Abstract
Vascular endothelial cells line the inner surface of blood vessels, functioning as the selective barrier between the blood and internal organs. Inflammation in endothelial cells impairs vascular functions, such as barrier function and the control of blood pressure, and enhances the recruitment of leukocytes, resulting in cardiovascular disease or cerebrovascular disease. Vascular inflammation is often initiated by enhanced generation of reactive oxygen species (ROS) and provokes additional oxidative stress. The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) coordinately activates the expression of antioxidant and xenobiotic detoxifying genes, thereby protecting the vascular cells from oxidative stress. A number of studies have investigated the role of Nrf2 in endothelial cells and revealed that endothelial Nrf2 is activated not only by well-known electrophilic Nrf2 inducers such as sulforaphane in broccoli but also by mechanical shear stress and by circulating insulin-like growth factor (IGF)-1. Nrf2 is activated in the endothelial cells of straight segments of vessels that are exposed to unidirectional laminar flow (L-flow), thus contributing to the atheroprotective property of these areas. In contrast, Nrf2 activation is defective and unactivated in cells in branched segments that are exposed to disturbed flow; these areas are atheroprone. We review the mechanisms for endothelial Nrf2 activation via physiological stimuli and its effects on vascular biology, and we discuss the roles of Nrf2 target genes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Function of Endothelial Cells
Vascular endothelial cells are a type of epithelial cells that line the cardiovascular system from the heart to the capillaries. A defective function of the endothelium is a key risk factor for cardiovascular disease (CVD) and initiates the development of atherosclerosis [1,2,3]. Endothelial cells have unique functions in vascular biology, as described below (Fig. 1).
1.1 Barrier Functions
Endothelial cells form a physical barrier that separates blood from each tissue. Communication between blood and tissue occurs through the delivery of molecules and circulating substances across a single layer of endothelial cells by bidirectional transport, either through endothelial transporters or the interspace between cells [4].
1.2 Control of Blood Pressure
Endothelial cells produce and secrete endothelium-derived relaxing factor (EDRF) (i.e., nitric oxide (NO)) and endothelium-derived contracting factor (EDCF) (i.e., endothelin) in response to various stimuli to adjust blood pressure [5,6,7]. In particular, NO is generated by the conversion of the amino acid L-arginine to NO and L-citrulline by the endothelial NO synthase (eNOS) [8, 9]. Once produced, NO rapidly diffuses across smooth muscle cells and induces relaxation by activating guanylate cyclase to produce cGMP [8, 9]. NO also protects against vascular injury, inflammation, and thrombosis, as mentioned below.
1.3 Thrombosis and Fibrinolysis
Endothelial cells are normally anticoagulant and antithrombotic because they produce and secrete antiplatelet agents, such as prostacyclin and NO [10]. Endothelial cells express heparan sulfate on the cell surface, which acts as a cofactor for activating antithrombin [11]. Endothelial cells are also involved in fibrinolysis by secreting tissue-type plasminogen activator that converts plasminogen to the active enzyme plasmin through constitutive and regulated pathways [10].
1.4 Angiogenesis
Vascular endothelial growth factor (VEGF) is an inducer of angiogenesis [12]. VEGF binds to the tyrosine kinase receptor VEGE receptor 2 (VEGFR2) of endothelial cells and stimulates the production of factors that increase vessel permeability (eNOS), proliferation/survival and migration. The repair of damaged organ and wound healing are achieved by regulated angiogenesis.
1.5 Leukocyte Recruitment
The interaction of endothelial cells and leukocytes is usually inhibited by basal levels of endothelial NO but activated in response to various stimuli, such as infection, certain mechanical stress, and ischemia reperfusion through the downregulation of NO or upregulation of adhesion molecules [2, 3, 13]. Proper leukocyte recruitment is necessary for regulated inflammation, while excessive or persistent reactions result in tissue-damaging inflammation that associated with the dysfunction of endothelial cells.
2 Inflammation in Endothelial Cells
2.1 Triggers of Inflammation in Endothelial Cells
Inflammation of endothelial cells is evoked by various pathological states, such as dyslipidemia, hypertension, and hyperglycemia, which are closely linked with lifestyle as well as infection (Fig. 1) [1,2,3]. In dyslipidemia, low-density lipoprotein (LDL) undergoes oxidative modification and is converted to oxidized LDL (oxLDL). oxLDL elicits the recruitment of leukocytes to the endothelial cells by decreasing intracellular NO, inducing the expression of adhesion molecules, chemokines, and proinflammatory cytokines [1,2,3]. On the other hand, the overexpression of angiotensin II (AII) is the cause of vascular dysfunction in renovascular hypertension. AII induces the production of superoxide anion (O2−) and activates the expression of the proinflammatory cytokines, monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1). In the case of hyperglycemia, advanced glycation end products (AGEs) bind to receptors for AGEs (RAGEs) and induce the production of proinflammatory cytokines in endothelial cells by activating the transcription factor NF-κB, the master regulator of inflammation. Hyperglycemia also promotes oxidative stress by enhancing the generation of reactive oxygen species (ROS) by multiple mechanisms [1,2,3, 14,15,16,17]. Importantly, the greater amount of glucose influx to endothelial cells results in increased flux of mitochondrial respiration substrates, leading to production of mitochondrial O2− [14,15,16] (Fig. 2). O2− activates poly (ADP-ribose) polymerase (PARP) by inducing DNA double-strand break to inhibit the activity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), one of glycolytic enzyme, by polyADP-ribosylation resulting in the production of AGEs, activation of PKC, and polyol pathway. Importantly, ROS such as O2− reduces NO bioavailability by directly reacting with NO leading to peroxynitrite (ONOO−) production or inducing eNOS uncoupling [18, 19].
