Abstract
Liver extracellular matrix (ECM) composition, topography and biomechanical properties influence cell-matrix interactions. The ECM presents guiding cues for hepatocyte phenotype maintenance, differentiation and proliferation both in vitro and in vivo. Current understanding of such cell-guiding cues along with advancement of techniques for scaffold fabrication has led to evolution of matrices for liver tissue culture from simple porous scaffolds to more complex 3D matrices with microarchitecture similar to in vivo. Natural and synthetic polymeric biomaterials fabricated in different topographies and porous matrices have been used for hepatocyte culture. Heterotypic and homotypic cell interactions are necessary for developing an adult liver as well as an artificial liver. A high oxygen demand of hepatocytes as well as graded oxygen distribution in liver is another challenging attribute of the normal liver architecture that further adds to the complexity of engineered substrate design. A balanced interplay of cell-matrix interactions along with cell-cell interactions and adequate supply of oxygen and nutrient determines the success of an engineered substrate for liver cells. Techniques devised to incorporate these features of hepatic function and mimic liver architecture range from maintaining liver cells in mm-sized tailor-made scaffolds to a more bottoms up approach that starts from building the microscopic subunit of the whole tissue. In this review, we discuss briefly various biomaterials used for liver tissue engineering with respect to design parameters such as scaffold composition and chemistry, biomechanical properties, topography, cell-cell interactions and oxygenation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracellular matrix (ECM) is the interactive foundation in which cells adhere, proliferate, migrate, differentiate and interact with other cells [1, 2]. Similar to other organ systems, liver ECM facilitates these processes by a fine balance of temporal and spatial distribution of cells and chemical composition [2, 3]. Furthermore, physical features such as topology, pore structure and biomechanical properties also play a significant role in determining cell physiology [1, 4, 5]. Hepatocytes are polarized cells, and this polarity needs to be maintained for optimal cell performance. In vivo liver ECM is known to play an active role in maintaining cell function and polarity by presenting a gradient of solid and soluble factors [6].
Thus, a fundamental requirement for in vitro culture of hepatocytes is a suitable ECM or scaffold that can maintain the hepatocyte phenotype [3, 7]. Scientists from various disciplines have come together in an attempt to recreate the complex cellular architecture of the native liver ECM and extend liver functionality in vitro to obtain a highly functional artificial liver tissue [8]. Alternative therapies such as an engineered functional liver support can be helpful in aiding liver regeneration and extending patient waiting time for a liver transplant [9]. These alternative models can also be instrumental in expediting drug toxicity studies [10, 11] and enhance understanding of normal and diseased liver physiology [12, 13].
However, rebuilding the liver ECM and microenvironment has some critical challenges. Liver, being a highly metabolic organ, performs a myriad of functions including synthesis, detoxification, etc. [8]. This is achieved by placing the hepatocytes as well the other cells in a complex milieu, carefully adapted to fulfilling the functional need [14]. A brief overview of liver basic architecture and ECM distribution will help clearly define the challenges of engineering liver microarchitecture.
Basic liver architecture and extracellular matrix composition
The basic functional unit of liver is the acinus. The acinus is constituted of radially transversing strings or plates of parenchymal/hepatocytes and non-parenchymal cells tied between two central veins and centered on a portal triad. It is a miniature model of the typical microenvironment and zonation that exist in liver. The acinus is delineated into three zones: periportal, midlobular and centrilobular. The zonation occurs as a consequence of the cellular arrangement along the microvasculature and the direction of blood flow. Each of these zones specializes in different metabolic functions as dictated by oxygen tension, presence of the CYP450 enzymes, matrix chemistry, solute gradients and gene expression [14].
Hepatocytes are the specialized endothelial cells of liver that perform a majority of liver functions [15]. Hepatocytes in the vicinity of sinusoids extend numerous microvilli into the space of Disse and come into direct contact with blood, facilitating exchange of nutrients [8]. The non-parenchymal cell population is mainly constituted of liver sinusoidal endothelial cells (LSECs), Kupffer cells and hepatic stellate cells (HSCs) [14, 15]. LSECs line the sinusoids of liver, which carry blood from the portal vein to central venule and deliver oxygen to the surrounding parenchyma [16]. Sinusoids are separated from the hepatic parenchyma by a protein-rich interface called the space of Disse. Fenestrae, a characteristic feature of LSECs, act like hepatic sieves that provide steric regulation of molecular transport into the parenchyma [17, 18]. A diagrammatic representation of a liver sinusoid is illustrated in Fig. 1. Kupffer cells are specialized macrophages that reside in liver sinusoids. They have a high phagocytic and endocytic activity and secrete several cytokines (IL 1, 6, TNF α), all of which play a critical role in defense, immunity and liver regeneration [18]. HSCs are also called fat storage cells or ito cells. They store vitamin A, produce components of ECM and control sinusoidal contractility. In case of an injury, activated HSCs secrete cytokines and growth factors responsible for liver fibrosis and deposition of ECMs, which contribute to scar formation [19]. Other cell types in liver include cholangiocytes and hepatic progenitor cells [14].
