Abstract
Background and purpose
The clinical role of postoperative adjuvant therapy in hepatocellular carcinoma (HCC) is still unclear. The purpose of our study was to explore the clinical value of postoperative adjuvant anti-programed cell death 1 antibody (PA-PD-1) on the prognosis of HCC patients with high relapse risks after surgery.
Patients and methods
Data of consecutive HCC patients with high recurrence risks treated with liver resection at our center during January 2019 and March 2021 were prospectively collected. Baseline differences were balanced between HCC patients with (PA-PD-1 group) or without PA-PD-1 (non-PD-1 group) after hepatectomy by propensity-score matching (PSM). Between these two groups, we compared overall survival (OS) and recurrence-free survival (RFS). Independent prognostic risk factors for OS and RFS were confirmed by Cox regression analysis, and subgroup analysis was also performed.
Results
47 pairs of patients with or without PD-1 treatment after hepatectomy were matched. After PSM, the 1-year and 2-year RFS was 58.4% and 44.1% in the PA-PD-1 group, and 34.0% and 21.3% in the non-PD-1 group (p = 0.008). The OS at 1 year and 2 years was 91.2% and 91.2% in the PA-PD-1 group, compared with 85.1% and 61.7% in the non-PD-1 group (p = 0.024). Multivariable analyses demonstrated that PA-PD-1 was an independent protective predictor associated with RFS and OS. Through subgroup analysis, we concluded that HCC patients with portal venous tumor thrombus (PVTT) or tumor size ≥ 5 cm significantly benefited from PA-PD-1 therapy in RFS and OS.
Conclusions
Adjuvant anti-PD-1 antibody can effectively improve the survival outcomes of HCC patients with high relapse risks after hepatectomy in this prospective observational study. This finding should be confirmed by results of the ongoing phase 3 randomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) makes up 75–85% of primary liver cancers and is among the deadliest malignant tumors in the world [1]. The established first-line therapeutic option for HCC patients is hepatectomy. However, up to 50%–70% of HCC patients undergoing radical hepatic resection experience relapse within 5 years [2,3,4], with 5-year survival rates of only 30%–50% [5,6,7]. Furthermore, patients with high relapse factors, for example, microvascular invasion (MVI), satellite nodules, multiple tumors, hepatic venous tumor thrombus (HVTT), portal venous tumor thrombus (PVTT), positive resection margin, alpha-fetoprotein (AFP) > 400 ng/mL, and large tumor (especially > 5 cm) showed obviously higher recurrence rate and worse prognosis [8,9,10]. Previous findings have revealed that the 5-year survival rates for HCC patients with lymph node metastasis, HVTT, and PVTT were only 20.8%, 6.5%, and 32.9%, respectively [11,12,13]. Therefore, effective postoperative adjuvant therapies are certainly important to reduce recurrence and improve long-term survival.
Although adjuvant treatment after surgery is not recommended by current guidelines on HCC [14, 15], previous studies including some randomized controlled trials (RCTs) did reveal that multiple treatment modalities, such as adjuvant transarterial chemoembolization (TACE), IFN-α, and direct-acting antiviral agents for hepatitis virus-infected HCC patients, have the influence of reducing recurrence and prolonging survival [16,17,18,19]. Immunotherapy has been previously explored as an adjuvant therapeutic option in HCC patients receiving radical surgery [20, 21]. A randomized trial of 150 postoperative HCC patients explored the clinical effectiveness of adoptive immunotherapy in reducing the frequency of tumor recurrence and reported that patients who received lymphocyte infusion gained obviously greater recurrence-free survival (RFS) than control patients [22].
Immune checkpoint inhibitor (ICI)-based combination regimens have shown potential survival benefits in advanced HCC [23,24,25]. Mechanically, they can play a role in enhancing human immune recognition and inhibiting tumor cell regeneration. The duration of response to ICIs has always been long, since activated T-lymphocyte can retain memories of their target [26], making it a promising approach for postoperative adjuvant therapy in HCC, especially for HCC patients with high relapse risks after initial hepatectomy. However, to date, postoperative adjuvant treatment with anti-PD-1 antibodies for HCC has not yet been reported. Our prospective observational study was set up to explore the clinical benefit of postoperative adjuvant anti-PD-1 antibody (PA-PD-1) on the prognosis of HCC with high relapse risks after liver resection.