2.2 Consequences of Inflammation in Endothelial Cells
Inflammation in endothelial cells is accompanied by the activation of NF-κB. NF-κB transcriptionally activates several genes such as cell adhesion molecules, chemokines and cytokines, resulting in recruitment of monocyte and cell proliferation [3]. Inflammation in endothelial cells promotes macromolecular transport by decreasing cell-to-cell and cell-to-matrix adhesion and by increasing centripetally directed tension (i.e., cell shrinkage), resulting in the formation of intercellular gaps [4]. Inflammation may also increase the selective transport of macromolecules through cells [4]. Such dysfunction of endothelial cells in coronary arteries results in diminished NO production, leading to impaired vasodilation and myocardial perfusion, which cause myocardial ischemia [1, 2].
3 Shear Stress
The vascular endothelial cells are constantly exposed to hemodynamic forces. Endothelial cells can sense hemodynamic forces because they have receptors that sense the flow and transduce mechanical signals [20,21,22,23]. The nature of the endothelial mechanical stress that occurs from the exposure to blood flow varies depending on the arterial architecture [21,22,23,24]. Steady laminar flow (L-flow) or pulsatile flow that occurs in the straight segments of the arterial tree is atheroprotective, whereas disturbed oscillatory flow (O-flow) in branch points are atheroprone because the respective shear stress has different effects on endothelial cells. L-flow confers pro-survival, cell-statics, and barrier function on endothelial cells, and it suppresses coagulation and leukocyte adhesion. On the other hand, O-flow gradually alters the endothelial cells toward proliferative and procoagulant properties, reduces barrier function, and increases the adhesion of leukocytes to endothelial cells. These differences are mainly attributable to the alteration of transcriptional regulation in endothelial cells through receptors for mechanical stress [20,21,22,23]. During L-flow, the transcription factor Krüppel-like transcription factor 2 (KLF2) is activated in endothelial cells, and KLF2 directly upregulates eNOS expression, resulting in increased NO production [25, 26]. In contrast, in endothelial cells that are exposed to O-flow, decreased KLF2 expression reduces eNOS-mediated NO production and results in inflammation and oxidative stress in these cells [27]. In addition to KLF2, increasing observations have demonstrated that Nrf2 (nuclear factor erythroid 2-related factor 2) is also a key player that acts as an endothelial mechanosensitive transcription factor.
4 Nrf2 Activation in Endothelial Cells
4.1 Transcription Factor Nrf2
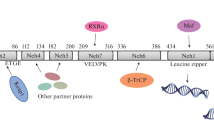
The transcription factor Nrf2 is a master regulator of cytoprotective response against oxidative stress and xenobiotics (Fig. 3). Under unstressed conditions, Nrf2 is ubiquitinated by the Keap1 (Kelch-like ECH-associated protein 1)-Cul3 E3 ligase and degraded by the proteasome [28,29,30,31]. Upon exposure to electrophiles or oxidants, Keap1-dependent Nrf2 degradation is attenuated because of the modification of reactive cysteine residues of Keap1, resulting in Nrf2 accumulation in the nucleus [32,33,34,35,36,37]. In addition, the glycogen synthase kinase 3 (GSK-3) phosphorylates Nrf2. Then, phosphorylated Nrf2 binds to the β-TrCP-Cul1 E3 ligase and is degraded via the ubiquitin-proteasome pathway in the nucleus [38, 39]. Because the phosphoinositide 3-kinase (PI3K)/Akt pathway, which is the intracellular signaling pathway related to cellular proliferation and survival, inhibits GSK-3 activity by phosphorylation, the protein level of Nrf2 is often augmented in proliferating cells that exhibit increased PI3K/Akt pathway activation [40, 41]. Accumulated Nrf2 in the nucleus heterodimerizes with small Maf proteins (sMaf) and binds to antioxidant or electrophile response elements (AREs/EpREs, respectively) in the gene regulatory region of many cytoprotective genes [42,43,44,45]. Nrf2 activates genes involved in the synthesis and conjugation of glutathione (GSH), antioxidant proteins/enzymes, drug-metabolizing enzymes, drug transporters, proteasome subunits, pentose phosphate pathway enzymes, and enzymes involved in nucleotide synthesis [42, 43, 46]. To examine the physiological effects of Nrf2 in vivo, Nrf2 gene targeting was performed [42]. Nrf2 knockout (KO) mice are viable and fertile under normal conditions, but these mice are susceptible to various environmental chemicals that provoke oxidative stress or bacterial endotoxin [42, 47, 48]. In contrast, Nrf2 activation by compounds that modify reactive cysteine of Keap1 confers protection against various stressors and diseases in mice [37, 49,50,51,52,53,54]. The anti-inflammatory activity of Nrf2 is often explained by its antioxidant protein/enzyme-inducing property because the exacerbated inflammation in Nrf2 KO mice is often alleviated by N-acetyl cysteine (NAC) [47]. However, it has been recently shown that Nrf2 represses interleukin (IL)-6 or IL-1β transcription by directly binding to their regulatory regions although the precise mechanisms are presently unclear [55].
