Abstract
The vascular endothelium plays a fundamental role in the health and disease of the cardiovascular system. The molecular mechanisms regulating endothelial homeostasis, however, remain incompletely understood. CCN3, a member of the CCN (Cyr61, Ctgf, Nov) family of cell growth and differentiation regulators, has been shown to play an important role in numerous cell types. The function of CCN3 in endothelial cells has yet to be elucidated. Immunohistochemical analysis of CCN3 expression in mouse tissues revealed robust immunoreactivity in the endothelium of large arteries, small resistance vessels, and veins. We found that CCN3 expression in human umbilical vein endothelial cells (HUVECs) is transcriptionally induced by laminar shear stress (LSS) and HMG CoA-reductase inhibitors (statins). Promoter analyses identified the transcription factor Kruppel-like factor 2 (KLF2) as a direct regulator of CCN3 expression. In contrast to LSS, proinflammatory cytokines reduced CCN3 expression. Adenoviral overexpression of CCN3 in HUVEC markedly inhibited the cytokine-mediated induction of vascular adhesion molecule-1 (VCAM-1). Consistent with this observation, CCN3 significantly reduced monocyte adhesion. Conversely, CCN3 knockdown in HUVECs resulted in enhancement of cytokine-induced VCAM-1 expression. Concordant effects were observed on monocyte adhesion. Gain and loss-of-function mechanistic studies demonstrated that CCN3 negatively regulates nuclear factor kappaB (NF-κB) activity by reducing its translocation into the nucleus and subsequent binding to the VCAM-1 promoter, suggesting that CCN3’s anti-inflammatory effects occur secondary to inhibition of NF-κB nuclear accumulation. This study identifies CCN3 as a novel regulator of endothelial proinflammatory activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vascular endothelium, the monolayer of cells lining all blood vessels, is critically involved in the maintenance of vascular homoeostasis (Gimbrone et al. 2000). The endothelium regulates vascular permeability, blood coagulation, and homing of immune cells to specific sites of the body. When functioning normally, the endothelium exerts tight control of these processes and maintains an anti-inflammatory, anti-adhesive, anti-coagulant luminal surface (Gimbrone et al. 2000). Dysfunction of the endothelium is a key pathophysiologic event in the development and progression of vascular disease states. Characteristic abnormalities include impaired endothelium-dependent vasodilation, enhanced expression of leukocyte adhesion molecules, increased elaboration of pro-coagulant factors, and abnormal vascular remodeling (Gimbrone et al. 2000). Despite considerable effort, the molecular mechanisms regulating endothelial homeostasis remain incompletely understood.
CCN (Cyr61, Ctgf, Nov) proteins are a group of secreted extracellular matrix-associated signaling proteins that are capable of mediating diverse biologic functions (Yeger and Perbal 2007). To date, six members have been identified in mammals; cysteine-rich protein 61 (Cyr61 or CCN1), connective tissue growth factor (Ctgf or CCN2), nephroblastoma overexpressed (Nov or CCN3), Wisp1 (CCN4), Wisp2 (CCN5), and Wisp3 (CCN6) (Yeger and Perbal 2007). These proteins possess four conserved structural domains, including: (1) an insulin like growth factor–binding protein (IGFBP) homology domain, (2) a von Willebrand factor type C (VWC) domain, (3) a thrombospondin type 1 (TSP-1) repeat homology domain, and (4) a C-terminal (CT) domain with sequence similarities to growth factor cysteine knots (Yeger and Perbal 2007). The structure of CCN5 differs from the others in that it lacks a CT domain. A growing body of evidence demonstrates that the CCN gene family plays critical roles in cell growth and differentiation processes such as mitosis, adhesion, apoptosis, extracellular matrix production, growth arrest, and migration. For example, CCN1 and CCN2 have been shown in vitro to support cell adhesion, induce cell migration, augment growth factor–induced cell proliferation, and, in vivo, to induce angiogenesis (Chen and Lau 2009). By serving as cell adhesion substrates, CCN1, CCN2, and CCN3 can induce apoptotic cell death in fibroblasts (Chen and Lau 2009). While members of this family have also been implicated in endothelial biology and processes such as angiogenesis (Lin et al. 2003), the precise role remains poorly understood.
CCN3 (also called nephroblastoma overexpressed) was originally isolated from nephroblastoma tissue in newborn chicks infected with the MAV-1 avian retrovirus (Joliot et al. 1992). CCN3 is normally expressed both in embryonic and adult tissues (Burren et al. 1999; Kocialkowski et al. 2001; Perbal 2001). In adults, CCN3 is expressed in diverse tissues, including the nervous system (brain and spinal cord), adrenocortical glands, skeletal and cardiac muscle, cartilage, bone, lung and kidney (Burren et al. 1999; Liu et al. 1999; Ellis et al. 2000; Kocialkowski et al. 2001; Su et al. 2001; Schild and Trueb 2004). CCN3 has been shown to exert multiple biological functions through diverse signaling (Perbal 2001; Sakamoto et al. 2002; Lin et al. 2003; Lombet et al. 2003; Fu et al. 2004; Gellhaus et al. 2004; Luo et al. 2006). Recently, an intriguing study highlighted an important role of CCN3 in stem cell renewal (Gupta et al. 2007). In the cardiovascular system, CCN3 expression has been demonstrated in fibroblasts, smooth muscle and endothelial cells (Kocialkowski et al. 2001; Su et al. 2001; van Roeyen et al. 2008). In rat smooth muscle cells, CCN3 acts as an adhesion factor and is induced upon vascular injury (Ellis et al. 2000; Su et al. 2001). CCN3 was also found to be activated in blood vessels and skin fibroblasts after vascular injury (Lin et al. 2005a). However the biologic function of CCN3 in endothelial cells (ECs) has not been well explored. Herein, using overexpression and siRNA-mediated knockdown approaches, we provide evidence that CCN3 regulates key aspects of endothelial anti-inflammatory function.