Materials and methods
Participant selection
Following the guidance of the Declaration of Helsinki (1964), this prospective observational study was conducted. Inclusion criteria are outlined as follows: (1) age 18–75 years; (2) underwent R0 resection and confirmed as HCC by postoperative pathology; (3) received radiological evaluations such as abdominal CT or MRI to confirm no tumor relapse or residual within 1 month after surgery; (4) Eastern Co-operative Oncology Group (ECOG) score ≤ 1; (5) Child–Pugh A or B; (6) presence of one or more high risk for relapse: MVI, PVTT, HVTT, satellite nodules, multiple tumor nodules (> 3 nodules), AFP > 400 ng/mL, and maximum tumor size exceeding 5 cm [9, 17, 27]. Following were the exclusion criteria: (1) known sarcomatoid HCC, combined cholangiocarcinoma, and HCC or fibrolamellar HCC; (2) evidence of distant metastasis or coexistence of other malignancies on baseline imaging; and (3) previously received systemic anti-cancer therapy for HCC. According to these criteria, patients were included and separated into two groups: (1) HCC patients undergoing hepatic resection with postoperative adjuvant anti-PD-1 antibody (PA-PD-1 group); (2) HCC patients undergoing hepatectomy without postoperative adjuvant anti-PD-1 antibody (non-PD-1 group). Data including demographic and clinical characteristics and follow-up information were collected and analyzed by two independent researchers (Yukun Sun and Shuifang Hu).
Adjuvant anti-PD-1 antibody treatment
PA-PD-1 antibody therapy was recommended according to the physician’s clinical knowledge and experience and ultimately determined by the patient. HCC patients in the PA-PD-1 group were treated with intravenous PD-1 blockade therapy at an interval of 21 days after each regimen. Patients continuously received PD-1 blockade treatment until unacceptable toxic reactions were measured following the CTCAE version 5, or disease progression occurred.
Postoperative follow-up and end points
HCC patients in the two groups received regular follow-ups after liver surgery. Postoperative surveillance visits were scheduled 1 month postoperatively to confirm disease-free status, every 2–3 months for the first 2 years, and then every 6–12 months thereafter. Follow-up examinations were conducted using physical examination, laboratory tests (including peripheral blood test, liver function, AFP), and abdominal radiological examinations (ultrasound, contrast-enhanced CT, or MRI). RFS and overall survival (OS) were used as primary end points. RFS was defined as the interval from the date of hepatectomy to the date of death due to any cause or last follow-up, whichever occurred first. OS was defined as the interval from the date of hepatectomy to the date of death due to any cause or last follow-up. The last follow-up date was based on the last hospital or outpatient visit or telephone record. Follow-up data collection was terminated on January 30, 2022.
HCC recurrences or metastases were diagnosed according to histological or cytological evidence or non-invasive examination recommended by the EASL [28]. Images were independently reviewed by two senior radiologists. The final recurrent diagnosis was determined upon review of all clinical data in the case of discrepancies in CT or MRI. Patients with recurrence of HCC underwent radiofrequency ablation, surgery, TACE, radiotherapy or systemic treatment, considering tumor characteristics (location, size, and number), general condition, and liver function.
Statistical analysis
To minimize selection bias and reduce the potential impact of confounders, we used a propensity-score matching (PSM) analysis, matching patients who received anti-PD-1 antibody after hepatectomy with those who did not receive treatment. Baseline variables with p values < 0.2 of the two groups were put into the PSM model to calculate the propensity score, including age, tumor number, BCLC classification, lymph node invasion, HVTT, liver cirrhosis, PVTT, adjuvant TACE, adjuvant radiotherapy, adjuvant hepatic arterial infusion chemotherapy, and adjuvant targeted therapy. HCC patients were matched in a ratio of one to one based on logistic regression of propensity scores. Continuous normally or non-normally distributed variables were represented as means ± standard deviations or medians with interquartile ranges (IQR) and compared through the Student’s t tests or the Mann–Whitney U tests, respectively. Comparisons of categorical variables, expressed as frequencies (percentages), were performed through Pearson's Chi-square tests or Fisher’s exact tests. RFS and OS curves were generated and compared between the non-PD-1 and PA-PD-1 groups through the Kaplan–Meier method and the log-rank test. Independent predictors for RFS and OS were confirmed by Cox regression analyses. Clinical factors with p < 0.1 in the univariate analysis were entered into multivariable analysis and considered for developing the multivariable Cox model for further screening. Subgroup analyses were performed through the Kaplan–Meier method, and the forest plot for subgroup analyses was described with estimated hazard ratios (HRs) and 95% confidence intervals (CIs). For statistical analysis, the software of SPSS (version 26.0) was employed. Rstudio, “survminer”, “survival”, and “forestplot” packages were used to analyze the data. Statistical tests were two sided with significance defined as p < 0.05.