Regulation of Nrf2. The molecular mechanisms for Nrf2 activation are shown. Nrf2 is repressed by Keap1 via ubiquitin-mediated proteosomal degradation in basal states. Upon oxidative stress, the reactive cysteine residues of Keap1 are modified by oxidants. Subsequently, Nrf2 degradation is attenuated, and newly synthesized Nrf2 translocates to the nucleus and activates its target genes. Nrf2 is also degraded by the GSK-3β-TrCP-dependent pathway, while the PI3K/Akt pathway suppresses the degradation and enhances Nrf2 activity
4.2 Shear Stress and Nrf2
cDNA subtraction and microarray analysis revealed that L-flow activates ARE-regulated genes, such as NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), ferritin, microsomal epoxide hydrolase, glutathione S-transferase, γ-glutamylcysteine ligase, and solute carrier family 7, member 11 in human aortic endothelial cells (HAECs) [56] or human umbilical vein endothelial cells (HUVECs) [57]. Exposure to L-flow induces the nuclear translocation of Nrf2 in endothelial culture cells [58,59,60,61] and in endothelial cells in the straight segments of the arterial tree [62, 63].
Several possible mechanisms have been suggested for Nrf2 activation by L-flow. We showed that the cyclooxygenase-2 (COX-2)-dependent generation of 15-deoxy- Δ12,14-prostaglandin J2 (15d-PGJ2), which belongs to the cyclopentenone-type prostaglandins with electrophilic character, is essential for the induction of Nrf2 target genes in endothelial cells in response to L-flow (Fig. 4a, b). L-flow induces Nrf2 accumulation, the increased binding of it to the ARE of NQO1, and increases target gene expression, such as NQO1, HO-1, and ferritin heavy chain (Fig. 4b–e). Exposure of endothelial cells to L-flow enhances the production of prostaglandin D2 (PGD2) and 15d-PGJ2 through the upregulation of cytosolic phospholipase A2, COX-2, and lipocalin-type PGD synthase [64,65,66], and specific COX-2 inhibitors (Nimesulide and NS-398) repress the production of PGD2 and Nrf2 activation. Of note, it was reported that 15d-PGJ2 covalently binds to Cys273 of Keap1 and suppresses Nrf2 degradation [34,35,36, 58, 67, 68]. Thus, although it is well known that 15d-PGJ2 possesses anti-inflammation roles by activating PPARγ and repressing NF-κB, the novel function involving Nrf2 adds to the anti-inflammatory function of 15d-PGJ2.
L-flow activates Nrf2 by producing 15d-PGJ2. (a) The metabolic pathway of arachidonic acid is shown. 15d-PGGJ2 exerts anti-inflammatory roles through the activation of PPARγ and the repression of Nrf2 and NF-κB. (b) The mRNA levels of Nrf2 target genes of HAECs exposed to L-flow, O-flow, or kept under a static condition for 24 h. FTH; ferritin heavy chain. (c) HAECs were exposed to respective flows, and their nuclear extracts were analyzed by Western blot analysis using the anti-Nrf2 antibody. (d) HAECs were exposed to respective flows for 24 h. A chromatin immunoprecipitation (ChIP) assay was carried out with the anti-Nrf2 antibody or normal rabbit IgG. (b–d) were reprinted from an already published report [58]. (e) Mechanism of Nrf2 activation by blood flow. Although both L-flow and O-flow induce Nrf2 accumulation in the nucleus, only L-flow, but not O-flow activates Nrf2 binding to ARE of target genes and activates their expression. One plausible explanation is the presence of factor X that is inducible by O-flow and inhibits Nrf2 binding to ARE
Exposure of HUVECs to L-flow induces Nrf2 nuclear translocation, which is inhibited by LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor and calphostin C, a protein kinase C (PKC) inhibitor [60, 62] (Fig. 5). These results demonstrated that PI3K and PKC are involved in the signaling pathway that leads to the nuclear translocation of Nrf2 in response to L-flow. As mentioned above, Nrf2 activity is enhanced by the activation of the PI3K/Akt pathway by inhibiting β-TrCP-dependent degradation in the nucleus [40, 41]. It was also reported that PKC phosphorylates Nrf2 and regulates Nrf2 target gene expression in response to oxidative stress [69, 70]. Such mechanisms may be involved in the regulation of Nrf2 activity in endothelial cells under L-flow condition.
Differential regulation of Nrf2 activity by blood flows. The left and right schemes show the several mechanisms for Nrf2 regulation in endothelial cells exposed to L-flow and O-flow, respectively. Erk5 also regulates KLF2 expression via MEF2 although it is not shown in the fig [71]
KLF2, a master regulator of endothelial cells, is also involved in Nrf2 activation [59] (Fig. 5). The siRNA-mediated knockdown of KLF2 partially decreases NQO1 expression under L-flow conditions, whereas KLF2 overexpression increases NQO1 expression both in the absence and presence of the Nrf2 inducer tert-butylhydroquinone (tBHQ). These results suggest that the concomitant activation of both the transcription factor Nrf2 and KLF2 might be required for efficient Nrf2 target gene expression.