Materials and methods
Cell culture and reagents
Human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAEC), human pulmonary artery endothelial cells (HPAEC) were acquired from Lonza (Walkersville, MD) and cultured in EBM-2 media according to instructions. Human microvascular endothelial cells (HMEC) were obtained from ATCC. Recombinant human IL-1β and TNFα was obtained from R&D systems (Minneapolis, MN). U937 cells were purchased from American Type Culture Collection and were cultured in RPMI 1640 with 2 mM L-glutamine and 10% fetal bovine serum. Antibodies recognizing VCAM-1, ICAM-1, p50, p65, Ku70 and IκBα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); and α-tubulin antibody was from Sigma (St. Louis, MO). Simvastatin was obtained from Calbiochem. All adenoviral constructs (Ad-GFP, Ad-Flag-KLF2, Ad-CCN3) were generated as described previously (SenBanerjee et al. 2004; Yan et al. 2006).
Transient transfection
The CCN3 promoter luciferase construct was kindly provided by Dr. C. Martinerie (France) (Lafont et al. 2002), this construct was used as template to clone the proximal 492 base pair CCN3 promoter into pGL3-Basic vector (Promega, Madison, WI). CCN3 promoter deletion constructs were generated by PCR. Site-specific mutagenesis was accomplished by using the QuikChange mutagenesis kit following the manufacture’s instruction (Stratagene, La Jolla, CA). HUVECs were plated at a density of 105 cells per well in 12-well plates 1 day prior to transfection. Transient transfection studies were performed using Lipofectamine 2000 reagent (Invitrogen) according to instructions by the manufacturer. A constant total of 1 μg plasmid DNA per well was used in transfections. Cells were harvested 48 h after transfection and assays were performed for luciferase activity, normalized to total protein in each sample. All transfections were performed in triplicate (n = 9).
siRNA transfection
Human CCN3-directed pooled siRNA oligo (Catlog # L-010527) and a non-specific control pooled siRNA (Catlog # D-001810) were purchased from Dharmacon (Lafayette, CO). HUVECs were plated 1 day before transfection in antibiotic-free EBM-2 medium. On the day of transfection, 100 nM of specific siRNA targeting human CCN3 or non-specific siRNA was incubated with Lipofectamine 2000 (Invitrogen) at room temperature for 30 min before adding to the HUVECs in OPTI-MEM (Invitrogen). Three hours later the medium was replaced by EBM-2 and cultured for an additional 48 h. Cells were treated with or without TNFα for indicated time and harvested for total protein or nuclear protein.
Western blot analysis
Cells were treated as described, and then harvested for total protein or nuclear protein. The resultant protein was subjected to western blot analyses using the indicated antibodies. CCN3 proteins in the conditioned medium were concentrated using heparin sepharose beads (Amersham, Uppsala, Sweden) as described by Chevalier et al. (Chevalier et al. 1998). Briefly, supernatants were incubated overnight with heparin and then washed 4 times in PBS containing protease inhibitors. Bound CCN3 was dissociated using 2-mercaptoethanol in Laemmli buffer, boiled for 10 min and then centrifuged. The supernatant was collected for western blot assay of the free protein. Heparin sepharose-concentrated samples and cellular extracts subjected to western blot analyses using CCN3 antibody.
Gel-shift studies
HUVECs were infected with control virus (Ad-GFP) or Ad-CCN3 for 48 h, followed by treatment with TNFα (10 ng/ml) for 30 min. Nuclear extracts were isolated from infected cells as previously described (Beg et al. 1993). Nuclear extracts were incubated for 30 min with an oligonucleotide probe corresponding to the NFκB consensus binding sites on human VCAM-1 promoter and then run on a polyacrylamide gel. Antibody against p65 was pre-incubated with nuclear extract for 20 min before adding radiolabeled probe.
Chromatin Immunoprecipitation (ChIP)
HUVECs were infected with Ad-KLF2-Flag or control virus (Ad-GFP) for 24 h, and native protein-DNA complexes were cross-linked by treatment with 1% formaldehyde for 15 min. The ChIP assay was carried out as reported earlier (Lin et al. 2005b). Briefly, equal aliquots of isolated chromatin were subjected to immunoprecipitation with a mouse anti-flag antibody, or mouse IgG control. The DNA associated with specific immunoprecipitates or with control IgG was isolated and used as a template for the PCR to amplify the human CCN3 promoter sequences containing the KLF binding sites. The primers used were: 5′-primer, TTG CAG CCC TGG TTG GAA 3′; 3′-primer, AGG TTT TAT AGC GCG CTC CT-3′.
