Abstract
Stress adaptation effect provides cell protection against ischemia induced apoptosis. Whether this mechanism prevents other types of cell death in stroke is not well studied. This is an important question for regenerative medicine to treat stroke since other types of cell death such as necrosis are also prominent in the stroke brain apart from apoptosis. We report here that treatment with 17-N-Allylamino-17-demethoxygeldanamycin (17AAG), an Hsp90 inhibitor, protected neural progenitor cells (NPCs) against oxygen glucose deprivation (OGD) induced cell death in a dose dependent fashion. Cell death assays indicated that 17AAG not only ameliorated apoptosis, but also necrosis mediated by OGD. This NPC protection was confirmed by exposing cells to oxidative stress, a major stress signal prevalent in the stroke brain. Mechanistic studies demonstrated that 17AAG activated PI3K/Akt and MAPK cell protective pathways. More interestingly, these two pathways were activated in vivo by 17AAG and 17AAG treatment reduced infarct volume in a middle cerebral artery occlusion (MCAO) stroke model. These data suggest that 17AAG protects cells against major cell death pathways and thus might be used as a pharmacological conditioning agent for regenerative medicine for stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regenerative and reparative therapy has emerged to be an important approach for stroke management (Banerjee et al. 2014, Chang et al. 2007, Lee et al. 2007, Steindler 2007). On one hand, the brain is capable of regenerating neurons after their maturation (Aimone et al. 2006, Allen et al. 2001, Eriksson et al. 1998), and stroke can stimulate neurogenesis (Chopp et al. 2007, Jin et al. 2006, Ohira et al. 2009). On the other hand, stem cell transplantation enhances neurogenesis in the brains and significantly improves cognitive functions in experimental animals (Horie et al. 2011, Roitberg et al. 2006, Theus et al. 2008, Yu et al. 2013). In addition, the newly generated or transplanted cells (hereafter “new cells”) also provide essential trophic support to the tissues at risk in the penumbra surrounding the infarct area (Chang et al. 2007, Haas et al. 2005). However the potential of regenerative and reparative therapy is severely hampered by the extremely low survival rate of the “new cells” due to the harsh environment of excessive oxidative stresses, inflammatory cytokines, and cytotoxic cells in the diseased brain (Bliss et al. 2006, Hicks et al. 2009, Kelly et al. 2004, Norgaard et al. 2006).
Harnessing the stress adaptation (hormesis) effect is a promising strategy for protection of the “new cells” (Hess et al. 2013, Marini et al. 2008, Theus et al. 2008). Pre-treatment or engineering before transplantation improves stem cell survival in the hostile brain environment (Mitrecic et al. 2012, Wang et al. 2011, Wang et al. 2009, Wei et al. 2005). A non-lethal episode of hypoxia before stroke onset substantially reduces the infarct volume in acute stroke, which is termed ischemic preconditioning (IPC) (Ratan et al. 2007, Rosova et al. 2008). IPC has limited clinical application and is suited for a small number of predictable situations, such as cardiac bypass (Hess et al. 2013). Remote ischemic preconditioning (RIPC) and ischemic postconditioning (IPoC) are clinically more relevant and show comparable neuroprotection in animal studies. RIPC is currently in several clinical trials (Hahn et al. 2011, Hess et al. 2013, Koch et al. 2011, Xie et al. 2013). In addition, pharmacological conditioning has been gaining attention as an alternative cell treatment method, in particular for those patients who have higher burdens on the blood and hearts which may not be suitable for ischemic conditioning (Hess et al. 2013).
Over the last two decades, numerous molecular targets were tackled in an effort to achieve neuroprotection in ischemic stroke, including excitotoxicity, inflammation, and apoptosis (Majid 2014, Moretti et al. 2014). However, the pathophysiology of stroke is very heterogenic and multiple cell death mechanisms exist at the same time (Mitsios et al. 2006, Shaller et al. 1980). Apoptosis is dominant in the penumbra while necrosis is prominent in the infarct area (Yuan 2009). These forms of cell death will inevitably affect the survival of the new cells because they are either injected or are found to migrate to the infarct area (Chang et al. 2007, Haas et al. 2005). Thus protection against all modes of cell death is essential for regenerative and reparative medicine for stroke.