Organization of a liver sinusoid depicting distribution and arrangement of various cell types. The blood flows from the portal triad toward the central vein, creating an oxygen gradient that leads to zonation in liver. The size of hepatocytes, sinusoidal lumen diameter and fenestrae changes from the periportal to perivenous zone
Natural ECM of liver consists of collagen (type I–IV), hyaluronans, laminin, fibronectin and elastin, all of which are distributed in a graded manner from zone 1–3 in an acinus [20]. Collagen type I forms the fundamental structure of the porous scaffold over which other matrix components attach. In the acinus, the periportal region is rich in fibrillar collagen (type I and III), laminins, vimentin, hyaluronan, and chondroitin sulfate proteoglycan (CS-PG) and heparin sulfate proteoglycan (HS-PG), with low levels of sulfation. This chemistry of ECM components transitions gradually in the space of Disse toward the pericentral region to a matrix enriched in collagen type IV and VI, syndecans 1 and 4 and highly sulfated proteoglycan specifically heparin PG. This gradient of matrix chemistry interplays synergistically with soluble factors that are mostly bound to glycosoaminoglycans (GAGs) to modulate cell behavior [6]. A biphasic response of soluble factors is mediated by the gradient distribution of sulfated proteoglycans, being mitogenic when bound to less sulfated PG while inducing growth arrest and differentiation when complexed with highly sulfated PG [2, 11].
Thus, a basic engineered liver construct needs an optimum distribution of ECM components in a gradient fashion so as to modulate hepatocyte functions and facilitate cell-cell interaction [21]. Heterotypic cell interactions have been rendered imperative to hepatocyte function in vivo and have shown favorable response in vitro as well [21]. Additionally, maintaining oxygen gradients and fluid flow for nutrient transport is mandatory to achieve the goal of functional liver tissue [22]. In this review, we provide an analysis of the factors that need to be considered when designing an artificial matrix for the liver as well as the biomaterials used in liver tissue engineering.
Designing an artificial liver biomatrix
The underlying importance of the cytoskeletal architecture has prompted many studies to reproduce hepatic plate-like architecture by varying substrate structure and chemistry [23], cell-cell interactions [24] and flow parameters [25, 26]. To re-establish cell polarity, hepatocytes have been cultured over different 2D and 3D matrix configurations with varying chemical and biological properties as well as with other non-parenchymal cells to promote heterotypic interactions [27]. Providing a 3D matrix with optimal adhesion ligands on both sides of cells allows for establishment of cellular polarity [5, 7]. Density of adhesion ligands [28] [29], the biomechanical [30], structural and surface properties [5] of the matrix are postulated to guide cell behavior under in vitro conditions similar to mechanisms found in vivo [31]. Thus, designing a matrix for growth of liver tissue in vitro involves several considerations:
-
(1)
Scaffold architecture: This involves multiple factors such as
-
Scaffold chemical composition—natural, synthetic and hybrid polymers;
-
Pore structure and porosity;
-
Biomechanical properties;
-
Scaffold chemistry—density and distribution of adhesion ligands
-
-
(2)
Heterotypic and homotypic cell interactions: coculture of hepatocytes with different liver cell types or non-liver cells.
-
(3)
Transport of nutrients and oxygenation: fluid flow and shear stress.
Biomaterial scaffolding
Different types of polymers have been used for making artificial matrices for liver reconstruction. These may be classified in broad categories of natural and synthetic polymers or a combination of both. These scaffolds have been molded into different topographies such as films, coatings, sponges, hydrogels, cryogels, nanofibers, etc. In addition to these, natural ECM matrices in the form of a decellularized biomatrix have been employed for culture of liver cells.