Tissue Preparation and Immunohistochemistry
C57BL/6 mice were used in this study. All animals were handled according to IACUC protocol (#2009-0108) approved by the Institutional Animal Care and Use Committee at Case Western Reserve University, which is certified by the American Association of Accreditation for Laboratory Animal Care. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Mice were anesthetized and tissues were harvested, rinsed in phosphate-buffered saline, fixed in 4% paraformaldehyde for 48 h, imbedded in paraffin, and 5 mm sections were cut. Immunohistochemical analysis of formalin-fixed tissues was performed with standard procedures using a rabbit polyclonal antibody against CCN3 (K19M, 1:200) (Chevalier et al. 1998). Adjacent sections were stained with nonimmune IgG or in the absence of primary antibody as negative control.
Laminar Shear Stress Experiments
Confluent HUVECs cultured in 60-mm dishes were serum-starved for 24 h. The cells were then exposed to static conditions or laminar flow (shear stress = 12 dyn/cm2) in a cone and plate viscometer for 24 h. Conditioned media was collected and subjected to heparin bead enrichment for CCN3 protein detection. Total RNA was isolated from cells for quantitative-PCR analysis.
RNA extraction and Quantitative PCR analysis
Cells were treated as indicated and then harvested for total RNA analysis. Total RNA was isolated from cultured cells with Trizol (Invitrogen) as described by the manufacturer. Total RNA (1 μg) was reverse transcribed using reverse transcriptase. The resulting cDNA was diluted to 100 μl and used in subsequent real time PCR reactions. Gene expression was assessed by the Taqman system from Roche. The primers used were as follows: CCN1: forward primer: AAGAAACCCGGATTTGTGAG, reverse primer: GCTGCATTTCTTGCCCTTT; CCN2: forward primer: CTCCTGCAGGCTAGAGAAGC, reverse primer: GATGCACTTTTTGCCCTTCTT; CCN3: forward primer: CAGCAACCAGACTGGCATC, reverse primer: GAATTTGCAGCTTGGCTGA; GAPDH: forward primer: AGCCACATCGCTCAGACAC, reverse primer: GCCCAATACGACCAAATCC.
In vitro leukocyte adhesion assays
HUVECs were plated on 0.1% gelatin coated 24 wells, then infected with adenovirus or transfected with siRNA as described above. U937 cells were labeled with fluorescent dye DiI (Invitrogen) in phenol red free RPMI 1640 medium. Forty-eight hours later, HUVECs were treated with IL-1β or TNFα (10 ng/ml) for 4 h, then cells were washed with PBS twice. U937 cells were then added to the plate in a final concentration of 2 × 105 cells per well. Thirty minutes later, nonadherent cells were removed by rinsing (Dulbecco’s PBS, 3 times), then the remaining cells were lysed with 1% Triton in PBS. The fluorescent intensity of the cell lysates was measured using Cytofluor II fluorescent plate reader (PerSeptive Biosystems). Results are expressed as the percentage of leukocytes bound to IL-1β - stimulated cells relative to that in the control group (Ad-GFP infected or non-specific siRNA oligo transfected).
Statistics
Data are expressed as mean +/- SE. For comparison between two groups, unpaired Student t tests were used. A value of P ≤ 0.05 was considered significant.
Results
Expression of CCN3 in endothelium
While CCN3 expression has been reported in ECs, a detailed analysis has not been undertaken (Chevalier et al. 1998; Lin et al. 2003; Gellhaus et al. 2006). To evaluate CCN3 expression in endothelial cells in vivo, we performed immunohistochemical studies on mouse vascular tissues. As shown in Fig. 1a, CCN3 protein was observed in large vessels including the ascending aorta, carotid arteries, and the thoracic aorta. The expression of CCN3 was also observed in medium-sized vessels such as coronary arteries and small pulmonary veins (Fig. 1a). In addition, and correlating with previously reported analyses (Kocialkowski et al. 2001), CCN3 was also found to be present in the heart as evidenced by the positive staining in intramyocardial vessels and the endocardium (illustrated in the cardiac valve leaflet panel). Finally, ECs from the small vessels (bronchial vein) also stained positive for CCN3 (Fig. 1a). Relatively weak staining was observed in the vessels from spleen and thymus gland (data not shown). We verified the expression of CCN3 in primary endothelial cell lines derived from various human vascular beds (aorta, pulmonary artery, human microvascular endothelial cells, and HUVECs) by western blot (Fig. 1b). The presence of CCN3 in ECs from diverse vascular beds supports the notion that CCN3 may play an important role in endothelial biology. HUVECs were used in subsequent experiments as they are one of the most extensively studied cell lines in endothelial biology.
CCN3 is expressed in arterial and venous endothelial cells. a A variety of tissues were harvested and sectioned, followed by staining with a CCN3 antibody generated from rabbit. Immunohistochemical analysis revealed that CCN3 is expressed in endothelial cells of small, medium, and large vessels and in endocardial endothelium. In each panel, an arrow points to the positively-stained endothelial cell. As negative controls, adjacent aorta sections were stained with nonimmune rabbit IgG or in the absence primary antibody. All tissues were harvested from C57BL/6 mice. Scale bar 50 μm. b CCN3 is expressed in various primary human endothelial cell lines. Culture medium from confluent monolayers of endothelial cells were harvested and subjected to heparin bead enrichment for western blot analysis using the CCN3 antibody. Protein loading was normalized to equal numbers of cells. HUVEC: human umbilical vein endothelial cells; HAEC: human aortic endothelial cells; HPAEC: human pulmonary artery endothelial cells; HMEC: human microvascular endothelial cells
CCN3 expression is regulated by laminar flow, statins and cytokines
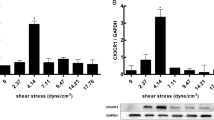
In order to investigate the role of CCN3 in regulating endothelial function, we assessed its expression under various conditions. Since endothelial cells in vessels are constantly exposed to blood flow, we hypothesized that CCN3 expression may be regulated by flow. To test this hypothesis, we performed experiments using laminar shear stress, a flow pattern that has been known to confer favorable properties to the endothelium (Berk 2008). As shown in Fig. 2a, exposure to laminar flow (12 dynes/cm2) for 24 h strongly induced CCN3 mRNA and protein level in HUVECs. The effect of laminar flow on CCN3 was specific to this member of the CCN family as we observed no effect on CCN1 and a modest decrease in CCN2 expression (data not shown).