17-N-Allylamino-17-demethoxygeldanamycin (17AAG) is a potent Hsp90 inhibitor currently undergoing a series of clinical trials against multiple cancers because it induces apoptosis selectively in cancer cells (Gallo 2006, Terasawa et al. 2005, Waza et al. 2006, Zhang and Burrows 2004). In contrast to these findings, several groups including ours have found that 17AAG favors cell survival when treated transiently or with lower doses (Koga et al. 2006, Kwon et al. 2008, Wang et al. 2011, Wen et al. 2008, Yano et al. 2008). Nonetheless, 17AAG’s function in stroke has not been studied although its toxic precursor, geldanamycin, exerts beneficial effects in animal stroke models (Kwon et al. 2008, Wen, Li, Zong, Yu et al. 2013).
In this study we investigated whether 17AAG induced neuroprotective effects in acute ischemic conditions. NPCs were used as an in vitro model and transient middle cerebral artery occlusion (MCAO) in mice an in vivo. Our focus was to determine whether low dose 17AAG prevented NPCs against ischemia induced cell death, especially necrosis.
Materials and methods
Materials
Mouse ES cells J1 was from the ES Cell core facility (Dr. Ali Eroglu), Medical College of Georgia, Georgia Regents University. 17-AAG was from LC laboratories (Woburn, MA). Antibodies against cleaved Caspase 3, pGSK-3β (Serine 9), tGSK-3β, LC3B, pAkt (Thr 308) , tAkt, pStat3, tStat3, pErk1/2, and tErk were from Cell Signaling (Beverly, MA). The antibody against β-Actin was from Santa Cruz (Santa Cruz, CA). Hoechst 33,258, H2O2, trypan blue solution, and glutamate monosulphate were from Sigma Aldrich (St. Louis, MO). The PI3K inhibitor LY294002 was from EMD Millipore (Billerica, MA). The Mek inhibitor U0126 was from SelleckChem (Houston, TX). The Alexa Fluor 488/647/Annexin V/ Dead cells apoptosis kit was from Life Technologies (Grand Island, NY). The hypoxia chamber was from StemCell Biotechnologies (Vancouver, BC, Canada).
Methods
In vitro NPC differentiation from mouse ES cells
Mouse ES cells were grown on γ–irradiated feeder fibroblasts, and neural differentiation was induced by serum deprivation of embryoid bodies as previously described (Bradley et al. 2012, Wang et al. 2011, Wang et al. 2005). Briefly, ES cells were grown on feeder fibroblast for 2–3 days and the feeder cells were removed by replating. Floating EBs were then generated by brief trypsinization of the feeder free ES cells and seeded into non-tissue culture treated petri dishes. After 4–6 days of culture, the floating EBs were plated onto tissue culture dishes and fetal bovine serum was withdrawn to trigger neural differentiation. The attached EBs were then replated again onto tissue culture dishes changed to NPC media containing DMEM-F12, N2 supplement, basic fibroblast growth factor, L-glutamine, non-essential amino acids, and pen/strep, to produce highly pure NPC populations.
OGD and glutamate treatment of NPCs
OGD treatment was modified from published procedures (Kaushal and Schlichter 2008). Differentiated NPCs was grown in glucose free NPC medium that has been bubbled with 5 % CO2 and 95 % N2 for 10 min, in a 37 °C environmental chamber (Stemcell Tech). The chamber was flushed with 5 % CO2 and 95 % N2 for 5 min, sealed, and then kept overnight. Cultures were returned to normal conditions. For the OGD-AAG treatment group, 17AAG was added to the glucose free NPC medium 1 h before OGD and remained in the culture medium throughout the experiment. Control group samples were placed in normal NPC medium and in an incubator with 5 % CO2 and 95 % normal air. Control group samples were placed in normal NP medium and in an incubator with 5 % CO2 and 95 % normal air. Glutamate treatment was also modified from a published procedure (Croslan et al. 2008). 17AAG was added to NPCs 1 h before adding 50 μM of glutamate. Cells exposed to OGD or glutamate exposure were collected for cell death analysis.
Cell death measurement
For trypan blue staining, cells were diluted with a 0.4 % Trypan Blue solution. Total number of dead cells (stained blue) and viable cells (unstained) were counted double blindly using a hemocytometer. To determine the number of necrotic cells, cells were stained with propidium iodide (PI), fixed by 1 % paraformaldehyde (PFA) in phosphate buffered saline (PBS), and counted by a 4-color Becton Dickinson FACSCalibur flow cytometer (Crawford et al. 2010) (BD Biosciences, San Jose, CA). Morphology alterations (mitochondria swelling, cell volume increase and plasma membrane rupture) were used to confirm the flow cytometry data of necrosis. To determine apoptosis, several methods were used: 1), Immunoblot analysis to detect cleaved caspase 3; 2), to count cells that had condensed nuclei; and 3), flow cytometry counting of Annexin V labeled cells by using an Alexa Fluor /Dead Cell Apoptosis Kit (Life Technologies) following manufacturer’s instructions (Crawford et al. 2010). Autophagy was detected by immnuoblot analysis of the microtubule-associated protein 1 light chain-3B (LC3B) (Chen et al. 2010).