Although extracellular signal-regulated kinase 5 (ERK5) has been reported to regulate endothelial integrity to protect endothelial cells from vascular dysfunction by activating the KLF2 pathway [71,72,73,74], ERK5 is also involved in Nrf2 activation [75] (Fig. 5). ERK5 inhibition either by knockdown using siRNA or by biochemical inactivation with a specific compound inhibits Nrf2 target gene expression in response to L-flow, whereas the activation of ERK5 increases the transcriptional activity and nuclear translocation of Nrf2. Furthermore, the direct interaction between ERK5 and Nrf2 under L-flow is observed. This interaction depends on the active state of ERK5, suggesting that ERK5 binds to Nrf2 and phosphorylates Nrf2, which may result in the dissociation of Nrf2 from the Keap1-Cul3 complex.
As a number of reports have shown ROS activates Nrf2, endothelial Nrf2 is also activated by ROS during exposure to L-flow (Fig. 4). Several reports have examined the intracellular ROS level using dichloro-dihydro-fluorescein diacetate (DCFH-DA). DCFH-DA is oxidized to the fluorescent dichlorofluorescein (DCF) by potent oxidants, such as hydroxyl radical (・OH), hydrogen peroxide (H2O2), O2−, nitric oxide (NO), and ONOO− [76]. DCF fluorescence was increased 30 min to 1 h after L-flow stimulation in HUVECs and HMVECs [77, 78]. O2− production detected by electron spin resonance (ESR) was also increased 1 h after L-flow stimulation, whereas it was decreased by 18 h after stimulation [79]. The xanthine oxidase inhibitor oxypurinol and the flavoprotein inhibitor diphenyleneiodonium, which inhibits both NAD(P)H oxidase and the mitochondrial respiratory chain, markedly suppressed the expression of Nrf2 target genes [77]. The deficiency of the NAD(P)H oxidase component p47 impaired O2− production in endothelial cells under L-flow [79]. Additionally, diphenylpyrenlphosphine (DPPP), a reducing compound of lipid hydroperoxides, also significantly suppressed Nrf2-regulated gene expression [77]. Such L-flow-induced ROS might directly attack the cysteine residues of Keap1 and repress the ubiquitin-proteasome-mediated degradation of Nrf2. NAC treatment also significantly attenuated the Nrf2 activation induced by L-flow, suggesting that oxidative stress is involved in the activation of Nrf2 [77]. Such L-flow-induced ROS might directly attack the cysteine residues of Keap1 and repress the ubiquitin-proteasome-mediated degradation of Nrf2. Although O-flow induces ROS production via NAD(P)H oxidase [79, 80] as well as L-flow, O2− accumulation persisted 18 h after exposure to O-flow [79]. Although Nrf2 accumulated in the nucleus with O-flow, this increase was concomitant with increases in ROS; however, Nrf2 target genes were not induced by O-flow [58] (Fig. 4b, c). Chromatin immunoprecipitation (ChIP) using the anti-Nrf2 antibody showed that Nrf2 binds to the NQO1 ARE in response to L-flow, but it is impaired under O-flow (Fig. 4d). This finding suggests the presence of certain factor(s) that could be induced by O-flow to prevent Nrf2 binding to the NQO1 ARE. Lee and colleagues provided several results that indicate these factors may be class I histone deacetylases (HDACs) [81](Fig. 5). The nuclear accumulation of class I and class II HDACs (HDAC-1/2/3 and HDAC-5/7, respectively) was observed in cultured endothelial cells under O-flow and rat aortic arch. O-flow induced the association of HDAC-1/2/3 with Nrf2 and Nrf2 deacetylation, probably by HDAC-1/2/3, and thus repressed Nrf2 binding to ARE of target genes and their transcription. O-flow also repressed KLF2 transcription by inducing the association of HDAC-3/5/7 and myocyte enhancer factor-2 (MEF2), a positive regulator of KLF2, and the deacetylation of MEF2. O-flow-mediated HDAC-1/2/3 accumulation also activated cyclin A and repressed p21CIP1 in endothelial cells, inducing their proliferation, although the precise mechanisms are yet to be determined. The O-flow-induced HDAC signaling is mediated by the PI3K/Akt pathway because HDAC induction was repressed by PI3K inhibitor LY294002. On the other hand, L-flow did not induce HDAC-1/2/3 accumulation in the nucleus, induced the phosphorylation of HDAC-5/7, and exported HDAC-5/7 from the nucleus. These findings demonstrate the importance of HDACs in regulating the oxidative, inflammatory, and proliferative responses of endothelial cells to O-flow.
4.3 Aging and Nrf2
Over three quarters of deaths from CVD occur among people older than the age of 65 years. In young organisms, the homeostatic response mediated by Nrf2 serves to attenuate vascular oxidative stress and limits the cellular damage caused by increased free radical production, diabetic conditions, and oxidized phospholipids [82, 83]. Ungvari et al. showed the carotid arteries of aged rhesus macaques exhibit significant oxidative stress, which was indicated by the increased 8-iso-PGF2α and 4-hydroxynonenal (HNE) content and decreased glutathione and ascorbate levels, compared with vessels of young macaques [84]. Oxidative stress in aged rhesus macaques induced inflammation phenotype characterized by NF-κB, IL-6, inducible NOS (iNOS), and intercellular adhesion molecule-1 (ICAM-1) activation but did not activate Nrf2 target genes, such as glutamate–cysteine ligase, catalytic subunit (GCLC), NQO1, and HO-1. Although these results were examined in carotid arteries that contain a variety of cells (e.g., endothelial cells, smooth muscle cells and migrated leukocytes), endothelial cells might be one of the responsible cells.