Endothelial CCN3 is regulated by laminar shear stress (LSS), statins and inflammatory cytokines. a CCN3 is induced by laminar shear stress. HUVECs were cultured to a confluent monolayer and exposed to static medium or LSS for 24 h prior to harvest for RNA and collection of conditioned medium. Quantitative PCR data is expressed as fold induction relative to basal expression by HUVEC under static conditions. N = 3 per group; * p < 0.001. Conditioned medium was enriched for CCN3 by precipitation with heparin beads and followed by western blot analysis with CCN3 antibody. Protein loading was normalized to equal numbers of cells. b CCN3 expression is induced by statins. HUVECs were treated with vehicle or simvastatin (5 μM) for 24 h before harvest for RNA and collection of conditioned medium. Quantitative PCR data is expressed as fold induction relative to vehicle. N = 3 per group; * p < 0.001. Conditioned medium was enriched with heparin bead and followed by western blot analysis with CCN3 antibody. Protein loading was normalized to equal numbers of cells. c TNFα reduces CCN3 expression. HUVECs were treated with TNFα (10 ng/ml) for 24 h and RNA was harvested for quantitative PCR assessment (N = 3 per group; * p < 0.001.); conditioned medium was collected for heparin bead enrichment and subsequent western blot analysis. Protein loading was normalized to equal numbers of cells
Accumulating evidence indicates that HMG-CoA reductase inhibitors (statins) have effects on endothelial cells that overlap those of laminar flow (Jain and Ridker 2005). To examine whether statins regulate CCN3 similarly to laminar flow, we treated HUVECs with simvastatin and assessed for CCN3 expression. Indeed, simvastatin significantly increased CCN3 mRNA and secreted protein levels (Fig. 2b).
Inflammatory cytokines confer gene regulatory effects that generally oppose those of laminar flow and statins. Thus, we assessed the effect of the proinflammatory cytokine TNFα on CCN3 expression in endothelial cells. Figure 2c shows that TNFα significantly reduces CCN3 expression in HUVECs.
CCN3 is transcriptionally regulated by KLF2
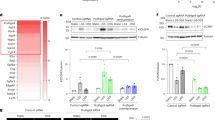
KLF2 is a member of the Kruppel-like family of zinc-finger proteins which function as transcriptional regulators in critical processes involved in cellular differentiation and tissue development (Atkins and Jain 2007). Studies from our laboratory demonstrate that KLF2 mediates the expression of a subset of genes regulated by laminar flow and statins in endothelial cells (Atkins and Jain 2007). We postulated that CCN3 might be transcriptionally regulated by KLF2. To assess the effect of KLF2 on CCN3 expression, we used adenovirus to overexpress KLF2 in HUVECs. As shown in Fig. 3a, b, forced expression of KLF2 strongly induces CCN3 mRNA and protein levels. In contrast, CCN1 and CCN2 mRNA were both downregulated by KLF2 (data not shown). To gain molecular insight into how KLF2 regulates CCN3, we performed transient transfection assays to assess whether KLF2 regulates the CCN3 promoter. Co-transfection of a −0.492kB CCN3 promoter reporter plasmid with a KLF2 expression plasmid resulted in a marked increase of promoter activity in HUVECs (Fig. 3c). A scanning of the proximal 492 bp region of human CCN3 promoter identified quite several potential KLF binding sites, to identify which sites are responsible for KLF2’s effects, we first generated CCN3 promoter deletion constructs (−376 bps, −293 bps), each constructs lost a few KLF putative sites. Then we performed transfection studies using these constructs to determine whether the induction of CCN3 promoter by KLF2 is retained. Our studies indicate that KLF2-mediated induction is retained within a −0.376kB-Luc fragment, but markedly reduced with a −0.293 kB-Luc promoter construct (Fig. 3c). These studies localize the KLF2-mediated activation of the CCN3 promoter to the region between −0.376 and −0.293 kB. Examination of this critical 83-bp region revealed the presence of two KLF sites. To determine the importance of these sites, promoter mutation studies were undertaken. Individual mutation of these two sites results in mild reduction of CCN3; however, the induction was markedly abrogated when both KLF sites were mutated. These data suggest that these two KLF sites mediate induction of the CCN3 promoter by KLF2. Finally, to verify that KLF2 can bind to the KLF sites, chromatin immunoprecipitation (ChIP) studies were undertaken. As shown in Fig. 3d, ChIP studies verify the ability of adenovirally overexpressed KLF2 to bind to the CCN3 promoter. Collectively, these results suggest that KLF2 regulates CCN3 in endothelial cells.
