; Schaefer et al. 2000; Stokes et al. 2001; Ebefors et al. 2011). Besides, decorin and biglycan deposits in fibrotic lesions were co-localized with collagen type I (Stokes et al. 2001), and they have also been localized in glomerular deposits of amyloid A (Moss et al. 1998). Various kidney diseases that different SLRPs were involved in were summarized in Table 1.
Decorin
In an immunohistochemical study of several matrix proteins, decorin was found to be the best predictor of the severity of interstitial fibrosis and renal failure (Lohr et al. 2012; Merline et al. 2011; Schaefer et al. 2000; Diamond et al. 1997; Vleming et al. 1995; De Heer et al. 2000). Urinary excretion of decorin was significantly increased in patients with membranous nephropathy, minimal change disease and IgA nephropathy, and urine decorin in these patients was negatively correlated to creatinine clearance (Schaefer et al. 2000; Kuroda et al. 2004).
Decorin is found having anti-fibrotic activities. Decorin interacts through its protein core with all three forms of TGF-β with dissociation constants in the nanomolar range and neutralizes TGF-β activities in several organs including kidney by interfering with TGF-β signaling (Schaefer 2011; Stokes et al. 2000; Border et al. 1992; Hildebrand et al. 1994; Yamaguchi et al. 1990). Alternatively, binding of TGF-β to decorin may serve as a reservoir by increasing the availability of this cytokine without the need of de novo synthesis at sites of fibrotic injury (Stokes et al. 2000, 2001). On the other hand, chronic exposure to circulating TGF-β caused an up-regulation of decorin in mouse kidney (Mozes et al. 1999). In addition, decorin inhibits connective tissue growth factor (CTGF) signaling in fibroblast, down-regulates microRNA miR-21, and inhibits apoptosis of renal tubular epithelial cells via the IGF type I receptor/Akt signaling pathway (Anders and Schaefer 2014), which all result in the alleviation of interstitial fibrosis (Anders and Schaefer 2014; Merline et al. 2011; Vial et al. 2011; Glowacki et al. 2013).
In diabetic nephropathy (DN), decorin was upregulated in the mesangial cells, and in response to high glucose stimulation decorin was increased in the mesangial and tubular cells cultured in vitro (Brunskill and Potter 2012; Mogyorosi and Ziyadeh 1998). Decorin deficiency, however, resulted in a much more severe DN with increased mesangial matrix expansion, elevated albuminuria, and increased TGF-β bioactivity in mice with streptozotocin induced diabetes, indicating that decorin is protective against DN (Brunskill and Potter 2012). Interestingly, the importance of decorin in DN was further corroborated by Cosmo and his colleagues’ finding that decorin gene 179 allelic variant was associated with a slower progression of renal disease in patients with type 1 diabetes (De Cosmo et al. 2002), indicating genetic mechanisms may also be involved in DN pathogenesis.
In unilateral ureteral obstruction (UUO) model, a well established model of renal inflammation and fibrosis, decorin expression became evident 36 hours after ligation and remained up-regulated throughout the whole experiment (Schaefer et al. 2002). Via specific effects on apoptosis through P27 signaling, TGF-β activity and collagen turnover, decorin had profound effects on the course and final outcome of ureteral kidney obstruction (Schaefer et al. 2002). Not so surprisingly, decorin deficient mice showed marked aggravation of renal fibrosis in ureteral obstruction, further stressing the importance of decorin in fibrotic renal disorders (Lohr et al. 2012; Danielson et al. 1997).
Due to its protective effects against fibrosis, several attempts have been made to explore the potential use of decorin in the treatment of fibrotic renal disorders (Border et al. 1992; Danielson et al. 1997; Costacurta et al. 2002; Huijun et al. 2005; Isaka et al. 1996): administration of exogenous decorin or transfection of decorin cDNA into skeletal muscle has been reported alleviating renal clinical and pathological manifestations including reduced proteinuria, ECM accumulation and glomerular TGF-β levels in experimental animal models (Border et al. 1992; Danielson et al. 1997; Isaka et al. 1996); decorin gene transfection in human mesangial cells down-regulates genes playing a role in fibrosis such as TGF-β1, collagen IV and fibronectin in this cell type (Costacurta et al. 2002); and ex vivo transfer of decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis (Huijun et al. 2005). These findings experimentally validate the use of decorin for gene therapy in treating renal diseases. Indeed, decorin deficiency led to the infiltration of large number of biglycan-expressing macrophages in the kidney (Schaefer et al. 2002) in a non-infectious animal model of renal inflammation.