Circulating levels of insulin-like growth factor (IGF)-1, which is produced in the liver, decline during aging, which significantly increases the risk for CVD in humans. The liver-specific knockdown of the Igf1 gene in mice exhibited decreased expression of Nrf2 mRNA and the Nrf2 target genes GCLC, NQO1, and HO-1, in vascular vessels, whereas IGF-1 treatment in cultured primary human coronary arterial endothelial cells (CAECs) activate the Nrf2 pathway [85]. This report also provided evidence that in endothelial cells, IGF-1 activates Nrf2 via an Akt1-dependent pathway. The decrease of Nrf2 activity because of IGF-1 hypofunction during aging may contribute to vascular impairments in aging.
5 The Effect of Nrf2 Activation in Endothelial Cells
The scavenging of ROS by Nrf2 would be one of its critical roles in endothelial cells because endothelial ROS leads to monocyte adhesion that results in the initiation of inflammation [79]. Nrf2 is also involved in the regulation of the adhesion molecule VCAM-1 [63]. Nrf2-mediated scavenging of ROS results in the activation of MAP kinase phosphatase-1 (MKP-1), followed by the inactivation of p38 mitogen-activated protein (MAP) kinase that positively regulates VCAM-1. The activation of Nrf2 by sulforaphane suppresses p38 activation and VCAM-1 expression in the susceptible regions of the aorta, suggesting the possible future clinical use for prevention of atherosclerosis. Nrf2 or NQO1 suppresses tumor necrosis factor-α (TNF-α)-induced activation of VCAM-1 gene expression [56], indicating that the increased expression of ARE-mediated genes has a potential atheroprotective effect and exerts anti-inflammatory functions in endothelial cells. Of note, HO-1 is a well-known Nrf2 target gene that has anti-inflammatory roles [86] (Fig. 6). HO-1 is a rate-limiting enzyme that catalyze the conversion of heme into biliverdin, iron, and carbon monoxide (CO) [87]. Biliverdin is rapidly converted by biliverdin reductase to the antioxidant bilirubin [88]. The heme-derived ferrous iron is directly sequestered and inactivated by co-induced ferritin [88]. Ferritin limits the generation of ROS by binding free labile iron that would otherwise participate in a Fenton reaction to promote the generation of ROS [89]. CO is a vasodilator and has profound effects on intracellular signaling processes, which culminate in anti-inflammatory, antiproliferative, antiapoptotic, and anticoagulative effects through modulating signaling pathways such as p38 MAPK, ERK, and JNK or activating guanylate cyclase [88]. Bilirubin and biliverdin scavenge ROS/reactive nitrogen species (RNS) and reduce ROS production through the direct inhibition of NAD(P)H oxidase [90]. Bilirubin inhibits the activation of O2− -producing NAD(P)H oxidase in a neutrophil cell-free system [91, 92]. Furthermore, HO-1 is proposed to inhibit eNOS uncoupling by reducing eNOS expression when its cofactor tetrahydrobiopterin availability is limited [93]. Humans with HO-1 deficiency showed hemolytic anemia characterized by marked erythrocyte fragmentation and intravascular hemolysis, with a paradoxical increase in serum haptoglobin and low bilirubin [94]. An abnormal coagulation/fibrinolysis system, associated with elevated thrombomodulin and von Willebrand factor, indicated the presence of severe and persistent endothelial damage. The expression of HO-1 induced by adenovirus or hemin inhibits the expression of proinflammatory genes, such as E-selectin and VCAM-1, by the inhibition of NF-κB activation [95]. Curcumin, a spice and coloring agent in food that activates Nrf2, induces HO-1 expression and confers cytoprotective effect against oxidative stress in bovine aortic endothelial cells [96]. This cytoprotective effect was considerably attenuated by protoporphyrin IX, an inhibitor of HO, indicating the importance of HO-1. Additional evidence for the role of HO in vasoregulation is provided by ex vivo models. The overexpression of HO-1 by adenovirus infection of the vessels inhibited phenylephrine-dependent vasoconstriction in isolated aortic rings, also in a fashion dependent on heme degradation but independent of NO production [97]. Hemin-induced HO-1 activation in microvascular endothelial cells exhibits protection against oxidative stress and leukocyte adhesion by a mechanism that depends on the antioxidant role of bilirubin [98]. In vivo Nrf2 activation in the athero-sensitive region of the aorta by sulforaphane induced HO-1 expression and suppressed p38-VCAM-1 signaling in an Nrf2-dependent manner [63]. The inhibition of HO-1 activity using zinc protoporphyrin did not influence the VCAM-1 suppression caused by sulforaphane in HUVECs. These results indicate that HO-1 is not essential for the p38-suppressing effects of sulforaphane and the other Nrf2-induced antioxidants can compensate for the absence of HO-1 activity. It has also been reported that Nrf2 and Oct-1 corporately suppress the transcription of the NAD(P)H oxidase 4 (Nox4) gene under L-flow condition, leading to the suppression of O2− production [61].