Results
Patient characteristics
From January 2019 to March 2021, 1001 HCC patients underwent surgery in our institution. Based on exclusion criteria, 483 patients were excluded, and 518 HCC patients with high relapse risks were included, with 51 patients undergoing PA-PA-1 treatment and 467 patients undergoing hepatectomy alone (Fig. 1). To balance the baseline differences, 94 patients were included in the two groups after 1:1 PSM. Detailed baseline and preoperative clinical characteristics of the non-PD-1 group compared with the PA-PD-1 group before and after PSM are described in Table 1. Before PSM, age, tumor number, BCLC classification, liver cirrhosis, and PVTT differed obviously between the two groups (all p < 0.05). Male patients, patients infected with hepatitis B virus, and patients with Child–Pugh A comprised the majority of patients (> 80%) in the two groups. Forty-seven pairs of HCC patients who did and did not receive anti-PD-1 antibody therapy after hepatectomy were selected from each group, after a 1:1 PSM, with a median follow-up duration of 15.3 months. The standardized differences between the PA-PD-1 and non-PA-PD-1 groups were much lower after PSM (Fig. S1). Among the 47 patients in the PA-PD-1group, 23 received tislelizumab, 21 received camrelizumab, 2 received pembrolizumab, and 1 received toripalimab. The median duration of PA-PD-1 was 4.17 months. The median number of cycles received was 5 (interquartile range 3–14) in the PA-PD-1 group. Potential confounding factors were balanced in the two groups (all p > 0.05).
Recurrence-free survival and overall survival
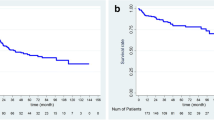
After PSM, the median RFS in the PA-PD-1 group was 17.67 (6.18–29.16) months, while it was 5.73 (4.31–7.15) months in the non-PA-PD-1 group. The corresponding 1-year and 2-year RFS were 58.40% and 44.13% in the PA-PD-1 group, and 34.04% and 21.28% in the non-PA-PD-1 group, respectively. In the PA-PD-1 group, the RFS was longer than that in the non-PD-1 group (p = 0.008, Fig. 2a). Both groups did not reach the median OS time. The corresponding 1-year and 2-year OS were 91.29% and 91.29% in the PA-PD-1 group, and 85.10% and 61.70% in the non-PD-1 group, respectively. Between the two groups, OS showed a statistically significant difference (p = 0.024, Fig. 2b).
Univariable and multivariable analysis
Univariable analysis revealed that PA-PD-1 (HR = 0.485; 95% CI = 0.282–0.836; p = 0.009), multiple tumor numbers (HR = 2.493; 95% CI = 1.463–4.248; p = 0.001), HVTT (HR = 2.605; 95% CI = 1.315–5.161; p = 0.006), and AFP > 400 ng/ml (HR = 2.111; 95% CI = 1.265–3.521; p = 0.004) were factors significantly related to RFS (Table 2). Similarly, multivariable analysis showed that PA-PD-1 (HR = 0.479; 95% CI = 0.276–0.832; p = 0.009), multiple tumor numbers (HR = 2.058; 95% CI = 1.199–3.533; p = 0.009), HVTT (HR = 2.029; 95% CI = 1.353–5.545; p = 0.005), and AFP > 400 ng/ml (HR = 2.029; 95% CI = 1.205–3.417; p = 0.008) were independent predictors of RFS (Table 2).
PA-PD-1 treatment (HR = 0.303; 95% CI = 0.101–0.908; p = 0.033), tumor size > 5 cm (HR = 3.453; 95% CI = 1.027–11.612; p = 0.045), and HVTT (HR = 2.709; 95% CI = 1.086–6.757; p = 0.033) were significantly related with OS in the univariate analysis. Multivariable analysis revealed that PA-PD-1 treatment (HR = 0.297; 95% CI = 0.095–0.922; p = 0.036) was a predictor of OS.
Safety
Of the 47 patients in the PA-PD-1 group, no patients discontinued the regimen owing to an adverse event. There were no treatment-related deaths in the PA-PD-1 group. Treatment-related adverse events of any grade occurred in 36 patients (76.60%) in the PA-PD-1 group. The incidence of grade 3 or 4 treatment-related adverse events was 21.27%. Detailed adverse events in the PA-PD-1 group are summarized in Table 3. Grade 3 or 4 treatment-related adverse events included increased ALT/AST (seven patients, 14.9%), hypoalbuminemia (two patients, 4.3%), anemia (one patient, 2.13%), and decreased neutrophils (one patient, 2.13%).