Transient middle cerebral artery occlusion (MCAO)
Transient MCAO was performed following published procedures (Frederix et al. 2007, Li et al. 2007). All experiments involving animals followed approved protocols by Georgia Regents University’s Institutional Animal Care and Use Committee. Three to four-month-old C57Bl6 male mice were anesthetized with ketamine and xylazine. Body temperature was maintained at 37 ± 1.0 °C throughout all surgical procedures using a heating pad. The right common carotid, external carotid artery, and internal carotid artery were exposed by a midline neck incision. A 6.0 silicon pre-coated monofilament nylon suture was introduced into the internal carotid artery to occlude the right middle cerebral artery to produce transient focal cerebral ischemia. The filament was withdrawn after 90 min to allow reperfusion. Twenty-four hours after reperfusion, mice were sacrificed by decapitation. The brains were removed and coronal slices stained with 2 % 2,3,5-triphenyltetrazolium chloride (TTC) and fixed.
Fluorescence microscopy
Live NPCs were stained with 1 μM PI for 30 min and fixed by 1 % PFA in PBS. Fluorescent and DIC images were taken by a Nikon TE300 microscope (Nikon, Japan), and edited by Photoshop CS2 Professional (Adobe).
Quantification and statistical analysis
Cell death was quantified by flow cytometry or double blinded cell counting. Data were expressed as mean + SD. Student’s t-test was used to determine their statistical significance. The infarct area in each slice was scanned and the volume of each slice was obtained by multiplying the infarct area by 2-mm thickness. Total infarct volume was determined by summing infarct volume of four consecutive slices by ImageJ, and was expressed as means + SD. Its statistical significance was analyzed using one way ANOVA, followed by Tukey’s post hoc test. A value of p < 0.05 was considered statistically significant.
Miscellaneous
For western blot analysis, protein concentrations were determined using the RC/DC protein assay, in accordance with the manufacturer’s (Bio-Rad) instructions. Equal amounts of protein were loaded on a 4–20 % gradient gel and SDS-PAGE was performed using the Laemmli method. β-actin (Actin) was used as loading control.
Results
Treatment with low dose 17AAG protected NPCs from OGD-induced cell death
Several groups including ours have reported that low dose 17AAG exerts cell protective effects in different cell types, including NPCs (Ansar et al. 2007, Koga et al. 2006, Wang et al. 2011, Yano et al. 2008). However, no study has been done on whether 17AAG protects cells against stroke related stresses. To evaluate this, we detected 17AAG’s role on NPCs during OGD. The NPCs used in our studies were derived from mouse embryonic stem cells which express neural stem cell marker Nestin (Bieberich et al. 2004, Wang et al. 2011). Overnight treatment of OGD was applied because this condition caused not only apoptosis but also necrosis in NPCs, which recapitulates the disease pathology. Trypan blue staining showed that 28.9 ± 2.5 % of NPCs survived after OGD treatment (Fig. 1a). Control NPCs show a ~30 % naturally-occurring cell death, which is consistent with previous studies (Fig. 1a). Treatment with a higher dose 17AAG (250nM, AAG250) severely worsened the viability of NPCs undergoing OGD (Fig. 1a). Interestingly, 17AAG at a dose of 10nM (AAG10) significantly increased NPC survival to 45.8 ± 3.4 % (a 59.0 % increase, Fig. 1a), suggesting that 17AAG triggers stress adaptation (hormetic) effect in stroke situations. Other doses (1nM and 50nM) had minimum effects. Therefore we used 10nM 17AAG for further experiments. This observation is consistent with previous studies that hormetic effect usually increases cell survival by 30–60 % (Calabrese 2013a, Calabrese 2013b, Wang et al. 2011).