Natural polymers
Collagen is the most abundant component constituting the ECM in any tissue type and particularly the liver. This makes collagen the most commonly used biomaterial for hepatocyte culture. Monolayer culture of hepatocytes over collagen gels [32] and collagen sandwiches [7] are two of the most common methods of growing hepatocytes in vitro and utilizes collagen type I as the base scaffold. It has been used in other forms such as microspheres, coatings for scaffolds and 3D matrices. Collagen, being a native protein found in the body, has many cell-binding motifs, low antigenicity, and high biocompatibility and biodegradability. A disadvantage with collagen as with other natural polymers is its low mechanical strength and high cost. A collagen-hepatocyte construct made up of multiple hepatic units (2,000–4,000 μm/500–1,000 μm diameter/height) engrafted in the subcutaneous space was shown to have enhanced vascularization [33].
Chitosan has been used as a popular matrix for hepatocyte culture mainly because of its resemblance to glycosaminoglycan. Chitosan scaffolds fabricated as foams, composites, hydrogels [34], microcarriers [35], membranes [36], micro- and nanofibers [37, 38] have been used to maintain hepatocytes in vitro. Chitosan, being a hydrophilic charged polymer, promotes spheroid formation in hepatocytes. Hybrid scaffolds of chitosan with collagen or of alginate with galactosylated chitosan have been used successfully for hepatocyte culture and spheroid formation [38].
Alginate-based scaffolds have been used to cultivate or microencapsulate hepatocytes to generate implantable constructs. Being a hydrophilic polymer, like chitosan, it promotes spheroid formation and thus enhances cell-cell interactions and hepatocyte function. Porous alginate scaffolds having 90 % porosity and a pore size of 100 μm are favorable for hepatocyte culture. They promote spheroid formation due to low adherence of the cells to the substrate. Seeding of hepatocytes at a high density (5.7 × 106 cells/cm3), using centrifugal force, has been observed to maintain hepatocyte viability and function for a longer time as compared to static seeding at low cell density (0.28 × 106 cells/cm3). Compared to synthetic hydrophobic matrices, cell seeding in hydrophilic porous matrices like alginate is less time consuming and simple [39, 40]. Hepatocyte aggregates encapsulated in alginate hydrogels cultured in a bioreactor and spinner flask for a period of 1 month were superior to unencapsulated aggregates in terms of liver functions. These studies show that use of an appropriate biocompatible ECM for hepatocyte culture influences cellular phenotype and functionality. Alginate used in this study was clinical grade and provided a good, thermostable, semi-permeable ECM [41].
A combination of alginate with galactosylated chitosan (AL-GC) in porous sponges has been shown to promote hepatocyte culture better than alginate sponge alone because of the presence of specific ligands for asialoglycoprotein receptor (ASGPR) in hepatocytes. Hepatocytes cultured in AL-GC foams formed large 100-μm aggregates and expressed connexin-32 and E-cadherin as markers for cell–cell contact, which was not observed in case of alginate only foams. Furthermore, coculture of hepatocytes with NIH3T3 fibroblasts in AL-GC foams showed enhanced liver-specific functions in comparison to alginate only sponges [42]. Composite scaffolds of collagen/chitosan/heparin [43], cross-linked collagen/chitosan [44], etc., have also been tested for hepatocyte culture in vitro.
Hyaluronic acid is also a main component of the space of Disse in liver. In that effect use of hyaluronic acid containing scaffolds for liver cell culture is a logical option to explore. Non-woven fabrics of hyaluronic acid esters were first used by Catapano et al. [45] for the culture of liver cells [45]. As a further modification, non-woven fabrics of hyaluronic acid were enriched in components of ECM by culturing fibroblasts in them and then used for hepatocyte culture. These ECM-enriched matrices increased the survival time for hepatocytes from 7 to 14 days in vitro while upon implantation the hepatocytes formed small aggregates and survived up to day 35 [46].
Synthetic matrices
Synthetic matrices have also been utilized for hepatocyte culture in vitro, for making implantable scaffolds as well as drug delivery vehicles [47]. A major advantage of using synthetic polymers over natural polymers for scaffold fabrication is the easy control of their physiochemical and biological properties. Some of the popularly used synthetic polymers are poly L-lactic acid (PLLA), poly (lactide-co-glycolide) (PLGA), poly(ethyleneglycol) (PEG) [28] and stimuli-responsive polymers such as poly(N-isopropylacrylamide) [48].