Subgroup analysis
Subgroup analyses stratified by different clinical variables were performed to further explore the efficacy of the PA-PD-1 for HCC patients after surgery.
The PA-PD-1 group consistently achieved longer RFS than the non-PA-PD-1 group in the subgroups of < 55 years (HR = 0.51, 95% CI = 0.27–0.96; Fig. 3a), tumor size ≥ 5 cm (HR = 0.41, 95% CI = 0.23–0.73), no HVTT (HR = 0.45, 95% CI = 0.26–0.79), PVTT presence (HR = 0.40, 95% CI = 0.18–0.88), no MVI (HR = 0.38, 95% CI = 0.15–0.93), no tumor satellites (HR = 0.55, 95% CI = 0.31–0.96), no liver cirrhosis (HR = 0.53, 95% CI = 0.28–0.98), and AFP < 400 ng/ml (HR = 0.41, 95% CI = 0.2–0.84).
Subgroup analysis of RFS (a) and OS (b) stratified by clinical parameters between the PA-PD-1 and non-PD-1 groups after PSM. RFS recurrence-free survival, OS overall survival, PA-PD-1 adjuvant anti-PD-1, PSM propensity-score matching, HVTT hepatic venous tumor thrombus, PVTT portal venous tumor thrombus, MVI microvascular invasion, AFP α-fetoprotein
In addition, patients may benefited from PA-PD-1 in OS if they had age ≥ 55 years (HR = 0.18, 95% CI = 0.04–0.78; Fig. 3b), tumor size ≥ 5 cm (HR = 0.21, 95% CI = 0.09–0.49), BCLC stage A and B (HR = 0.25, 95% CI = 0.07–0.93), no HVTT (HR = 0.25, 95% CI = 0.1–0.64), PVTT presence (HR = 0.15, 95% CI = 0.04–0.5), no tumor satellites (HR = 0.36, 95% CI = 0.14–0.94), no liver cirrhosis (HR = 0.36, 95% CI = 0.14–0.95), and AFP < 400 ng/ml (HR = 0.18, 95% CI = 0.05–0.67).
Discussion
Recently, PD-1 inhibitors have been developed as postoperative adjuvant treatment in some cancers due to their ability to boost patients’ immune systems; for instance, pembrolizumab has been approved for melanoma and renal cell carcinoma based on conclusions from the KEYNOTE-716 study (NCT03553836) and phase III KEYNOTE-564 study (NCT03142334), respectively [29, 30]. However, clinical evidence of PA-PD-1 therapy for HCC is still lacking, although many clinical trials exploring the efficacy of PA-PD-1 treatment are currently ongoing, such as durvalumab (NCT03847428), pembrolizumab (NCT03867084), atezolizumab (NCT04102098), and nivolumab (NCT03383458).
As we know, this is the first prospective observational study to explore the effect of PA-PD-1 therapy, demonstrating that HCC patients with high recurrence risks after hepatectomy had obviously improved OS and RFS in the PA-PD-1 group compared with the non-PD-1 group. With 1:1 PSM, the two groups were well balanced for potential clinical variables affecting tumor relapse, such as tumor size, number, MVI, BCLC stage, AFP, and HVTT. Therefore, the significant reduction in the risk of recurrence is attributable to PA-PD-1 therapy. Furthermore, univariable and multivariable analyses revealed that PA-PD-1 treatment was an independent predictor of both RFS and OS. This finding suggests that PA-PD-1 could be a promising therapy for HCC patients with high risk of relapse after hepatectomy. In accordance with the present results, a phase II prospective multicenter trial reported at the 2021 ASCO Annual Meeting showed that the 1-year RFS was 76.7% and the median RFS was 26 months (95% CI 23.9–28.1 months) for HCC patients who received nivolumab after liver resection, which greatly improved postoperative quality of life [31]. Patients in this multicenter study had a more favorable RFS than those in our study (76.5% vs 58.40%), partly because the HCC patients in our study had at least one of the recurrence risk factors, meaning they were more susceptible to tumor recurrence. In fact, subgroup analysis of the phase 2 clinical trial also demonstrated that patients with an immunosuppressive tumor microenvironment had worse RFS, which further indicates the importance of postoperative immunotherapy for HCC patients with high relapse factors after hepatectomy [31].