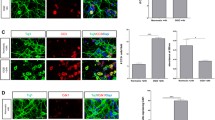
17AAG protects NPCs against major forms of cell death induced by OGD a: Trypan blue staining and counting of NPCs survived after OGD. 17AAG doses: AAG250, 250nM; AAG50, 50nM; AAG10, 10nM, and AAG1, 1nM. Note that 10nM 17AAG increased NPC survival. b: Western blot analysis to detect cleaved caspase 3 (Casp3) and LC3B. β-actin (Actin) was used as a loading control. c: Quantification of apoptosis by counting cells with condensed nuclei. Note 17AAG treatment reduced NPC apoptosis to 39.2 ± 2.3 % from 51.9 % ±9.0 %. d: PI staining and DIC images of NPCs undergoing OGD, note necrotic cells retained PI and exhibited a typical necrosis morphology: swollen and ruptured cells and organelles. e: A higher magnification image of inset show in D. f: a typical histogram from the flow cytometry of NPCs stained with PI. Necrotic cells stained PI because their cell membrane was ruptured. NPCs were then fixed and analyzed by a flowcytometer. g: Quantification of PI positive NPCs treated with OGD and the pan-caspase inhibitor Z-VAD (ZVAD). N = 3. In A, C, and G, *, p < 0.05; **, p < 0.01
We then determined the cell death mechanism (s) by which 17AAG protected NPCs from following OGD. Activated caspase 3 (Casp3) is a hallmark of apoptosis, and apoptotic cells display a condensed nuclei morphology (Bieberich et al. 2003, Bieberich et al. 2004). Fig. 1b shows that treatment with 10nM 17AAG reduced the level of Casp3 under OGD conditions, suggesting reduced apoptosis (Fig. 1b). This was validated by quantification of cells with condensed nuclei, which indicated that 17AAG treatment significantly reduced the number of apoptotic cells (from 51.9 ±8.2 % to 39.2 ± 2.3 %) induced by OGD (Fig. 1c). To measure necrosis, we examined the cell morphology and stained live cells by propidium iodine (PI). Necrotic cells stain positive for PI because their cell membranes are ruptured. Figure 1d and e show that ~1/3 of NPCs exhibit a typical necrotic morphology of swollen and ruptured membrane after OGD (upper panel of Fig. 1d, and e), while 17AAG treatment attenuated this phenotype, with majority of the NPCs retaining their regular morphology with extended bipolar processes (lower panel, Fig. 1d). Flow cytometry quantification showed that 17AAG treatment significantly reduced the number of PI-positive cells induced by OGD by 42.9 % (from 36.8 ± 2.4 % to 21.0± 2.5 %, Fig.1f and g). To exclude secondary apoptosis (since these types of cells also retain PI), we treated cells with a pan-caspase inhibitor, Z-VAD. Z-VAD treatment reduced the PI-positive NPCs by ~15 %, when compared to OGD + AAG (from 36.8 ± 2.4 % to 31.4 ± 2.3 %), demonstrating that majority of PI-positive cells were necrotic cells, which were significantly reduced by 17AAG treatment (Fig. 1g). The above data indicate that 17AAG protects NPCs against OGD-induced apoptosis and necrosis, major cell death forms in ischemic stroke.
17AAG treatment attenuated oxidative stress-mediated cell death in NPCs
Oxidative stress and glutamate exposure are two of the major triggers of brain damage during ischemic stroke (Davalos et al. 1997, Kostandy 2012, Pradeep et al. 2012). However, exposure to high levels of glutamate (up to 0.8 mM) did not induce NPC apoptosis or necrosis as determined by Annexin V/PI staining followed by flow cytometry (Fig. 2a and b). This observation is consistent with a previous report (Brazel et al. 2005). Hence we focused on oxidative stress-mediated cell death to further investigate 17AAG’s role in NPCs. Figure 2c shows that 43.7 ± 4.3 % of H2O2-treated cells retained PI (blue line), which was significantly reduced to 19.8 ± 3.5 % by 17AAG treatment (green line, Fig. 2c), indicating that 17AAG ameliorated oxidative stress-mediated necrosis. Similarly, treatment with 17AAG significantly attenuated H2O2 induced apoptosis as detected by Annexin V-Alexa Fluro 488 labeling (Fig. 2d), which was further confirmed by immunoblot analysis of cleaved Casp3 (Fig. 2e and f).