6 Vascular Diseases and Nrf2
Although dyslipidemia and hyperglycemia provokes inflammation in endothelial cells (See Sect. 2.1), Nrf2 activation is protective against these stresses. oxLDL induced the expression of MCP-1, VCAM-1, and ICAM-1 in HUVECs and also provokes cell death. Pretreatment of polyphenol genistein (4′,5,7-trihydroxyisoflavone) increased HO-1 expression and simultaneously suppressed MCP-1, VCAM-1, and ICAM-1 expression and restored the cell viability in an Nrf2-dependent manner [99]. Mimicking hyperglycemia in human microvascular endothelial cells (HMEC-1) increased the generation of ROS, which was prevented by Nrf2 inducer sulforaphane [100]. ROS accumulation was increased further by either knockdown of Nrf2 or transketolase, one of the pentose phosphate pathway (PPP) enzymes. PPP generates not only ribose 5-phosphate (R5P), a critical substrate for the nucleotide synthesis, but also NADPH as reducing equivalents. These results suggest that Nrf2-driven PPP activation generates NADPH, which contributes to scavenging of ROS induced by hyperglycemia. Additionally, Nrf2-mediated antioxidant defense has a critical role in fetal endothelial cells that are exposed to hyperglycemia [101]. HUVECs cultured from gestational diabetes mellitus (DM) pregnancies showed reduced rates of cell proliferation to reach confluency [102]. Further proteomic analysis revealed that proteins involved in redox homeostasis were significantly altered in gestational DM and associated with increased mitochondrial superoxide generation, protein oxidation, DNA damage, and diminished GSH synthesis [101]. In accordance, induction of Nrf2 was impaired in response to 4-HNE. Decreased DJ-1, involved in Nrf2 protein stability, and increased GSK3β phosphorylation in gestational DM cells may account for the deficits in Nrf2 nuclear accumulation. These results suggest that forced expression of Nrf2 might protect fetal endothelial cells from hyperglycemia. Of note, the therapeutic potential of sulforaphane against diabetic endothelial dysfunction of Goto-Kakizaki (GK) rats, an animal model of non-obese type 2 diabetes, was reported [103]. Although GK rats exhibited significantly lower levels of Nrf2 in endothelial cells in aorta and mesenteric arteries and higher levels of oxidative stress and endothelial dysfunction, administration of sulforaphane improved NO bioavailability and decreased oxidative damage, AGEs, and HbA1c levels. In addition, sulforaphane taken as broccoli sprout concentrates decreases fasting glucose levels and glycated HbA1c in obese patients with dysregulated type 2 diabetes. Mechanistically, administration of sulforaphane improved glucose tolerance and insulin sensitivity of high fat diet-treated rats and suppressed glucose production via suppressing gene expression involved in gluconeogenesis [104]. Collectively, these data suggested the potential role of Nrf2 both in preventing type 2 diabetes and hyperglycemia-mediated vascular injuries associated with diabetes.
7 Conclusion
Many reports have demonstrated that L-flow activates endothelial Nrf2, which preserves vascular homeostasis and confers resistance to atherosclerosis. Nrf2 activation provides endothelial cells with various functions and outcomes, such as antioxidant defense against ROS, suppression of ROS production and leukocyte recruitment, and protection from endothelial dysfunction. Although Nrf2 is not induced by O-flow, the forced activation of endothelial Nrf2 by phytochemicals such as sulforaphane reduces endothelial inflammation in atheroprone areas, suggesting Nrf2 as a potential therapeutic target for preventing vascular diseases.
References
Libby P. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. https://doi.org/10.1161/hc0902.104353.
Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–69. https://doi.org/10.7150/ijbs.7502.
Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–36. https://doi.org/10.1161/CIRCRESAHA.115.306301.
Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L419–22. https://doi.org/10.1152/ajplung.2000.279.3.L419.
Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86(9):3375–8.
Gardiner SM, Compton AM, Bennett T, Palmer RM, Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990;15(5):486–92.
Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25(4):219–24. https://doi.org/10.1016/j.tips.2004.02.008.
Moncada S, Higgs EA, Hodson HF, Knowles RG, Lopez-Jaramillo P, McCall T, Palmer RMJ, Radomski MW, Rees DD, Schulz R. The l-arginine: nitric oxide pathway. J Cardiovasc Pharmacol. 1991;17(Suppl.3):S1–9.
Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20(6):295–302. https://doi.org/10.1016/j.tem.2009.03.005.
Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. https://doi.org/10.1186/s12872-015-0124-z.
Weitz JI. Heparan sulfate: antithrombotic or not? J Clin Invest. 2003;111(7):952–4. https://doi.org/10.1172/JCI200318234.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–80. https://doi.org/10.1124/pr.56.4.3.
Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88(11):4651–5.
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. https://doi.org/10.1038/35008121.
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–57. https://doi.org/10.1172/jci18127.
Reusch JE. Diabetes, microvascular complications, and cardiovascular complications: what is it about glucose? J Clin Invest. 2003;112(7):986–8. https://doi.org/10.1172/jci200319902.
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ. The role of Nrf2 in cardiovascular function and disease. Oxidative Med Cell Longev. 2017;2017:9237263. https://doi.org/10.1155/2017/9237263.
Förstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387(12):1521–33. https://doi.org/10.1515/BC.2006.190.