CCN3 is transcriptionally regulated by KLF2. a KLF2 increases CCN3 mRNA levels. HUVECs were infected with control virus (Ad-GFP) or KLF2 adenovirus (Ad-KLF2), and 48 h later, total RNA was harvested for quantitative PCR analysis. N = 3 per group; * p < 0.001 b KLF2 increases CCN3 protein levels. Confluent HUVECs were infected with control virus (Ad-GFP) or KLF2 adenovirus (Ad-KLF2), and 48 h later, conditioned medium was collected and subjected to heparin bead enrichment and western blot analysis. c KLF2 regulates CCN3 promoter activity. Cells were harvested 48 h after transfection for luciferase activity assay. N = 9 per group; d KLF2 binds to CCN3 promoter. Chromatin immunoprecipitation assays were performed as described in Methods using extracts from HUVECs infected with control (Ad-GFP) or Flag-KLF2 (Ad-KLF2)
CCN3 regulates cytokine-induced pro-inflammatory gene VCAM-1 expression
The experiments described above demonstrate that CCN3 is induced by both biomechanical (laminar flow) and pharmacologic stimuli (statins). Furthermore, we found that KLF2, a factor known to inhibit endothelial cell pro-inflammatory inflammation, increases CCN3. These findings led us to hypothesize that CCN3 may play roles in regulating inflammatory processes. To test this hypothesis, we carried out experiments to assess the effects of manipulation of CCN3 on the expression of inflammatory genes. One of the earliest events in endothelial inflammation induced by cytokines such as TNFα and IL-1β is the expression of adhesion molecules on the cell surface. VCAM-1 is one of the key adhesion molecules induced by cytokines and plays a central role in initiating atherosclerosis. To determine the effect of CCN3 on endothelial activation in response to inflammatory cytokines, HUVECs were infected with Ad-GFP or Ad-CCN3 for 48 h, stimulated with TNFα or IL-1β for an additional 4 h, and assessed for VCAM-1 protein abundance. As shown in Fig. 4a, adenoviral overexpression of CCN3 strongly inhibited the induction of VCAM-1 and ICAM-1 by TNFα and IL-1β. Consistent with this observation, CCN3 markedly reduced TNFα mediated induction of cell surface expression of VCAM-1 (Fig. 4c). The significance of endogenous CCN3 on VCAM-1 was assessed in experiments utilizing siRNA-mediated knockdown of CCN3. HUVECs were transfected with siRNA against CCN3 or with a non-specific siRNA. Forty-eight hours later, cells were then exposed to TNFα for 1 or 2 h. As shown in Fig. 4b, CCN3 protein secretion by HUVECs is strongly reduced in the presence of a specific siRNA. No effect was seen on CCN1 and CCN2 expression (data not shown). Furthermore, as illustrated in Fig. 4b, deficiency of CCN3 resulted in augmented expression of VCAM-1 by HUVECs upon TNFα stimulation. However, no significant effect was observed on ICAM-1, suggesting the mechanisms underlying the regulation of VCAM-1 and ICAM-1 by CCN3 might be different. This data suggests that endogenous CCN3 protein may have an important function in limiting the deleterious effects of pro-inflammatory cytokines.
CCN3 has an anti-inflammatory effect on the endothelium. a CCN3 overexpression inhibits VCAM-1 expression. HUVECs were infected with the indicated adenovirus, stimulated with TNFα or IL-1β for 4 h, and the expression of indicated factors assessed by western blot analysis. Exo-CCN3 refers to exogenous CCN3, which was assayed in the cell lysate. * p < 0.001. ** p < 0.05. b Knockdown of CCN3 leads to enhanced VCAM-1 expression upon TNFα stimulation. HUVECs were transfected with 100 nM of non-specific (NS) or specific human CCN3 siRNA (siCCN3). Forty-eight hours after transfection, cells were exposed to vehicle or TNFα for 1 and 2 h, total protein harvested, and VCAM-1 levels assessed by western blot analysis. These blots are representative of 3 independent experiments. * p < 0.05. c CCN3 reduces surface expression of VCAM-1 on endothelial cells. HUVECs were infected with Ad-GFP or Ad-CCN3 for 48 h, stimulated with TNFα for 4 h, and the cell surface expression of VCAM-1 assessed by flow cytometry. APC + indicates cells stained positive for VCAM-1. d Effect of CCN3 overexpression on monocyte adhesion to endothelial cells. Leukocyte adhesion assays were performed as described in Methods. Results are expressed as percentage of leukocytes bound to HUVECs relative to that of cells infected with Ad-GFP. N = 6 per group; * p < 0.001. e CCN3 deficiency increases monocyte binding. HUVECs were transfected with siRNA as above, treated with TNFα, and monocyte adhesion assays performed. Results are expressed as percentage of leukocytes bound to HUVECs relative to that of cells transfected with non-specific siRNA. N = 6 per group; * p < 0.001
CCN3 decreases leukocyte adhesion
VCAM-1 mediates lymphocyte and leukocyte attachment to endothelial cells (Springer 1994). To determine whether the effect of CCN3 on VCAM-1 protein expression is translated to a functional consequence in regard to leukocyte adhesion, we performed a leukocyte adhesion assay. Confluent HUVECs were infected with Ad-GFP or Ad-CCN3 for 48 h, followed by stimulation with IL-1β or TNFα for 4 h, then the HUVEC monolayers were incubated with fluorescent dye-labeled human monocytic cells (U937). As shown in Fig. 4d, overexpression of CCN3 in HUVECs strongly inhibits leukocyte adhesion to IL-1β activated endothelium. In contrast, the knockdown of CCN3 results in augmented VCAM-1 expression (Fig. 4b) and monocyte adhesion to the activated endothelial monolayer (Fig. 4e).