17AAG protects NPCs against apoptosis and necrosis induced by H 2 O 2. a: Glutamate (0.8 mM) (GMS) did not induce NPC apoptosis as measured by Annexin V staining followed by flow cytometry. b: Glutamate (0.8 mM) did not induce NPCnecrosis measured by PI staining followed by flow cytometry. c: 17AAG ameliorated H2O2 induced necrosis in NPCs measured by PI staining followed by flow cytometry. Con (pink), vehicle treated; H2O2 (blue, 100μMH2O2 treated; and H2O2 + AAG (green), 17AAG pretreated and added 100μMH2O2) the number of necrotic cells to 19.8 ± 3.5 % of total cells, a 54.7 % reduction (green line, Fig. 2c). d: Treatment with 17AAG attenuated H2O2 induced apoptosis as determined by Annexin V-Alexa Fluro 488 labeling. e: Western blot analysis of cleaved Casp3. f: Densitometry analysis of the bands shown in E. *, p < 0.05
Low dose 17AAG activates multiple pro-survival pathways in vitro
Previous studies have shown that GA and 17AAG activated PI3K/Akt and MAPK pathways through transient stimulation of Src kinase (Koga et al. 2006, Wang et al. 2011). However whether 17AAG activates these pathways during ischemic conditions was not known. We measured the level of phospho-GSK-3β (pGSK-3β), one of the substrates of Akt kinase (Bhat et al. 2004, Koga et al. 2006), after OGD. Figure 3a and b show that GSK-3β phosphorylation was up-regulated by 10nM 17AAG. To confirm that the pro-survival pGSK-3β was induced by 17AAG during OGD, we detected the level of pAkt and found it was increased by 17AAG treatment (Fig. 3a and b). We also blocked the pathway by LY294002, a PI3K inhibitor and found that LY294002 prevented GSK-3β phosphorylation triggered by 17AAG (Fig. 3c and d). These data validate that 17AAG activates the PI3K/Akt pathway to protect NPCs during OGD.
17AAG activates multiple pro-survival pathways during OGD. a: Western blot analysis of the proteins as indicated in figure. b: Densitometry analysis of the bands in A. c: LY294002 prevented GSK-3β phosphorylation triggered by 17AAG. d: Densitometry analysis of the bands in C. e: The Mek inhibitor U0126 attenuated ERK phosphorylation triggered by 17AAG. f: Densitometry analysis of the bands in E. *, p < 0.05; **, p < 0.01
To determine whether MAPK pathway was activated, we determined the level of pERK1/2, a MAPK substrate. Figure 3a and b show that ERK was activated by 10nM 17AAG during OGD. The involvement of MAPK pathway in 17AAG mediated survival was validated by a Mek inhibitor U0126 (Figs. 3e and f).
The level of pStat3 remained unchanged, indicating low dose 17AAG did not activate the JAK/Stat3 pathway (Figs. 3a and b).
Pretreatment of 17AAG activates multiple pro-survival pathways and reduced infarct size in vivo
The above data indicate that low dose 17AAG protects neural cells against major types of cell death pathways induced by stroke-related stresses. To determine whether 17AAG protects against stroke-induced damage in vivo, we intraperitoneally (IP) injected 17AAG into 3 month old male C57Bl/6 mice before introducing MCAO. Figure 4a and b show that, similar to our in vitro studies, 17AAG reduced infarct size caused by MCAO in a dose dependent manner, with 0.2 mg/kg significantly reducing the infarct size by 57.7 %, while 0.05 mg/kg and 1 mg/kg had marginal effects.
17AAG reduces infarct volume in MCAO mouse brains. a: 17AAG reduced infarct size caused by MCAO in a dose-dependent manner. 17AAG of different doses was IP injected into 3 month old male C57Bl/6 mice before introducing MCAO. 17AAG doses were: AAG0.05, 0.05 mg/kg body weight; AAG0.2, 0.02 mg/kg body weight; AAG1, 1 mg/kg body weight. Note 0.2 mg/kg 17AAG significantly reduced the infarct volume, while 0.05 mg/kg and 1 mg/kg had little effects. b: Quantification of the infarct volume with image J. Sample sizes were: Con, N = 9; AAG0.05, N = 7; AAG0.2, N = 11; AAG1, N = 10. c: Western blot analysis of indicated proteins on whole brain lysates of mice injected with 0.2 mg/kg 17AAG. Note 17AAG greatly increased pGSK-3β and pERK levels in brains of mice without MCAO (compare lane 2 to lane 1) as well as those after MCAO (compare lane 4 to lane 3). Actin was used as a loading control. d: Densitometry analysis of the bands shown in C. *, p < 0.05, **, p < 0.01
To determine whether PI3K/Akt and MAPK pathways were activated in vivo, we collected brain tissues from mice injected with 0.2 mg/kg 17AAG and performed western blot analyses. Figure 4c and d show that the levels of pGSK-3β and pERK were both up-regulated by 17AAG in brains of mice without MCAO (compare lane 2 to lane 1). More importantly, these kinases remained more activated than those without 17AAG administration after MCAO (compare lane 4 to lane 3). These results demonstrate that 17AAG activates these two pathways in vivo to protect against stroke-induced brain damage.