PLLA and PLGA are biodegradable polyesters that have been used both for hepatocyte culture and hepatocyte implantation. The biodegradation rate of these polymers can be modulated based on the ratio of PLLA–PLGA, crystallinity of the polymer and molecular weight. A number of studies have been carried out using PLGA and PLLA scaffolds for hepatocyte culture. However, very limited success has been achieved using these polymers. Most of the studies were done more than a decade ago, and evidence of recent investigations is rare. Mostly composite PLGA–PLA scaffolds or scaffolds with some surface modifications have been used. PLGA foams have been shown to perform equivalent to a collagen sandwich culture of hepatocytes, while the foams coated with gelatin or collagen show a decrease in function [49, 50]. Hepatocyte culture in foams made of PLA, gelatin, polyurethane and calcium alginate have been shown to have a liver-specific function. Combination of PLGA scaffolds with hydrophilic polymers such as poly(vinylalcohol) (PVA) has been shown to improve hepatocyte seeding. A composite PLLA-PLGA scaffold coated with PVA supported long-term engraftment of hepatocytes after transplantation in the mesentery in a rodent injury model [51]. Recently, a PLLA nanofibrous scaffold coated with collagen type 1 was used for primary hepatocyte culture. The results demonstrated the effect of surface topography on hepatocyte function and adhesion, with the nanofibrous scaffold being superior to the non-porous PLLA collagen-coated scaffold [52].
PLLA–PLGA-based scaffolds have been utilized as a matrix in flow bioreactor configurations and have been shown to promote spheroid formation of human and rat hepatocytes. Such precultured rat hepatocytes, when transplanted on a PLLA scaffold, had less initial cell loss and regained 100 % cell mass in 6 months when implanted into pockets of mesenteric leaves in syngenic rats [53]. A liver organoid tissue was constructed in a bioreactor over a polylactic acid fabric by combining collagen fibrils, fibroblasts and HepG2 cells. The construct, on implantation into mice, showed repopulation with oval or spherical hepatocytes and engraftment with surrounding tissue. A condensed collagen fibril network was formed holding a dense network of hepatocytes in the presence of fibroblasts. Such constructs could be useful in studying cell-cell interactions and the role of various cytokines and growth factors [54].
Despite the obvious advantage of hepatocyte culture, PLGA scaffolds suffer from an inherent disadvantage of acidic environments within the scaffold, resulting from their degradation products that initiate peptide degradation, stimulate inflammation and result in poor tissue engraftment.
PEG is a widely used hydrophilic, biocompatible polymer used for tissue engineering applications. A two-dimensional micropatterned system composed of α-lactosyl PLA and PEG brushes (patterned over glass substrate 20 × 20 mm2) has been used for culturing heterospheroids of primary hepatocytes and bovine endothelial cells. The system maintained hepatocyte phenotype and liver-specific functions for a month. The heterospheroids had a diameter of 100 μm, and the polymer brushes were 100 μm apart. This PLA–PEG substrate can be modified by changing surface and chemical properties to modulate cell behavior [55]. Recently, the substrate has been used for culturing hepatic cells and making implantable constructs. Bipotential mouse embryonic liver cells (BMELs) encapsulated within a PEG hydrogel were able to differentiate into hepatic lineage, and their gene expressions could be modulated by siRNA. Coculture of hepatocytes within the PEG hydrogel improved the viability of the cells because of stabilization of cell-cell interactions. Photopolymerizable PEG hydrogels have been used for generating a network of hepatocytes and fibroblasts arranged in a defined network. The same system has been used for generating an array of BMELs. The system is advantageous as it allows reasonable control over cell orientation by a combination of photopatterning and dielectrophoretic patterning methods [56].
Poly(caprolactone) (PCL) [57] has been used for culturing hepatic cells. Being a hydrophobic polymer, cell seeding is difficult. Methods have been adopted to improve the seeding efficiency in such synthetic scaffolds. Seeding can be facilitated by using avidin-biotin binding, which has a very strong K d (K d = 10−15) as compared to integrin-fibronectin (10−6) and integrin-laminin (10−9). Such a system has been tested in both small 3D PLLA (0.1 cm3) scaffolds and large PCL constructs of 500 cm3 made using selective laser sintering (SLS). In PCL scaffolds with customized 3D branching and a joining flow-channel network, the technique of avidin-biotin binding when combined with centrifugal force greatly enhances cell attachment. However, the seeding density and level of liver specific functions in the larger PCL scaffolds were lower than those of the smaller PLLA scaffolds, which could be due to the limited oxygen supply.
Elastin-like polypeptides (ELPs) conjugated to positively charged polyelectrolytes have been shown to promote spheroid formation and enhanced hepatocyte function under in vitro conditions. Furthermore, alternating multilayers of ELP and polyelectrolytes have shown potential as substrates for hepatocyte culture [58].