Biglycan
Sequestered in ECM as a potential inflammatory trigger under normal circumstances, biglycan is released from ECM or de novo synthesized by macrophages and launches a sterile inflammatory response upon tissue damage or stress (Iozzo and Schaefer 2015; Hsieh et al. 2017). Enhanced interstitial and to a lesser degree glomerular expression and deposition of biglycan has been described in certain fibrotic renal diseases such as DN and mesangioproliferative glomerulonephritis (Schaefer et al. 2000; Stokes et al. 2000, 2001; Okuda et al. 1990; Schaefer et al. 2001). Several studies in various experimental models of sterile inflammatory kidney diseases such as ischemia/reperfusion injury and chronic renal allograft rejection also reveal a striking concurrence of biglycan expression and the extent of renal injury (Merline et al. 2009; Moreth et al. 2014). In a transient transgenic mouse model where full length and fully glycanated biglycan was de novo synthesized by hepatocytes, Schaefer et al. found that renal parenchyma was preferentially targeted by circulating soluble biglycan with profound consequences including the sequential recruitment of neutrophils, macrophages and T cells, and the production of CXCL1,CXCL2 , CCL2 and CCL5 in a TLR 2/4 dependent manner (Hsieh et al. 2017; Zeng-Brouwers et al. 2014). Besides TLR 2/4 signaling pathway, involvement of biglycan in oxidative stress and complement activation also plays an important role in renal injury (Babelova et al. 2009; Moreth et al. 2010).
As decorin, biglycan interacts through its protein core with all three isoforms of TGF-β (Schaefer 2011; Stokes et al. 2000; Border et al. 1992; Hildebrand et al. 1994; Yamaguchi et al. 1990). Besides, TGF-β stimulates the expression of biglycan in all renal cell types studied so far in vitro and in vivo (Schaefer et al. 2001, 2002; Schaefer 2011). However, the role of biglycan in fibrosis is not quite well understood as decorin (Babelova et al. 2009; Schaefer 2011; Bedke et al. 2007; Wang et al. 2010; Kiss et al. 2010).
In UUO model, same as decorin, biglycan expression became evident 36 hours after ligation and remained up-regulated throughout the whole experiment, and biglycan up-regulation was even more pronounced in decorin deficient mice, suggesting compensation exists among different SLRPs (Schaefer et al. 2002). Biglycan up-regulation seems protective in UUO model as indicated by the loss of elastic properties of renal tissue evidenced by cystic dilation of Bowman’s capsule and proximal tubules as well as hemorrhaging into renal pelvis in the absence of biglycan (Schaefer 2011; Schaefer et al. 2004). However, increased renal biglycan content, which also occurs in all stages of DN (Nastase et al. 2014), has been thought contributing to renal lipid accumulation and the development of DN (Thompson et al. 2011).
By linking innate and adaptive immune responses together through interacting with TLR2/4, biglycan contributes to the pathogenesis of lupus nephritis (LN), and genetic elimination of biglycan in lupus-prone mice improved systemic and renal outcomes by lowering levels of autoantibodies, reducing enlargement of spleen and lymph nodes, and preventing renal damage and albuminuria. In consistency, biglycan overexpression aggravated renal tissue damage and led to organ failure in these mice (Moreth et al. 2010; Schaefer 2011). In human patients with LN, plasma levels of circulating biglycan were elevated 5 fold compared with controls and higher levels of circulating biglycan were associated with albuminuria, increased plasma levels of CXCL13 and renal inflammation and damage (Moreth et al. 2010). These findings clearly indicate that biglycan participates in and aggravates the progression of LN. As in LN, when rats with Thy-1 nephritis were treated with biglycan delivered by a similar way as decorin was applied (Border et al. 1992), more severe glomerular lesions associated with enhanced infiltration of mononuclear cells, overexpression of glomerular α1 chains of collagen I and IV, and elevated albuminuria were developed (Schaefer 2011). All these findings indicate that the role of biglycan in fibrotic renal diseases is complicated and may vary among different etiologies.