Pierini D, Bryan NS. Nitric oxide availability as a marker of oxidative stress. Methods Mol Biol. 2015;1208:63–71. https://doi.org/10.1007/978-1-4939-1441-8_5.
Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol. 2017;219(2):382–408. https://doi.org/10.1111/apha.12725.
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LCM, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81(3):177–99. https://doi.org/10.1016/s0079-6107(02)00052-4.
Barakat A, Lieu, D. Differential responsiveness of vascular endothelial cells to different types of fluid mechanical shear stress. Cell Biochem Biophys. 2003;38(3):323–43. https://doi.org/10.1385/cbb:38:3:323.
Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–87. https://doi.org/10.1152/physrev.00047.2009.
Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2004;101(52):18262. https://doi.org/10.1073/pnas.0409060101.
Topper JN, Cai J, Falb D, Gimbrone MA Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93(19):10417–22. https://doi.org/10.1073/pnas.93.19.10417.
SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199(10):1305–15. https://doi.org/10.1084/jem.20031132.
Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341(4):1244–51. https://doi.org/10.1016/j.bbrc.2006.01.089.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. https://doi.org/10.1101/gad.13.1.76.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8(4):379–91. https://doi.org/10.1046/j.1365-2443.2003.00640.x.
Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–9. https://doi.org/10.1128/MCB.24.16.7130-7139.2004.
Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, et al. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433(2):342–50. https://doi.org/10.1016/j.abb.2004.10.012.
Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent Ubiquitination of Nrf2 and for stabilization of Nrf2 by Chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–51. https://doi.org/10.1128/mcb.23.22.8137-8151.2003.
Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, et al. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28(8):2758–70. https://doi.org/10.1128/MCB.01704-07.
Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29(2):493–502. https://doi.org/10.1128/MCB.01080-08.
Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, et al. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med. 2012;53(4):817–27. https://doi.org/10.1016/j.freeradbiomed.2012.06.023.
Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, et al. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol. 2015;36(2):271–84. https://doi.org/10.1128/MCB.00868-15.
Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292(41):16817-24. https://doi.org/10.1074/jbc.R117.800169.
Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–33. https://doi.org/10.1128/MCB.01204-10.
Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32(32):3765–81. https://doi.org/10.1038/onc.2012.388.
Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. https://doi.org/10.1016/j.ccr.2012.05.016.
Taguchi K, Hirano I, Itoh T, Tanaka M, Miyajima A, Suzuki A, et al. Nrf2 enhances cholangiocyte expansion in Pten-deficient livers. Mol Cell Biol. 2014;34(5):900–13. https://doi.org/10.1128/MCB.01384-13.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–22.
Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36(10):1208–13. https://doi.org/10.1016/j.freeradbiomed.2004.02.075.
Katsuoka F, Motohashi H, Ishii T, Aburatani H, Engel JD, Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol. 2005;25(18):8044–51. https://doi.org/10.1128/MCB.25.18.8044-8051.2005.
Yamazaki H, Katsuoka F, Motohashi H, Engel JD, Yamamoto M. Embryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins. Mol Cell Biol. 2012;32(4):808–16. https://doi.org/10.1128/MCB.06543-11.
Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34(6):340–6. https://doi.org/10.1016/j.tips.2013.04.005.
Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116(4):984–95. https://doi.org/10.1172/JCI25790.
Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046.
Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol. 2010;30(12):3016–26. https://doi.org/10.1128/MCB.01591-09.
Ohkoshi A, Suzuki T, Ono M, Kobayashi T, Yamamoto M. Roles of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev Res. 2013;6(2):149–59. https://doi.org/10.1158/1940-6207.CAPR-12-0401-T.
Miyazaki Y, Shimizu A, Pastan I, Taguchi K, Naganuma E, Suzuki T, et al. Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant. 2014;29(4):783–91. https://doi.org/10.1093/ndt/gfu002.
Satoh H, Moriguchi T, Saigusa D, Baird L, Yu L, Rokutan H, et al. NRF2 intensifies host defense systems to prevent lung carcinogenesis, but after tumor initiation accelerates malignant cell growth. Cancer Res. 2016;76(10):3088–96. https://doi.org/10.1158/0008-5472.CAN-15-1584.
Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, et al. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci U S A. 2015;112(39):12169–74. https://doi.org/10.1073/pnas.1509158112.
Yamazaki H, Tanji K, Wakabayashi K, Matsuura S, Itoh K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol Int. 2015;65(5):210–9. https://doi.org/10.1111/pin.12261.
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. https://doi.org/10.1038/ncomms11624.
Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278(2):703–11. https://doi.org/10.1074/jbc.M203161200.
Warabi E, Wada Y, Kajiwara H, Kobayashi M, Koshiba N, Hisada T, et al. Effect on endothelial cell gene expression of shear stress, oxygen concentration, and low-density lipoprotein as studied by a novel flow cell culture system. Free Radic Biol Med. 2004;37(5):682–94. https://doi.org/10.1016/j.freeradbiomed.2004.05.020.
Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, et al. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280(29):27244–50. https://doi.org/10.1074/jbc.M502551200.
Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, et al. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(7):1339–46. https://doi.org/10.1161/ATVBAHA.108.165811.
Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai YC, Chao YJ, et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J Biomed Sci. 2009;16:12. https://doi.org/10.1186/1423-0127-16-12.
Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, et al. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res Cardiol. 2011;106(4):551–61. https://doi.org/10.1007/s00395-011-0170-3.
Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–33. https://doi.org/10.1161/CIRCRESAHA.107.152942.
Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol 2009;29(11):1851–7. https://doi.org/10.1161/ATVBAHA.109.193375.
Pearce MJ, McIntyre TM, Prescott SM, Zimmerman GA, Whatley RE. Shear stress activates cytosolic phospholipase A2 (cPLA2) and MAP kinase in human endothelial cells. Biochem Biophys Res Commun. 1996;218(2):500–4. https://doi.org/10.1006/bbrc.1996.0089.
Taba Y, Sasaguri T, Miyagi M, Abumiya T, Miwa Y, Ikeda T, Mitsumata M. Fluid shear stress induces lipocalin-type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ Res. 2000;86(9):967–73. https://doi.org/10.1161/01.res.86.9.967.
Inoue H. Transcriptional and posttranscriptional regulation of Cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22(9):1415–20. https://doi.org/10.1161/01.atv.0000028816.13582.13.
McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107(44):18838–43. https://doi.org/10.1073/pnas.1007387107.
Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, et al. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286(16):14019–27. https://doi.org/10.1074/jbc.M110.190710.
Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277(45):42769–74. https://doi.org/10.1074/jbc.M206911200.
Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278(45):44675–82. https://doi.org/10.1074/jbc.M307633200.
Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116(1):49–58. https://doi.org/10.1172/JCI24787.
Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced anti-inflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102(5):538–45. https://doi.org/10.1161/CIRCRESAHA.107.156877.
Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, et al. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4). J Biol Chem. 2010;285(34):26199–210. https://doi.org/10.1074/jbc.M110.103127.
Clark PR, Jensen TJ, Kluger MS, Morelock M, Hanidu A, Qi Z, et al. MEK5 is activated by shear stress, activates ERK5 and induces KLF4 to modulate TNF responses in human dermal microvascular endothelial cells. Microcirculation. 2011;18(2):102–17. https://doi.org/10.1111/j.1549-8719.2010.00071.x.
Kim M, Kim S, Lim JH, Lee C, Choi HC, Woo CH. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem. 2012;287(48):40722–31. https://doi.org/10.1074/jbc.M112.381509.
Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27(1–2):146–59.
Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42(2):260–9. https://doi.org/10.1016/j.freeradbiomed.2006.10.043.
Jones CI 3rd, Zhu H, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng. 2007;35(5):683–93. https://doi.org/10.1007/s10439-007-9279-9.
Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, et al. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278(47):47291–8. https://doi.org/10.1074/jbc.M305150200.
McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–7. https://doi.org/10.1152/ajpheart.00515.2003.
Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, et al. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A. 2012;109(6):1967–72. https://doi.org/10.1073/pnas.1121214109.
Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300(4):H1133–40. https://doi.org/10.1152/ajpheart.00402.2010.
Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, et al. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30(5):1007–13. https://doi.org/10.1161/ATVBAHA.110.204354.
Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66(8):866–75. https://doi.org/10.1093/gerona/glr092.
Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67(4):313–29. https://doi.org/10.1093/gerona/glr164.
Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–55. https://doi.org/10.1016/s1471-4906(03)00181-9.
Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748–55.
Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. https://doi.org/10.1152/physrev.00011.2005.
Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267(25):18148–53.
Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys Acta. 1991;1076(3):369–73.
Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010;49(2):186–95. https://doi.org/10.1016/j.yjmcc.2010.04.011.
Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, et al. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010;78(9):905–19. https://doi.org/10.1038/ki.2010.265.
Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 2009;284(46):31579–86. https://doi.org/10.1074/jbc.M109.009175.
Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103(1):129–35. https://doi.org/10.1172/JCI4165.
Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172(6):3553–63. https://doi.org/10.4049/jimmunol.172.6.3553.
Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28(8):1303–12.
Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;693–8(7):6. https://doi.org/10.1038/89068.
Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85(8):663–71.
Zhang HP, Zheng FL, Zhao JH, Guo DX, Chen XL. Genistein inhibits ox-LDL-induced VCAM-1, ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1. Arch Med Res. 2013;44(1):13–20. https://doi.org/10.1016/j.arcmed.2012.12.001.
Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57(10):2809–17. https://doi.org/10.2337/db06-1003.
Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, Mayr M, Siow RC, Mann GE. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013;62(12):4088–97. https://doi.org/10.2337/db13-0169.
Sobrevia L, Cesare P, Yudilevich DL, Mann GE. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: association with membrane hyperpolarization. J Physiol. 1995;489(Pt 1):183–92.
Pereira A, Fernandes R, Crisostomo J, Seica RM, Sena CM. The Sulforaphane and pyridoxamine supplementation normalize endothelial dysfunction associated with type 2 diabetes. Sci Rep. 2017;7(1):14357. https://doi.org/10.1038/s41598-017-14733-x.
Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9(394):eaah4477. https://doi.org/10.1126/scitranslmed.aah4477.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yamazaki, H., Itoh, K. (2020). Nrf2 in the Regulation of Endothelial Cell Homeostasis During Inflammation. In: Deng, H. (eds) Nrf2 and its Modulation in Inflammation. Progress in Inflammation Research, vol 85. Springer, Cham. https://doi.org/10.1007/978-3-030-44599-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-44599-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44597-3
Online ISBN: 978-3-030-44599-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)