CCN3 inhibits NF-κB mediated activation
NF-κB is critical for the induction of VCAM-1 expression in response to inflammatory stimuli (Cybulsky et al. 2001). Under resting conditions, NF-κB resides in the cytoplasm as a heterodimer of the p50 and p65 subunits. A family of inhibitors termed IκBα bind to NF-κB dimers, thereby retaining the entire complex in the cytoplasm. Upon stimulation by proinflammatory cytokines such as TNFα, IκBα becomes phosphorylated and subsequently degraded, thus liberating NF-κB heterodimers which can then translocate to the nucleus to alter target gene expression (Maniatis 1997).
To delineate whether CCN3 plays a role in regulating the NFκB pathway, we first undertook CCN3 overexpression studies. HUVECs were infected with control and CCN3 adenovirus for 48 h, exposed to TNFα for 30 min, then nuclear extract and cytoplasmic proteins were harvested and subjected to western blot analysis. As shown in Fig. 5a, CCN3 overexpression markedly inhibits the nuclear accumulation of p65 following TNFα treatment, however no significant effect on cytoplasmic p50 and p65 was observed. Consistent with this observation, the degradation of IκBα was reduced. Next, to assess whether CCN3 can affect binding of NF-κB to DNA, gel-shift assays were performed using an NF-κB site derived from the VCAM-1 promoter. These assays show that in addition to inhibiting accumulation of p65 in the nucleus, CCN3 attenuates the formation of NFκB-DNA complex (Fig. 5b). To determine the effect of CCN3 deficiency on the NFκB pathway, cells were treated with TNFα after siRNA-mediated knockdown of CCN3. Western blot analysis of nuclear extracts were performed to assess for p65 translocation. As shown in Fig. 5c, knockdown of CCN3 resulted in an increase of p65 nuclear accumulation. Collectively, these observations suggest that alteration of CCN3 levels affects NFκB signaling.
CCN3 regulates NFκB signaling. a CCN3 overexpression reduces p65 accumulation in the nucleus. HUVECs were infected with control virus (Ad-GFP) and CCN3 adenovirus (Ad-CCN3) for 48 h, exposed to TNFα for 30 min, and nuclear and cytoplasmic protein extracted for assessment by western blot. Ku70 and tubulin were used for nuclear and cytosolic loading control respectively. * p < 0.01, ** p < 0.05. b CCN3 decreases p65 accumulation in the nucleus. Cells were treated as in (A), nuclear extracts were isolated and subjected to gel-shift analysis. SS indicates super-shift with p65 antibody, WT indicates unlabeled oligonucleotide. These blots are representative of n = 3 independent experiments. c CCN3 knockdown results in enhanced p65 nuclear accumulation. HUVECs were transfected with 100nM of non-specific (NS) or specific human CCN3 siRNA (siCCN3). Forty-eight hours after transfection, cells were exposed to vehicle or TNFα as indicated. Nuclear extracts were isolated and western blot performed. NE = nuclear extract. These blots are representative of 3 independent experiments. * p < 0.05
Discussion
Since the discovery of CCN3 more than a decade ago(Joliot et al. 1992), this molecule has been shown to play important roles in bone development, tumorigenesis, and hematopoiesis (Perbal 2001; McCallum et al. 2006; Perbal 2006b; Chen and Lau 2009) through its ability to negatively regulate cell proliferation (Bleau et al. 2007), A more recent study implicated CCN3 as an essential regulator of human hematopoietic stem or progenitor cell function(Schild and Trueb 2004; McCallum et al. 2006; Perbal 2006b; Gupta et al. 2007). However, the function of CCN3 in vascular biology has been under-explored. Toward that end, we demonstrate in this study that CCN3 is expressed in endothelial cells from a variety of vascular beds. Furthermore, we show that the expression of CCN3 in endothelial cells is differentially regulated by biomechanical, biochemical, and pharmacologic stimuli. Functionally, we provide evidence that CCN3 regulates a key endothelial adhesion molecule, VCAM-1, and as a result, affects monocyte adhesion to endothelial cells. Our studies identify a novel role for CCN3 in regulating endothelial inflammatory activation.
Laminar shear stress and statins have been demonstrated to be anti-inflammatory agents, having the ability to both induce anti-inflammatory factors and down-regulate pro-inflammatory factors (Jain and Ridker 2005; Berk 2008). In light of the observation that both laminar shear stress and statins increase CCN3 expression, we hypothesized that CCN3 might possess anti-inflammatory functions. Indeed, our data is consistent with this hypothesis. We found that CCN3 overexpression in endothelial cells markedly attenuates cytokine-mediated induction of VCAM-1 levels, while deficiency enhances VCAM-1 expression. This is an intriguing observation given that VCAM-1 is critical in recruiting immune cells such as macrophages to the vessel wall – a key initiating event in the development of atherosclerosis (Hajra et al. 2000). In the future it will be important to assess CCN3 expression in early and mature atherosclerotic lesions to determine if loss of CCN3 expression inversely correlates with VCAM-1 expression in vivo. Along this line, it is interesting to note that CCN3 was shown to physically interact with IL-33 (Perbal 2006a), a potent activator of T-helper 2 (Th2), known for its cardio- and athero-protective effects (O’Neill 2008).