Discussion
Stress adaptation (or hormesis) is a common paradigm found throughout nature. An initial exposure to a stressful stimulus results in an adaptive response by which a second more severe exposure produces a minimal response (Wheeler and Wong 2007). Research from IPC, RIPC and IpoC has demonstrated that stress adaptation provides substantial neuroprotection in animal studies of stroke (Hougaard et al. 2013, Wei et al. 2005, Wei et al. 2013, Yu et al. 2013). Apart from ischemia, a number of other methods have been reported to exert hormetic effect in stroke. For example, a number of pharmacological agents are found to induce the hormetic effect in stroke, including Ginkgolide B, adenosine, isoflurane, estrogen, 3-nitropropionic acid, and kainate (Della-Morte et al. 2009, Lee et al. 2008, Nagy et al. 2011, Raval et al. 2006, Wu et al. 2009). In addition, hypothermia induced pharmacologically by a neurotensin receptor agonist HPI-201 protects the brain from damages caused by intracerebral hemorrhage (Wei et al. 2013). These studies demonstrated that pharmacological conditioning can have a comparable stress adaptation effect in protecting damages induced by ischemia.
Due to the complexity of acute stroke, protection of cells against all forms of cell death is crucial to improve the outcome of regenerative and reparative medicine. The hormetic effect induced by Hsp90 inhibitors may be of advantage in this regard because it potentially affects many of the pathways mediated by Hsp90, its cochaperones and the ever growing list of client proteins (Camphausen and Tofilon 2007, Dezwaan and Freeman 2008, Jarosz and Lindquist 2010).
Data presented in this study demonstrated that 17AAG not only protected NPCs from apoptosis induced by OGD and oxidative stress, but also necrosis (Figs. 1 and 2). More interestingly, treatment of 17AAG significantly reduced the infarct volume in the MCAO model (Fig. 4a and b), indicating that low dose 17AAG is a potential conditioning agent for stroke treatment. The observation that 17AAG treatment reduced infarct volume suggests that low dose 17AAG protects other neural cells, especially neurons, from stroke mediated damage. This is a very interesting phenomenon and will be part of future studies.
In terms of signal transduction, low dose 17AAG activated Akt/GSK-3β and MAP kinase pathways, under stroke related stresses in vitro and under MCAO in vivo (Figs. 3 and 4c and d). These two cell signaling pathways are generally pro-survival and are severely affected by ischemic stroke (Sawe et al. 2008, Zhao et al. 2006).
Our purpose of choosing NPCs as an in vitro model to study low-dose 17AAG’s function in stroke-related stresses is to use NPCs for regenerative medicine for stroke. Establishing that 17AAG triggers cytoprotective mechanisms in NPCs against both apoptosis and necrosis under stroke conditions, we will commence cell transplantation studies to further investigate the synergistic effect of combining stress adaptation and cell therapy in stroke treatment.
In summary, we have demonstrated that 17AAG treatment protects NPCs from major forms of cell death mechanisms induced by OGD and stroke related stresses in vitro and reduces infarct size in vivo. These data demonstrate that pharmacological conditioning with 17AAG could be a more efficient approach for stroke management.
Abbreviations
- 17AAG:
-
17-N-Allylamino-17-demethoxygeldanamycin
- NPCs:
-
neural progenitor cells
- OGD:
-
oxygen glucose deprivation
- IPC:
-
ischemic preconditioning
- RIPC:
-
Remote ischemic preconditioning
- IPoC:
-
ischemic postconditioning
- MCAO:
-
middle cerebral artery occlusion
- PI:
-
propidium iodide
References
Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9(6):723–727
Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH, Barlow C (2001) Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev 15(5):554–566
Ansar S, Burlison JA, Hadden MK, Yu XM, Desino KE, Bean J, Neckers L, Audus KL, Michaelis ML, Blagg BS (2007) A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg Med Chem Lett 17(7):1984–1990
Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A, Nicholls J, Williamson DA, Jensen SL, Gordon M, Habib N, Chataway J (2014) Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl Med.