The tumor relapse rate within 5 years after hepatic resection for HCC is as high as 50–70%, which is related to the possible existence of small dissemination or multicentric occurrence before surgery [3, 32, 33]. The relapse of HCC is classified as early relapse (within two years) and late relapse (after two years) based on the time to recurrence after hepatectomy. Intrahepatic tumor metastasis is related to the aggressiveness of the primary cancer and is the primary reason for early recurrence [34]. Because of the short duration of treatment and follow-up, we only provide data on early recurrence and overall survival in the first 2 years. Our multivariable analyses showed that multiple tumor numbers (≥ 3), HVTT, and AFP > 400 ng/ml were other factors significantly related to RFS, indicating that these factors affect early recurrence. Previous studies reported that early tumor recurrence is more likely to occur with the characteristics of tumor pathology, such as poor cell differentiation, multiple tumors, large tumor size, MVI, and satellite lesions [35,36,37]. We are presently unable to arrive at conclusions about the predictive factors for late recurrence because of the short follow-up duration, but previous literature has reported on them. A multicenter retrospective study of 734 HCC patients after curative hepatectomy demonstrated that sex of male, cirrhosis, multiple tumors, satellite nodules, tumor size exceeding 5 cm, and MVI were independent predictors of late relapse [10]. Regardless of whether HCC patients with high risk factors of early or late relapse would benefit more from immunotherapy, our subgroup analysis suggested that PA-PD-1 significantly improved RFS and OS for patients with PVTT or tumor size ≥ 5 cm.
In this study, the resection criteria were referred to clinical guidelines from China but not Western countries [38]. Therefore, some patients (including patients with PVTT) outside resection criteria according to Western guidelines also underwent hepatectomy and were included in our study. Since HCC patients generally have more advanced stages in China and resection is one of the most commonly used treatment, surgery was performed in many selected advanced patients in real clinical scenario. Indeed, due to emerging evidence showing that some selected patients with advanced HCC could still benefit from surgery, guidelines from Asian countries including China now recommend surgery for selected advanced patients as first-line treatment for better prognosis [39, 40]. However, it should be noted that the different resection criteria in Western countries and China can affect the generalizability of our results.
In this study, PA-PD-1 was planned to be continued until disease progression. However, we found that the actual duration of PA-PD-1 was 4.17 months, while the median RFS was much longer. This was mainly because of the study design, since the discontinuation of PA-PD-1 before disease progression was allowed at patients’ will. Patients receiving at least one cycle of PA-PD-1 were included in the final analysis. Besides, we included patients receiving PA-PD-1 with or without other adjuvant therapies. Although we performed PSM using variables included adjuvant therapies other than PA-PD-1 to balance them between the two groups, these treatments could cause bias when interpreting the efficacy and toxicity of PA-PD-1. Synergic effects could exist between PA-PD-1 and other treatments such as anti-angiogenic agents, which might make the survival benefit of PA-PD-1 more significant. On the other hand, the incidence and severity of adverse events would also increase in patients receiving multiple treatments in the PA-PD-1 group. Therefore, the duration, efficacy, and toxicity of PA-PD-1 alone should be further studied.
There are several limitations. First, the observational, non-randomized study has its inherent flaws. For instance, the treatment (anti-PD-1 antibody with or without other therapies) and also the follow-up were flexible to some extent. Although we applied PSM including treatment variables, it was still difficult to completely avoid some biases. Among the four ongoing phase 3 clinical trials investigating the efficacy of PA-PD-1 treatment in HCC mentioned above, three (NCT03847428, NCT04102098, and NCT03383458) were also conducted in patients with high risks of recurrence as our study. Besides, two of them (NCT03847428 and NCT04102098) combined anti-PD-1 antibody and bevacizumab as adjuvant therapy. Therefore, the results of these trials would be very important to validate our findings. Moreover, the number of patients is insufficient (47 patients in each group after PSM) and the follow-up period was short. Third, the selection criteria of patients receiving hepatectomy were different between Western and Eastern countries, which can affect the generalizability of our results. Thus, research with more patients and longer follow-ups are needed to verify our results.
Conclusion
PA-PD-1 is a potentially new and effective intervention in improving survival outcomes for HCC patients with high relapse factors after hepatectomy. Further randomized controlled trials investigating its efficacy and safety are required to confirm the finding and to better understand the value of postoperative adjuvant PD-1 in patients with HCC.