A second important observation from our studies is that CCN3 is able to inhibit NFκB activation. NFκB plays a central role in regulating endothelial inflammation and atherosclerosis through affecting inflammatory gene expression, including VCAM-1 (Collins and Cybulsky 2001). Importantly, we found that CCN3 does not alter the expression of key components of NFκB, namely, p50, p65 and IκBα. However, when endothelial cells were challenged with cytokines, CCN3 does delay the degradation of IκBα, and consequently less p65 is translocated to the nucleus, and VCAM-1 expression is reduced. The precise basis for these effects on NFκB activation will require further investigation. CCN3 has been detected in the nucleus, cytoplasm, and extracellular space (Perbal 1999; Planque et al. 2006; Yeger and Perbal 2007) and thus the effects observed on IκBα could occur through one of a number of mechanisms. For example, whether the expression or function of other components of the NFκB signaling pathway (such as IKKα, IKKβ, and IKKγ) are altered by CCN3 expression is not known. Additionally, since IκB is a direct target of NFκB, it is possible that nuclear CCN3 may alter this regulatory process. Clearly, additional studies will be required to fully understand the relationship between CCN3 and the NFκB pathway.
A third important observation derived from our studies relates to the transcriptional regulation of CCN3. Previous studies indicate that AP-1 is critical for TGFβ-mediated reduction of CCN3 expression in adrenocortical cells (NCI H295R) (Lafont et al. 2002). In addition, steroidogenic factor-1 (SF-1) has been shown to transcriptionally down-regulate CCN3 in NCI H295R cells (Doghman et al. 2007). In our studies, we provide evidence that a member of the Kruppel-like factors (KLF) regulates CCN3. KLFs are transcription factors that bind DNA and regulate gene expression (Atkins and Jain 2007). Previous studies from our group and others have identified one member of this family named KLF2 to be a critical regulator of endothelial gene expression and function (Atkins and Jain 2007). In this paper we demonstrate that KLF2 regulates CCN3 expression (Fig. 3). Furthermore, the confluence of results from our promoter deletion studies and chromatin immunoprecipitation assays suggest CCN3 is a direct target of KLF2 action. The importance of KLF2 as a regulator of CCN3 in vivo will require further investigation. Systemic and endothelial specific deletion of KLF2 results in embryonic death (Atkins and Jain 2007). As such, the generation of inducible deletion of KLF2 in an endothelial specific manner will be required to determine the importance of this molecule in regulating CCN3 in vivo. The regulation of CCN3 by KLF2 also has implications for other cell types. For example, like CCN3, members of the KLF family (KLF2, KLF4, and KLF5) have been implicated in regulating cell pluripotency of embryonic stem cells and induced pluripotent cells (iPS) (Zhao and Daley 2008). Thus, the KLF-CCN3 regulatory axis may have biological implications well beyond the confines of blood vessel wall.
In summary, the findings in this study provide the functional evidence highlighting the importance of CCN3 gene in regulating endothelial cell inflammation. Future in vivo studies are warranted to confirm the role of CCN3 in endothelial biology. Our data also suggest that manipulation of CCN3 expression may be beneficial in the treatment of vascular inflammatory disorders.
Abbreviations
- CCN:
-
Cyr61, Ctgf, Nov
- CCN3/NOV:
-
Nephroblastoma overexpressed
- CCN1/Cyr61:
-
Cysteine-rich protein 61
- CCN2/Ctgf:
-
Connective tissue growth factor
- KLF:
-
Kruppel-like factor
- HUVEC:
-
Human umbilical vein endothelial cells
- VCAM-1:
-
Vascular adhesion molecule-1
- NF-κB:
-
Nuclear Factor Kappa B
- EC:
-
Endothelial cell
- TNFα:
-
Tumor necrosis factor- α
- IL-1β:
-
Interleulin-1 β
- EBM:
-
Endothelial basal medium
- GFP:
-
Green fluorescent protein
References
Atkins GB, Jain MK (2007) Role of Kruppel-like transcription factors in endothelial biology. Circ Res 100:1686–1695
Beg AA, Finco TS, Nantermet PV, Baldwin AS Jr (1993) Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol 13:3301–3310
Berk BC (2008) Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation 117:1082–1089
Bleau AM, Planque N, Lazar N, Zambelli D, Ori A, Quan T, Fisher G, Scotlandi K, Perbal B (2007) Antiproliferative activity of CCN3: involvement of the C-terminal module and post-translational regulation. J Cell Biochem 101:1475–1491
Burren CP, Wilson EM, Hwa V, Oh Y, Rosenfeld RG (1999) Binding properties and distribution of insulin-like growth factor binding protein-related protein 3 (IGFBP-rP3/NovH), an additional member of the IGFBP Superfamily. J Clin Endocrinol Metab 84:1096–1103
Chen CC, Lau LF (2009) Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol 41:771–783
Chevalier G, Yeger H, Martinerie C, Laurent M, Alami J, Schofield PN, Perbal B (1998) novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol 152:1563–1575
Collins T, Cybulsky MI (2001) NF-kB: pivotal mediator or innocent bystander in atherogenesis? J Clin Investig 107:255–264
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS (2001) A major role for VCAM-1, but not ICAM-1, in early atherosclerosis.[comment]. J Clin Investig 107:1255–1262
Doghman M, Arhatte M, Thibout H, Rodrigues G, De Moura J, Grosso S, West AN, Laurent M, Mas JC, Bongain A, Zambetti GP, Figueiredo BC, Auberger P, Martinerie C, Lalli E (2007) Nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic factor, is down-regulated in childhood adrenocortical tumors. J Clin Endocrinol Metab 92:3253–3260