Finally, temperature-responsive surfaces made using poly(NiPAAm) have been used to generate scaffold-free hepatic cell sheets. The hepatic cell sheets could be composed of only hepatocytes or contain a patterned coculture of hepatocyte and non-parenchymal cells [59, 60]. Implantation of 2D constructs in the subcutaneous space in mice has shown formation of 2D liver tissue, which is viable up to 200 days. A stratified 3D structure can be obtained by combining multiple such hepatocyte cell sheets in vivo [59].
Hybrid scaffolds
Hybrid scaffolds made up of thiolated heparin and PEG have been used for hepatocyte encapsulation and have been shown to maintain hepatocyte function for up to 20 days. Heparin is a natural component of the liver biomatrix and sequesters various growth ligands. Thus, heparin-based scaffolds are suitable for inclusion of growth factors in implantable scaffolds. These hybrid scaffolds were modified to include immobilized hepatocyte growth factor (HGF), which enhanced the hepatocyte function and survival within the hydrogel [61]. A summary of the various biomaterials used for hepatic tissue engineering is presented in Table 1.
Decellularized liver biomatrix
A further advancement in the field of scaffold fabrication for tissue engineering is use of decellularized matrix derived from native organs of cadaveric donors. A distinct advantage of decellularized matrix is its intact natural ECM composition, native vasculature/architecture and function allowing efficient recellularization [62], thus using a natural cellular platform to grow cells. Uygun et al. [63] first reported the creation of a transplantable liver graft by recellularizing a decellularized liver biomatrix with 200 million rat hepatocytes. The recellularized graft supported liver-like functions with an efficiency comparable to normal liver in vitro. The graft could be successfully transplanted in vivo in rats [63]. Furthermore, culture of human hepatocytes in porcine-derived decellularized matrix and coculture of hepatocytes and LSECs in decellularized matrices have been done successfully. Although this was by far one of most successful attempts to culture liver cells in vitro, transplanted grafts can only survive for a period of 2–8 h mainly because of clotting initiation due to exposed collagen. Thus, further improvisations are sought to adapt these matrices in clinical settings [64].
Scaffold chemistry
Role of adhesion ligands
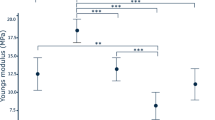
Although synthetic polymers offer several advantages, they lack cell-binding moieties. Apart from the exact ECM composition, an important parameter controlling the balance between the maintenance of the differentiation state and proliferation rate of hepatocytes is the density of ECM components. Low ECM component densities promote the differentiated state of hepatocytes along with spheroid formation, whereas high densities promote cell adherence and proliferation [31]. In order to optimize hepatocyte–matrix interactions, various ECM ligands have been incorporated in the polymer systems making up the scaffold. Coating of natural polymers over synthetic scaffolds, non-specific adsorption of growth factors, conjugation of sugar residues (lactose, glucose, heparin, galactose), cell adhesive sequences (integrin binding RGD), etc., are some of the examples of presenting appropriate ECM ligands to the hepatocytes. Of these, RGD and galactose conjugation have been most widely explored because of their potential to modulate hepatocellular adhesion. Adhesion of such ligands to synthetic substrates lacking cell adhesion ligands allows fine control over cell adhesion behavior and provides an opportunity to study these interactions. Sandwich culture of hepatocytes in a synthetic matrix composed of polyethyleneterpthalate modified with GRGDS for the top layer and galactosylated PET for the bottom layer has been shown to maintain hepatocyte function for 14 days and to be superior to conventional collagen sandwich culture [65]. Futhermore, a PET surface modified with both adhesion ligands RGD and galactose has been shown to promote formation of spheroids when the ratio of RGD:galactose is 1:1,000 [66], while at a ratio of 1:1 a monolayer is formed [29] (Fig. 2). Similar enhancement has also been shown in RGD-conjugated PLLA scaffolds [67].