Conclusions and future directions
In this review, we summarized and discussed the current knowledge regarding SLRPs in host immunity and renal diseases. From being recognized as just a molecule maintaining the integrity of ECM to currently being known participating in various signaling pathways, SLRPs are found involved in various cellular and disease processes after two decades of research. Armed with more knowledge about this family, we should be able to explore the potential therapeutic strategies in the treatment of the diseases in which different SLRPs are involved.
For the sake of developing future therapeutics, certain questions still remain to be addressed. For example, under normal conditions, the levels of circulating decorin and biglycan are undetectable while during inflammation or injury, their levels are up-regulated. So the questions are what triggers their up-regulation when injury starts and what dampens them when injury goes away. Could the change of their levels in circulation or in urine or in other body fluids serve as a biomarker for predicting the status of disease activity? Are SLRPs involved in other human disease processes not discussed in this review? Indeed, podocan, a member of the class V SLRP family, was reported up-regulated in the sclerotic glomerular lesion of experimental HIV-associated nephropathy (Ross et al. 2003). Besides, it was also found involved in the pathogenesis of coronary heart diseases (Hutter et al. 2013). Other SLRPs including lumican, fibromodulin, and osteoglycin were found participating in the pathogenesis of heart diseases as well (Christensen et al. 2018). Considering the important role of SLPRs in inflammation which is also essential for tumor initiation and growth, it is not surprising that SLRPs take part in tumorigenesis. Indeed, by regulating MMP activities and/or increasing angiogenesis et al, decorin, biglycan and lumican were reported influencing tumor growth and progression (Schaefer et al. 2017; Pietraszek et al. 2014). Influence of SLRP on exsome trafficking and autophagy is an emerging area of research in recent years (Schaefer et al. 2017; Karamanos 2017). Besides, future studies on the complex interactions among SLRPs and the orchestration of their downstream signaling events are needed. In addition, data regarding the functions of the SLRPs other than decorin and biglycan in different cellular and disease processes are sparse, and studies on them are warranted since functional compensation or redundancy among different SLRPs do exist, and double or even triple knockout models may need for these studies. Obviously, answers to above questions will fasten the discovery and development of new treatment methods.
Abbreviations
- SLRP:
-
Small leucine rich proteoglycans
- ECM:
-
Extracellular matrix
- LRR:
-
Leucine rich repeat
- PAMP:
-
Pathogen associated molecular pattern
- DAMP:
-
Danger associated molecular pattern
- HIF-2 α:
-
Hypoxia-inducible factor 2α
- SLE:
-
Systemic lupus erythematosus
- RANTES:
-
Regulated upon activation, normal T cell expressed and secreted
- MyD88:
-
Myeloid differentiation factor 88
- TRIF β:
-
TIR-domain-containing adaptor-inducing interferon β
- TLR:
-
Toll-like receptor
- TNF α:
-
Tumor necrosis factor α
- MIP:
-
Macrophage inflammatory protein
- NLRP3:
-
NLR family, pyrin domain containing 3
- NOX:
-
NADPH oxidases
- ROS:
-
Reactive oxygen species
- TGF:
-
Transforming growth factor
- miR:
-
Micro-RNA
- PDCD:
-
Programmed cell death protein
- MCP-1:
-
Monocyte chemoattractant protein-1
References
Al Haj Zen A, Lafont A, Durand E, Brasselet C, Lemarchand P, Godeau G et al (2003) Effect of adenovirus-mediated overexpression of decorin on metalloproteinases, tissue inhibitors of metalloproteinases and cytokines secretion by human gingival fibroblasts. Matrix Biol 22:251–258
Albig AR, Roy TG, Becenti DJ, Schiemann WP (2007) Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis 10:197–216
Anders HJ, Schaefer L (2014) Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25:1387–1400
Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF et al (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284:24035–24048
Bedke J, Kiss E, Schaefer L, Behnes CL, Bonrouhi M, Gretz N et al (2007) Beneficial effects of CCR1 blockade on the progression of chronic renal allograft damage. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 7:527–537
Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD et al (1992) Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360:361–364
Brunskill EW, Potter SS (2012) Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol 13:70