Bhat RV, Budd Haeberlein SL, Avila J (2004) Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem 89(6):1313–1317
Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG (2003) Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol 162(3):469–479
Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG (2004) Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol 167(4):723–734
Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, Sun GH, Ma M, Masel J, Kleppner SR, Schallert T, Palmer T, Steinberg GK (2006) Transplantation of hNT neurons into the ischemic cortex: cell survival and effect on sensorimotor behavior. J Neurosci Res 83(6):1004–1014
Bradley E, Bieberich E, Mivechi NF, Tangpisuthipongsa D, Wang G (2012) Regulation of embryonic stem cell pluripotency by heat shock protein 90. Stem Cells 30(8):1624–1633
Brazel CY, Nunez JL, Yang Z, Levison SW (2005) Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience 131(1):55–65
Calabrese EJ (2013a) Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut 182:452–460
Calabrese EJ (2013b) Hormetic mechanisms. Crit Rev Toxicol 43(7):580–606
Camphausen K, Tofilon PJ (2007) Inhibition of Hsp90: a multitarget approach to radiosensitization. Clin Cancer Res 13(15 Pt 1):4326–4330
Chang YC, Shyu WC, Lin SZ, Li H (2007) Regenerative therapy for stroke. Cell Transplant 16(2):171–181
Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM (2010) Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A 107(44):18880–18885
Chopp M, Zhang ZG, Jiang Q (2007) Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke 38(2 Suppl):827–831
Croslan DR, Schoell MC, Ford GD, Pulliam JV, Gates A, Clement CM, Harris AE, Ford BD (2008) Neuroprotective effects of neuregulin-1 on B35 neuronal cells following ischemia. Brain Res 1210:39–47
Davalos A, Castillo J, Serena J, Noya M (1997) Duration of glutamate release after acute ischemic stroke. Stroke 28(4):708–710
Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA (2009) Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159(3):993–1002
Dezwaan DC, Freeman BC (2008) HSP90: the Rosetta stone for cellular protein dynamics? Cell Cycle 7(8):1006–1012
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317
Frederix K, Chauhan AK, Kisucka J, Zhao BQ, Hoff EI, Spronk HM, Ten Cate H, Wagner DD (2007) Platelet adhesion receptors do not modulate infarct volume after a photochemically induced stroke in mice. Brain Res 1185:239–245
Gallo KA (2006) Targeting HSP90 to halt neurodegeneration. Chem Biol 13(2):115–116
Haas S, Weidner N, Winkler J (2005) Adult stem cell therapy in stroke. Curr Opin Neurol 18(1):59–64
Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN (2011) Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke 42(10):2960–2962
Hess DC, Hoda MN, Bhatia K (2013) Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke 44(4):1191–1197
Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J (2009) Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci 29(3):562–574
Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK (2011) Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29(2):274–285
Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Thomsen RB, Jonsdottir K, Mouridsen K, Hansen TM, Cho TH, Nielsen TT, Botker HE, Ostergaard L, Andersen G (2013) Remote ischemic perconditioning in thrombolysed stroke patients: randomized study of activating endogenous neuroprotection - design and MRI measurements. Int J Stroke 8(2):141–146
Jarosz DF, Lindquist S (2010) Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science 330(6012):1820–1824
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA (2006) Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A 103(35):13198–13202
Kaushal V, Schlichter LC (2008) Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 28(9):2221–2230
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK (2004) Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A 101(32):11839–11844
Koch S, Katsnelson M, Dong C, Perez-Pinzon M (2011) Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke 42(5):1387–1391
Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L (2006) Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc Natl Acad Sci U S A 103(30):11318–11322
Kostandy BB (2012) The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci 33(2):223–237
Kwon HM, Kim Y, Yang SI, Kim YJ, Lee SH, Yoon BW (2008) Geldanamycin protects rat brain through overexpression of HSP70 and reducing brain edema after cerebral focal ischemia. Neurol Res 30(7):740–745
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU (2007) Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 25(5):1204–1212
Lee JJ, Li L, Jung HH, Zuo Z (2008) Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology 108(6):1055–1062
Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD (2007) Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38(11):2992–2999
Majid A (2014) Neuroprotection in stroke: past, present, and future. ISRN Neurol 2014:515716
Marini AM, Jiang H, Pan H, Wu X, Lipsky RH (2008) Hormesis: a promising strategy to sustain endogenous neuronal survival pathways against neurodegenerative disorders. Ageing Res Rev 7(1):21–33
Mitrecic D, Nicaise C, Klimaschewski L, Gajovic S, Bohl D, Pochet R (2012) Genetically modified stem cells for the treatment of neurological diseases. Front Biosci (Elite Ed) 4:1170–1181
Mitsios N, Gaffney J, Kumar P, Krupinski J, Kumar S, Slevin M (2006) Pathophysiology of acute ischaemic stroke: an analysis of common signalling mechanisms and identification of new molecular targets. Pathobiology 73(4):159–175
Moretti A, Ferrari F, Villa RF (2014) Neuroprotection for ischaemic stroke: Current status and challenges. Pharmacol Ther.