Scanning electron micrograph (SEM) of cell morphology on different substrata after 5 days of culture; a collagen-coated PET; b RGD conjugated to PET; c galactose-conjugated PET; d RGD and galactose (1:500) conjugated to PET; e RGD and galctose (1:1,000) conjugated to PET. This illustrates the importance of the nature of adhesion ligands and their density distribution affecting cell behavior (reproduced with permission from [66])
Galactosylated matrices for liver tissue engineering
Asialoglycoprotein receptors (ASGPR) have been identified on the surface of hepatocytes [68]. Incorporation of galactose as a hepatocyte-attaching moiety to polyacrylamide gels was first tested as a technique to attach liver cells via receptor-mediated mechanism [69]. Lactose monohydrate, lactobionic acid, aminohexyl–D galactopyranoside, 1-amino-1-deoxy-β-D-galactose and 1-amino-1-deoxy-β-d-lactose have been used to introduce galactose ligands [70]. Dissacharide terminal β-galactose and monoscahrride terminal galactose moieties have higher affinity for hepatocytes than α-galactose of disaccharide [71]. Galactose ligand density and orientation have both been shown to influence hepatocyte attachment and function [70]. Since then, several synthetic polymers such as polystyrene [72], poly(ethylene oxide) [73], PLGA [74], PET [75], polyvinylidene difluoride (PVDF) [76] and polyamidoamine dendrimers [77] have been modified with galactose and have been shown to enhance hepatocyte survival, spheroid formation and function.
Mechanical stiffness
There is increasing evidence that matrix biomechanical properties govern cell behavior. These signals are transduced by matrices to the cells by means of the cell adhesion ligands and cytoskeleton. Such gradients of mechanical stiffness are also found in native liver ECM. The ECM in the vicinity of hepatocytes and endothelial cells is soft, while that in the vicinity of HSCS and cholangiocytes is more rigid [78]. As an exemplification of mechanically controlled cell response, hepatic stem cells differentiate into hepatocytes on soft surfaces while cholangiocytes are seen on rigid surfaces [11]. However, few studies have been conducted to study the effect of material stiffness on hepatocyte maintenance. The results of the studies are rather contradictory and vary depending upon the parameters and stiffness range examined. Hepatocytes cultured on polyelectrolyte multilayers (PEMs) had decreasing albumin production with increasing stiffness of the PEM [30]. However, another study exploring the effect of graded mechanical compliance of polyacrylamide gels on hepatocyte function found an increase in hepatocyte function with an increase in hydrogel stiffness [79]. In a recent study, hepatocytes cultured on thiolated heparin and PEG gel with mechanical stiffness between 10 and 110 kPa had five times higher levels of albumin on softer gels compared to a stiffer heparin gel [80]. A change in matrix rigidity is also seen under several pathological conditions. However, little is known about the effect of this change on cell performance. Many more insightful studies are required to understand the relationship of mechanical stiffness and hepatocyte response. These would also be helpful in developing models of diseased liver and understanding the pathophysiology of disease development and progression.
Scaffold porosity and pore structure
A crucial factor involved in designing an appropriate liver matrix is the pore architecture. One of the earlier studies elucidating the importance of pore size on hepatocyte culture involved the use of porous collagen sponges of subcellular (10 μm), cellular (18 μm) and supercellular (82 μm) pore sizes to study the impact of pore size on hepatocyte morphology and function. In this study, it was observed that hepatocytes cultured in subcellular and cellular ranges were more restricted to the surface and had limited degrees of cell-cell contact, whereas those cultured in the supercellular range had more spread out configurations and infiltrated into the pores [81]. This study was crucial in establishing the effect of pore size on the cellular spreading and cytoskeletal organization of cells. In another study, alginate scaffolds fabricated using the freeze-drying method was used to study the effect of pore structure on hepatocyte culture. Hepatocytes took up a round morphology in isotropic, spherical pores, but lined the pores in scaffolds where the pores were elongated in shape [82].
In this respect, our group has been extensively studying the use of cryogels as potential matrices for liver tissue engineering. Cryogels are polymeric matrices synthesized at sub-zero temperatures from the monomeric or polymeric precursors that belong to any gel-forming system. Typically, cryogels have been classified as supermacroporous gels, primarily because of their unique feature of an interconnected porous network, with a broad range of porosity ranging anywhere between 1.0 and 100 μm. It is this unique feature of cryogels that makes them a promising biomaterial in the area of tissue engineering. Our group has explored the use of cryogels for various different types of tissues such as bone [83], cartilage [84] and neural tissue [85] with many promising results. Currently, we are studying the use of the cryogel matrices poly(N-isopropylacrylamide)-chitosan, poly(acrylonitrile)-chitosan and poly(acrylamide)-chitosan for use in liver tissue engineering (Fig. 3). Pore interconnectivity is a unique and attractive feature of cryogel matrices, increasing the overall surface area-to-volume ratio of the gel and allowing unhindered convectional transport of solutes, contrary to the diffusion of solutes in conventional hydrogel systems.