Carlson EC, Lin M, Liu CY, Kao WW, Perez VL, Pearlman E (2007) Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem 282:35502–35509
Chen J, Wong-Chong J, SundarRaj N (2011) FGF-2- and TGF-beta1-induced downregulation of lumican and keratocan in activated corneal keratocytes by JNK signaling pathway. Invest Ophthalmol Vis Sci 52:8957–8964
Christensen G, Herum KM, Lunde IG (2018) Sweet, yet underappreciated: Proteoglycans and extracellular matrix remodeling in heart disease. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.01.001
Costacurta A, Priante G, D'Angelo A, Chieco-Bianchi L, Cantaro S (2002) Decorin transfection in human mesangial cells downregulates genes playing a role in the progression of fibrosis. J Clin Lab Anal 16:178–186
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136:729–743
De Cosmo S, Tassi V, Thomas S, Piras GP, Trevisan R, Cavallo Perin P et al (2002) The Decorin gene 179 allelic variant is associated with a slower progression of renal disease in patients with type 1 diabetes. Nephron 92:72–76
De Felice M, Esposito L, Pucci B, Carpentieri F, De Falco M, Rossi M et al (2003) Biochemical characterization of a CDC6-like protein from the crenarchaeon Sulfolobus solfataricus. J Biol Chem 278:46424–46431
De Heer E, Sijpkens YW, Verkade M, den Dulk M, Langers A, Schutrups J et al (2000) Morphometry of interstitial fibrosis. Nephrol Dial Transplant 15(Suppl 6):72–73
Dellett M, Hu W, Papadaki V, Ohnuma S (2012) Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance. Develop Growth Differ 54:327–340
Diamond JR, Levinson M, Kreisberg R, Ricardo SD (1997) Increased expression of decorin in experimental hydronephrosis. Kidney Int 51:1133–1139
Ebefors K, Granqvist A, Ingelsten M, Molne J, Haraldsson B, Nystrom J (2011) Role of glomerular proteoglycans in IgA nephropathy. PLoS One 6:e18575
Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L (2013) Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J 280:2165–2179
Frey H, Moreth K, Hsieh LT, Zeng-Brouwers J, Rathkolb B, Fuchs H et al (2017) A novel biological function of soluble biglycan: Induction of erythropoietin production and polycythemia. Glycoconj J 34:393–404
Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM et al (2013) Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 8:e58014
Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ et al (2009) Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 185:743–754
Gubbiotti MA, Neill T, Frey H, Schaefer L, Iozzo RV (2015) Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol 48:14–25
Hayashi Y, Call MK, Chikama T, Liu H, Carlson EC, Sun Y et al (2010) Lumican is required for neutrophil extravasation following corneal injury and wound healing. J Cell Sci 123:2987–2995
Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA et al (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302(Pt 2):527–534
Hsieh LT, Nastase MV, Roedig H, Zeng-Brouwers J, Poluzzi C, Schwalm S, et al (2017) Biglycan- and Sphingosine Kinase-1 Signaling Crosstalk Regulates the Synthesis of Macrophage Chemoattractants. Int J Mol Sci. https://doi.org/10.3390/ijms18030595
Huijun W, Long C, Zhigang Z, Feng J, Muyi G (2005) Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol 78:17–24
Hutter R, Huang L, Speidl WS, Giannarelli C, Trubin P, Bauriedel G et al (2013) Novel small leucine-rich repeat protein podocan is a negative regulator of migration and proliferation of smooth muscle cells, modulates neointima formation, and is expressed in human atheroma. Circulation 128:2351–2363
Iozzo MAGaRV (2015) Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept. Matrix Biol 48:6–13
Iozzo RV, Schaefer L (2015) Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42:11–55
Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA et al (1996) Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 2:418–423
Kalamajski S, Oldberg A (2009) Homologous sequence in lumican and fibromodulin leucine-rich repeat 5-7 competes for collagen binding. J Biol Chem 284:534–539
Karamanos NK (2017) Matrix pathobiology-central roles for proteoglycans and heparanase in health and disease. FEBS J 284:7–9
Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA et al (2000) Decorin binds near the C terminus of type I collagen. J Biol Chem 275:21801–21804
Kikuchi A, Tomoyasu H, Kido I, Takahashi K, Tanaka A, Nonaka I et al (2000) Haemopoietic biglycan produced by brain cells stimulates growth of microglial cells. J Neuroimmunol 106:78–86
Kiss E, Popovic ZV, Bedke J, Adams J, Bonrouhi M, Babelova A et al (2010) Peroxisome proliferator-activated receptor (PPAR) gamma can inhibit chronic renal allograft damage. Am J Pathol 176:2150–2162