Nagy D, Kocsis K, Fuzik J, Marosi M, Kis Z, Teichberg VI, Toldi J, Farkas T (2011) Kainate postconditioning restores LTP in ischemic hippocampal CA1: onset-dependent second pathophysiological stress. Neuropharmacology 61(5–6):1026–1032
Norgaard R, Kassem M, Rattan SI (2006) Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci 1067:443–447
Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S (2009) Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci.
Pradeep H, Diya JB, Shashikumar S, Rajanikant GK (2012) Oxidative stress–assassin behind the ischemic stroke. Folia Neuropathol 50(3):219–230
Ratan RR, Siddiq A, Smirnova N, Karpisheva K, Haskew-Layton R, McConoughey S, Langley B, Estevez A, Huerta PT, Volpe B, Roy S, Sen CK, Gazaryan I, Cho S, Fink M, LaManna J (2007) Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med 85(12):1331–1338
Raval AP, Bramlett H, Perez-Pinzon MA (2006) Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience 141(4):1721–1730
Roitberg BZ, Mangubat E, Chen EY, Sugaya K, Thulborn KR, Kordower JH, Pawar A, Konecny T, Emborg ME (2006) Survival and early differentiation of human neural stem cells transplanted in a nonhuman primate model of stroke. J Neurosurg 105(1):96–102
Rosova I, Dao M, Capoccia B, Link D, Nolta JA (2008) Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26(8):2173–2182
Sawe N, Steinberg G, Zhao H (2008) Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res 86(8):1659–1669
Shaller CA, Jacques S, Shelden CH (1980) The pathophysiology of stroke: a review with molecular considerations. Surg Neurol 14(6):433–443
Steindler DA (2007) Stem cells, regenerative medicine, and animal models of disease. Ilar J 48(4):323–338
Terasawa K, Minami M, Minami Y (2005) Constantly updated knowledge of Hsp90. J Biochem (Tokyo) 137(4):443–447
Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP (2008) In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol 210(2):656–670
Wang G, Krishnamurthy K, Tangpisuthipongsa D (2011) Protection of murine neural progenitor cells by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin in the low nanomolar concentration range. J Neurochem 117(4):703–711
Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E (2005) Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem 280(28):26415–26424
Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC (2009) Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells 27(12):3021–3031
Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G (2006) Modulation of Hsp90 function in neurodegenerative disorders: a molecular-targeted therapy against disease-causing protein. J Mol Med 84(8):635–646
Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM Jr, Yu SP, Choi DW (2005) Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis 19(1–2):183–193
Wei S, Sun J, Li J, Wang L, Hall CL, Dix TA, Mohamad O, Wei L, Yu SP (2013) Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice. Neuroscience 252:489–500
Wen XR, Li C, Zong YY, Yu CZ, Xu J, Han D, Zhang GY (2008) Dual inhibitory roles of geldanamycin on the c-Jun NH2-terminal kinase 3 signal pathway through suppressing the expression of mixed-lineage kinase 3 and attenuating the activation of apoptosis signal-regulating kinase 1 via facilitating the activation of Akt in ischemic brain injury. Neuroscience 156(3):483–497
Wheeler DS, Wong HR (2007) Heat shock response and acute lung injury. Free Radic Biol Med 42(1):1–14
Wu X, Qian Z, Ke Y, Du F, Zhu L (2009) Ginkgolide B preconditioning protects neurons against ischaemia-induced apoptosis. J Cell Mol Med 13(11–12):4474–4483
Xie R, Wang P, Ji X, Zhao H (2013) Ischemic post-conditioning facilitates brain recovery after stroke by promoting Akt/mTOR activity in nude rats. J Neurochem 127(5):723–732
Yano A, Tsutsumi S, Soga S, Lee MJ, Trepel J, Osada H, Neckers L (2008) Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc Natl Acad Sci U S A 105(40):15541–15546
Yu SP, Wei Z, Wei L (2013) Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 4(1):76–88
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14(4):469–477
Zhang H, Burrows F (2004) Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med 82(8):488–499
Zhao H, Sapolsky RM, Steinberg GK (2006) Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol 34(3):249–270
Acknowledgment
We are grateful to Dr. Weiguo Li, Dr. Quanguang Zhang, Dr. Jianghe Yuan, and Dong Han for technical assistance. We thank Dr. Lin Mei for institutional support. This project is partly supported by a Scientist Training Program award from Medical College of Georgia and partly by a Scientist Development Grant award from American Heart Association to GW.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bradley, E., Zhao, X., Wang, R. et al. Low dose Hsp90 inhibitor 17AAG protects neural progenitor cells from ischemia induced death. J. Cell Commun. Signal. 8, 353–362 (2014). https://doi.org/10.1007/s12079-014-0247-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-014-0247-5