Coculturing of different liver cell types on scaffolds
Introduced by Langenbach et al. [86] in 1979, where primary hepatocytes were cultured on top of a feeder layer of fibroblasts, and further elucidated by Guguen-Guillouzo et al. [87] wherein hepatocytes were mixed with epithelial cells in culture, the role of cell-cell interactions in the hepatocyte phenotype has been actively pursued. Coculture of hepatocytes with different nonparenchymal cells (SECs, Kupffer cells, stellate cells) and nonhepatic cell types (epithelial cells and fibroblasts) has been shown to have a positive effect on maintenance of cell life in vitro [24]. Coculture of hepatocytes with fibroblasts has shown beneficial effects in 3D scaffolds [42]. A recent study by Yamada et al. [88] extended the use of coculture configuration of hepatocytes with Swiss 3T3 cells to a three-dimensional platform. Swiss 3T3 cells and hepatocytes suspended in a sodium-alginate solution were introduced into a micro-fluidic channel to synthesize hydrogel fibers that contained hepatocytes at the center sandwiched between layers of 3T3 cells. The fibers closely mimicked hepatic cords found in liver lobules in vivo and maintained expression of hepatocyte-specific genes over a 90-day period.
Inspite of the success in maintaining the hepatocyte phenotype by coculture with fibroblasts and nonparenchymal cells, these systems are unable to recapitulate all the features of hepatocyte function. Moreover, there are reports indicating that coculturing does lead to the loss of certain key hepatic functions such as certain cytochrome P450 isoforms [11]; hence, there is a need to look into other hemodynamic, microenvironmental factors to maintain a near in vivo-like condition for maintenance of hepatocyte culture.
In a recent report, coculture of hepatic endoderm cells (derived from human iPS cells) and human umbilical vein endothelial cells and human mesenchymal stem cells in a 2D configuration led to tissue self-organization into 3D liver buds in 72 h. The gene expression profiles in the in vitro liver buds were similar to liver buds derived from human fetal liver cells. Implantation of the in vitro-generated liver buds led to formation of highly vascularized and functional liver tissue [89]. Such potential approaches can overcome some of the limitations of classical coculture strategies. Moreover, it demonstrates the potential of iPS cells as a cell source for generating functional tissue.
Oxygenation in scaffolds
Hepatocytes are high-oxygen-demanding cells, and various techniques have been designed to ensure a homogeneous and constant supply of oxygen to hepatocyte biomatrices in vitro. For this purpose, several perfusion bioreactors have been designed that augment the oxygen supply, increase cell survival, promote cell-cell interactions and mimic the in vivo hepatic environment. Dissolved oxygen in medium is insufficient to fulfill the requirements of hepatocyte culture (in vivo oxygen supply = 2,000 nmol/ml; in vitro dissolved oxygen <200 nmol/ml) [22]. Culture of hepatocytes under high oxygen tension is shown to be beneficial in numerous studies [16, 90]. To meet the high oxygen demand of hepatocytes, various strategies have been devised, such as use of hemoglobin-based oxygen carriers (bovine RBCs) [91], incorporation of synthetic oxygen carriers such as perfluorocarbons (PF) [92], use of the oxygen-permeable PDMS membranes [93] or use of fluoropolymer membranes [94] in microfluidic devices. An important parameter controlling the oxygen supply is fluid flow, which also mediates shear stress. Thus, an optimum balance between oxygen supply and shear stress is necessary, which is controlled by the fluid flow rate. It has been demonstrated that shear stress above 5 dyn/cm2 greatly affects some of the liver-related functions, while others are not affected as much until shear stress reaches around 10 dyn/cm2 [95]. Many strategies have been employed to reduce shear stress while maintaining the oxygen tension. These include incorporation of microgrooves in the flat plate bioreactor [96], use of circular inlets/outlets to ensure homogeneous distribution of stress [97], use of separate oxygen supply chambers so that lower flow rates can be used for medium supply [98] and use of a 3D porous framework that allows for spontaneous reorganization into multicellular layers that have higher resistance to shear stress than large spheroids [99].
Conclusion
The field of tissue engineering has progressed significantly recently with concomitant advancement of the techniques and understanding of cellular phenomena. This has also benefited the field of liver tissue development, though the ultimate goal has not been achieved. Hepatocyte culture longevity has been extended up to months with minimal proliferation in vitro. Methods to cultivate cells have evolved from simple suspension or monolayer culture to more complex micropatterned and microfluidic culture.