Kitaya K, Yasuo T (2009) Dermatan sulfate proteoglycan biglycan as a potential selectin L/CD44 ligand involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J Leukoc Biol 85:391–400
Kou I, Nakajima M, Ikegawa S (2010) Binding characteristics of the osteoarthritis-associated protein asporin. J Bone Miner Metab 28:395–402
Kresse H, Liszio C, Schonherr E, Fisher LW (1997) Critical role of glutamate in a central leucine-rich repeat of decorin for interaction with type I collagen. J Biol Chem 272:18404–18410
Kuriyama S, Lupo G, Ohta K, Ohnuma S, Harris WA, Tanaka H (2006) Tsukushi controls ectodermal patterning and neural crest specification in Xenopus by direct regulation of BMP4 and X-delta-1 activity. Development 133:75–88
Kuroda M, Sasamura H, Kobayashi E, Shimizu-Hirota R, Nakazato Y, Hayashi M et al (2004) Glomerular expression of biglycan and decorin and urinary levels of decorin in primary glomerular disease. Clin Nephrol 61:7–16
Lohr K, Sardana H, Lee S, Wu F, Huso DL, Hamad AR et al (2012) Extracellular matrix protein lumican regulates inflammation in a mouse model of colitis. Inflamm Bowel Dis 18:143–151
Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD et al (2009) Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol 60(Suppl 4):5–13
Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG et al (2011) Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 4:ra75
Mogyorosi A, Ziyadeh FN (1998) Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. Am J Phys 275:F827–F832
Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J et al (2010) The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest 120:4251–4272
Moreth K, Iozzo RV, Schaefer L (2012) Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle 11:2084–2091
Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT et al (2014) Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol 35:143–151
Morris SA, Almeida AD, Tanaka H, Ohta K, Ohnuma S (2007) Tsukushi modulates Xnr2, FGF and BMP signaling: regulation of Xenopus germ layer formation. PLoS One 2:e1004
Moss J, Shore I, Woodrow D (1998) An ultrastructural study of the colocalization of biglycan and decorin with AA amyloid fibrils in human renal glomeruli. Amyloid: The International Journal of Experimental and Clinical Investigation: The Official Journal of the International Society of Amyloidosis 5:43–48
Mozes MM, Bottinger EP, Jacot TA, Kopp JB (1999) Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol 10:271–280
Nastase MV, Young MF, Schaefer L (2012) Biglycan: a multivalent proteoglycan providing structure and signals. J Histochem Cytochem 60:963–975
Nastase MV, Iozzo RV, Schaefer L (2014) Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology. Biochim Biophys Acta 1840:2460–2470
Nastase MV, Janicova A, Roedig H, Hsieh LT, Wygrecka M, Schaefer L (2018) Small leucine-rich proteoglycans in renal inflammation: two sides of the coin. J Histochem Cytochem 66:261–272
Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK et al (2011) Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. Int J Biochem Cell Biol 43:928–935
Ohta K, Kuriyama S, Okafuji T, Gejima R, Ohnuma S, Tanaka H (2006) Tsukushi cooperates with VG1 to induce primitive streak and Hensen's node formation in the chick embryo. Development 133:3777–3786
Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S et al (2011) Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A 108:14962–14967
Okuda S, Languino LR, Ruoslahti E, Border WA (1990) Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest 86:453–462
Pietraszek K, Chatron-Colliet A, Brezillon S, Perreau C, Jakubiak-Augustyn A, Krotkiewski H, et al (2014) Lumican: A new inhibitor of matrix metalloproteinase-14 activity. FEBS Lett 588(23):4319–4324
Popovic ZV, Wang S, Papatriantafyllou M, Kaya Z, Porubsky S, Meisner M et al (2011) The proteoglycan biglycan enhances antigen-specific T cell activation potentially via MyD88 and TRIF pathways and triggers autoimmune perimyocarditis. J Immunol 187:6217–6226
Rehn AP, Chalk AM, Wendel M (2006) Differential regulation of osteoadherin (OSAD) by TGF-beta1 and BMP-2. Biochem Biophys Res Commun 349:1057–1064
Rehn AP, Cerny R, Sugars RV, Kaukua N, Wendel M (2008) Osteoadherin is upregulated by mature osteoblasts and enhances their in vitro differentiation and mineralization. Calcif Tissue Int 82:454–464
Ross MD, Bruggeman LA, Hanss B, Sunamoto M, Marras D, Klotman ME et al (2003) Podocan, a novel small leucine-rich repeat protein expressed in the sclerotic glomerular lesion of experimental HIV-associated nephropathy. J Biol Chem 278:33248–33255