Data availability
The data used in the study are available from the corresponding author upon reasonable request.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- CIs:
-
Confidence intervals
- HCC:
-
Hepatocellular carcinoma
- HRs:
-
Hazard ratios
- HVTT:
-
Hepatic venous tumor thrombus
- MVI:
-
Microvascular invasion
- RFS:
-
Recurrence-free survival
- TACE:
-
Transarterial chemoembolization
- OS:
-
Overall survival
- PA-PD-1:
-
Postoperative adjuvant anti-programed cell death 1 antibody
- PSM:
-
Propensity-score matching
- PVTT:
-
Portal venous tumor thrombus
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382
Lee SY, Konstantinidis IT, Eaton AA, Gönen M, Kingham TP, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford). 2014;16:943–953
Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955
Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205:27–36
Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476–482
Lee JG, Kang CM, Park JS, Kim KS, Yoon DS, et al. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J. 2006;47:105–112
Shinkawa H, Tanaka S, Takemura S, Amano R, Kimura K, et al. Nomograms predicting extra- and early intrahepatic recurrence after hepatic resection of hepatocellular carcinoma. Surgery. 2021;169:922–928
Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24
Xu XF, Xing H, Han J, Li ZL, Lau WY, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154:209–217
Amini N, Ejaz A, Spolverato G, Maithel SK, Kim Y, Pawlik TM. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg. 2014;18:2136–2148
Chen ZH, Zhang XP, Feng S, Feng JK, Chai ZT, et al. Liver resection versus intensity-modulated radiation therapy for treatment of hepatocellular carcinoma with hepatic vein tumor thrombus: a propensity score matching analysis. Hepatobiliary Surg Nutr. 2021;10:646–660
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–943
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, et al. 2018. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md.) 67:358–80
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. 2018. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182-236
Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842
Wang Z, Ren Z, Chen Y, Hu J, Yang G, et al. Adjuvant transarterial chemoembolization for hbv-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074–2081
Wei W, Jian PE, Li SH, Guo ZX, Zhang YF, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond Engl). 2018;38:61
Yin J, Li N, Han Y, Xue J, Deng Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655
Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36–41
Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91.e6
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952
Qin S, Ren Z, Meng Z, Chen Z, Chai X, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207
2018. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236
Luke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–1729
Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385:683–694
Kudo M, Ueshima K, Nakahira S, Nishida N, Ida H, et al. 2021. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. Journal of Clinical Oncology 39:4070-
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022
Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–551
Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: current status and prospects. Genes Dis. 2020;7:359–369
Shimoda M, Tago K, Shiraki T, Mori S, Kato M, et al. Risk factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg. 2016;40:2466–2471
Lee HY, Rhim H, Lee MW, Kim YS, Choi D, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23:190–197
Huang L, Li J, Yan J, Cao J, Liu C, et al. Early recurrence after curative resection in oligonodular hepatocellular carcinoma. Hepatogastroenterology. 2013;60:28–31
Zhou J, Sun H, Wang Z, Cong W, Wang J, et al. 2020. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver cancer 9:682–720
Cheng S, Chen M, Cai J, Sun J, Guo R, et al. 2020. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver cancer 9:28–40
Sun J, Guo R, Bi X, Wu M, Tang Z, et al. 2022. Guidelines for Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus in China (2021 Edition). Liver cancer 11:315–28
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82072029), the National high level talents special support plan—“Ten thousand plan”–Young top-notch talent support program (No. 2019).
Author information
Authors and Affiliations
Contributions
ZW Peng, SL Shen, and J Wu conceived and designed this study; material acquisition, data collection, analysis, and interpretation were carried out by W Chen, SF Hu, ZL Liu, YK Sun, and ZW Peng. The initial draft of the article was written by W Chen, SF Hu, ZL Liu, and YK Sun. And ZW Peng revised it. Final approval of the manuscript was obtained from all authors.
Corresponding authors
Ethics declarations
Conflict of interest
Wei Chen, Shuifang Hu, Zelong Liu, Yukun Sun, Jian Wu, Shunli Shen, and Zhenwei Peng declare that they do not have any conflict of interests.
Informed consent in studies with human subjects
All enrolled participants provided written informed consent for data collection and analysis.
Ethical approval
The study was conducted under the guidance of the Declaration of Helsinki (1964) and was approved by the institutional research ethics committee of the First Affiliated Hospital of Sun Yat-sen University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Hu, S., Liu, Z. et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int 17, 406–416 (2023). https://doi.org/10.1007/s12072-022-10478-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10478-6