Ellis PD, Chen Q, Barker PJ, Metcalfe JC, Kemp PR (2000) Nov gene encodes adhesion factor for vascular smooth muscle cells and is dynamically regulated in response to vascular injury. Arterioscler Thromb Vasc Biol 20:1912–1919
Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC (2004) CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanism of connexin-mediated growth suppression. J Biol Chem 279:36943–36950
Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, Winterhager E (2004) Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem 279:36931–36942
Gellhaus A, Schmidt M, Dunk C, Lye SJ, Kimmig R, Winterhager E (2006) Decreased expression of the angiogenic regulators CYR61 (CCN1) and NOV (CCN3) in human placenta is associated with pre-eclampsia. Mol Hum Reprod 12:389–399
Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G (2000) Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci 902:230–239, discussion 239-240
Gupta R, Hong D, Iborra F, Sarno S, Enver T (2007) NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science 316:590–593
Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI (2000) The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA 97:9052–9057
Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4:977–987
Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B (1992) Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 12:10–21
Kocialkowski S, Yeger H, Kingdom J, Perbal B, Schofield PN (2001) Expression of the human NOV gene in first trimester fetal tissues. Anat Embryol (Berl) 203:417–427
Lafont J, Laurent M, Thibout H, Lallemand F, Le Bouc Y, Atfi A, Martinerie C (2002) The expression of novH in adrenocortical cells is down-regulated by TGFbeta 1 through c-Jun in a Smad-independent manner. J Biol Chem 277:41220–41229
Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF (2005a) Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem 280:8229–8237
Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF (2003) CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem 278:24200–24208
Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA Jr, Balasubramanian V, Garcia-Cardena G, Jain MK (2005b) Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 96:e48–e57
Liu C, Liu XJ, Crowe PD, Kelner GS, Fan J, Barry G, Manu F, Ling N, De Souza EB, Maki RA (1999) Nephroblastoma overexpressed gene (NOV) codes for a growth factor that induces protein tyrosine phosphorylation. Gene 238:471–478
Lombet A, Planque N, Bleau AM, Li CL, Perbal B (2003) CCN3 and calcium signaling. Cell Commun Signal 1:1
Luo X, Ding L, Chegini N (2006) CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod 12:245–256
Maniatis T (1997) Catalysis by a multiprotein IkappaB kinase complex.[comment]. Science 278:818–819
McCallum L, Price S, Planque N, Perbal B, Pierce A, Whetton AD, Irvine AE (2006) A novel mechanism for BCR-ABL action: stimulated secretion of CCN3 is involved in growth and differentiation regulation. Blood 108:1716–1723
O’Neill LA (2008) The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev 226:10–18
Perbal B (1999) Nuclear localisation of NOVH protein: a potential role for NOV in the regulation of gene expression. Mol Pathol 52:84–91
Perbal B (2001) NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 54:57–79
Perbal B (2006a) New insight into CCN3 interactions–nuclear CCN3: fact or fantasy? Cell Commun Signal 4:6
Perbal B (2006b) The CCN3 protein and cancer. Adv Exp Med Biol 587:23–40
Planque N, Long Li C, Saule S, Bleau AM, Perbal B (2006) Nuclear addressing provides a clue for the transforming activity of amino-truncated CCN3 proteins. J Cell Biochem 99:105–116
Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K (2002) The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem 277:29399–29405
Schild C, Trueb B (2004) Three members of the connective tissue growth factor family CCN are differentially regulated by mechanical stress. Biochim Biophys Acta 1691:33–40
SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA Jr, Garcia-Cardena G, Jain MK (2004) KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199:1305–1315
Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301–314
Su BY, Cai WQ, Zhang CG, Martinez V, Lombet A, Perbal B (2001) The expression of ccn3 (nov) RNA and protein in the rat central nervous system is developmentally regulated. Mol Pathol 54:184–191
van Roeyen CR, Eitner F, Scholl T, Boor P, Kunter U, Planque N, Grone HJ, Bleau AM, Perbal B, Ostendorf T, Floege J (2008) CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int 73:86–94
Yan X, Baxter RC, Perbal B, Firth SM (2006) The aminoterminal insulin-like growth factor (IGF) binding domain of IGF binding protein-3 cannot be functionally substituted by the structurally homologous domain of CCN3. Endocrinology 147:5268–5274
Yeger H, Perbal B (2007) The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal 1:159–164
Zhao R, Daley GQ (2008) From fibroblasts to iPS cells: induced pluripotency by defined factors. J Cell Biochem 105:949–955
Funding
This work was supported by NIH grants HL72952, HL75427, HL76754, HL086548, HL084154, and P01 HL48743 (to M.K.J.); HL087595 (to Z.L.); and HL088740 (to G.B.A.); and HL083090 (to A.H.) and a Robert Wood Johnson/Harold Amos Medical Faculty Development grant (to G.B.A.) and American Heart Association grants 0635579 T (to Z.L.) and 0725297B (to D.K.). BP was supported by the Ministere de la Recherche et de la Technologie (France), and acknowledges Professor G. Fisher (Ann Arbor- University of Michigan) for hosting.
Conflict of interest
none declared.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, Z., Natesan, V., Shi, H. et al. A novel role of CCN3 in regulating endothelial inflammation. J. Cell Commun. Signal. 4, 141–153 (2010). https://doi.org/10.1007/s12079-010-0095-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-010-0095-x