Schaefer L (2011) Small leucine-rich proteoglycans in kidney disease. J Am Soc Nephrol 22:1200–1207
Schaefer L, Iozzo RV (2008) Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem 283:21305–21309
Schaefer L, Iozzo RV (2012) Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev 22:56–57
Schaefer L, Hausser H, Altenburger M, Ugorcakova J, August C, Fisher LW et al (1998) Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int 54:1529–1541
Schaefer L, Grone HJ, Raslik I, Robenek H, Ugorcakova J, Budny S et al (2000) Small proteoglycans of normal adult human kidney: distinct expression patterns of decorin, biglycan, fibromodulin, and lumican. Kidney Int 58:1557–1568
Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J et al (2001) Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J 15:559–561
Schaefer L, Macakova K, Raslik I, Micegova M, Grone HJ, Schonherr E et al (2002) Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol 160:1181–1191
Schaefer L, Mihalik D, Babelova A, Krzyzankova M, Grone HJ, Iozzo RV et al (2004) Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol 165:383–396
Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M et al (2005) The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115:2223–2233
Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L et al (2007) Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol 170:301–315
Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV (2017) Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J 284:10–26
Shao H, Lee S, Gae-Scott S, Nakata C, Chen S, Hamad AR et al (2012) Extracellular matrix lumican promotes bacterial phagocytosis, and Lum-/- mice show increased Pseudomonas aeruginosa lung infection severity. J Biol Chem 287:35860–35872
Silverstein DM, Travis BR, Thornhill BA, Schurr JS, Kolls JK, Leung JC et al (2003) Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int 64:25–35
Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM (2009) Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol 46:830–839
Stokes MB, Holler S, Cui Y, Hudkins KL, Eitner F, Fogo A et al (2000) Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int 57:487–498
Stokes MB, Hudkins KL, Zaharia V, Taneda S, Alpers CE (2001) Up-regulation of extracellular matrix proteoglycans and collagen type I in human crescentic glomerulonephritis. Kidney Int 59:532–542
Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK (2010) Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 277:3904–3923
Thompson J, Wilson P, Brandewie K, Taneja D, Schaefer L, Mitchell B et al (2011) Renal accumulation of biglycan and lipid retention accelerates diabetic nephropathy. Am J Pathol 179:1179–1187
Tomoeda M, Yamada S, Shirai H, Ozawa Y, Yanagita M, Murakami S (2008) PLAP-1/asporin inhibits activation of BMP receptor via its leucine-rich repeat motif. Biochem Biophys Res Commun 371:191–196
Vial C, Gutierrez J, Santander C, Cabrera D, Brandan E (2011) Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem 286:24242–24252
Vij N, Roberts L, Joyce S, Chakravarti S (2005) Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci 46:88–95
Vleming LJ, Baelde JJ, Westendorp RG, Daha MR, van Es LA, Bruijn JA (1995) Progression of chronic renal disease in humans is associated with the deposition of basement membrane components and decorin in the interstitial extracellular matrix. Clin Nephrol 44:211–219
Wang S, Schmaderer C, Kiss E, Schmidt C, Bonrouhi M, Porubsky S et al (2010) Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis Model Mech 3:92–103
Wu F, Vij N, Roberts L, Lopez-Briones S, Joyce S, Chakravarti S (2007) A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem 282:26409–26417
Yamaguchi Y, Mann DM, Ruoslahti E (1990) Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346:281–284
Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L (2014) De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol 35:132–142
Acknowledgements
National Natural Science Foundation of China (grant No.: 81660279, 81701629) , Jiangxi Department of Science and Technology (grant No.: 20151BAB205062 ,20161ACB21016, 20171BCB23088 and 20181ACH80002) and by the startup funding for the scholars studying and coming back from abroad from Ministry of Education, P.R.C (grant No.: 2015060020102070).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, W., Wan, J., Li, M. et al. Small leucine rich proteoglycans in host immunity and renal diseases. J. Cell Commun. Signal. 13, 463–471 (2019). https://doi.org/10.1007/s12079-018-0489-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-018-0489-8