Although the culture life span has increased, many metabolic functions are not maintained as in in vivo conditions. Proliferation of hepatocytes in vitro is still up to a very minimal level, and building up the required cell mass remains an unmet challenge. Progress has been made in understanding the role of oxygen in hepatic cell cultures. However, finding an optimum solution for presenting an oxygen gradient is in progress. Though a lot of progressive work has been carried out in the area of scaffold design for liver tissue engineering, there is still scope for more designs to be explored that can mimic the existing native liver ECM as closely as possible. With continuous progress in understanding cell behavior and contributions from diverse fields, that there will be further progress in the field is unequivocal.
References
Owen SC, Shoichet MS. Design of three-dimensional biomimetic scaffolds. J Biomed Mater Res A 2010;94(4):1321–1331
Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J 1995;9(14):1401–1410
Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006;7(3):211–224
Griffith LG. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann N Y Acad Sci 2002;961:83–95
Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J 1996;10(13):1471–1484
Reid LM, Fiorino AS, Sigal SH, Brill S, Holst PA. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology 1992;15(6):1198–1203
Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J 1989;3(2):174–177
Nahmias Y, Berthiaume F, Yarmush ML. Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol 2007;103:309–329
Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science 2002;295(5557):1005–1009
Soldatow VY, Lecluyse EL, Griffith LG, Rusyn I. Models for liver toxicity testing. Toxicol Res (Camb) 2013;2(1):23–39
LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol 2012;42(6):501–548
Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, et al. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J 2009;23(7):2155–2164
Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology 2006;43(2):257–265
Grisham JW. In Arias I, Wolkoff AW, Boyer J, Shafritz DA, Fausto N, Alter H, et al., editors. Organizational Principles of The Liver. 5th ed. Singapore: Wiley; 2009. 3–10
Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. Semin Immunopathol 2009;31(3):399–409
Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, and gene expression: achieving in vivo levels of drug clearance and HCV infection in vitro. J Hepatol 2010;52:S374
Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 2002;1(1):1
Smedsrod B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, et al. Cell biology of liver endothelial and Kupffer cells. Gut 1994;35(11):1509–1516
Huebert RC, Shah VH. Hepatic endothelial cells. In Dufour J-F, C P-A, editors. Signaling Pathways in Liver Diseases. Berlin: Springer–Verlag; 2010. 79–91
Martinez-Hernandez A, Delgado FM, Amenta PS. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest 1991;64(2):157–166
Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, et al. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology 1999;29(1):90–100
Stevens KM. Oxygen requirements for liver cells in vitro. Nature 1965;206(980):199
Ananthanarayanan A, Narmada BC, Mo X, McMillian M, Yu H. Purpose-driven biomaterials research in liver-tissue engineering. Trends Biotechnol 2011;29(3):110–118
Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 1999;13(14):1883–1900
Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng 2002;78(3):257–269
Allen JW, Khetani SR, Bhatia SN. In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol Sci 2005;84(1):110–119
Moghe PV, Coger RN, Toner M, Yarmush ML. Cell-cell interactions are essential for maintenance of hepatocyte function in collagen gel but not on matrigel. Biotechnol Bioeng 1997;56(6):706–711
Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci USA 2011;108(29):11842–11847
Du Y, Chia SM, Han R, Chang S, Tang H, Yu H. 3D hepatocyte monolayer on hybrid RGD/galactose substratum. Biomaterials 2006;27(33):5669–5680
Chen AA, Khetani SR, Lee S, Bhatia SN, Van Vliet KJ. Modulation of hepatocyte phenotype in vitro via chemomechanical tuning of polyelectrolyte multilayers. Biomaterials 2009;30(6):1113–1120
Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol 1992;151(3):497–505
Michalopoulos G, Cianciulli HD, Novotny AR, Kligerman AD, Strom SC, Jirtle RL. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res 1982;42(11):4673–4682
Zhao Y, Xu Y, Zhang B, Wu X, Xu F, Liang W, et al. In vivo generation of thick, vascularized hepatic tissue from collagen hydrogel-based hepatic units. Tissue Eng C 2010;16(4):653–659
Li JL, Pan JL, Zhang LG, Guo XJ, Yu YT. Culture of primary rat hepatocytes within porous chitosan scaffolds. J Biomed Mater Res A 2003;67A(3):938–943
Li KG, Wang Y, Miao ZC, Xu DY, Tang YF, Feng MF. Chitosan/gelatin composite microcarrier for hepatocyte culture. Biotechnol Lett 2004;26(11):879–883
Elcin YM, Dixit V, Gitnick G. Hepatocyte attachment on biodegradable modified chitosan membranes: in vitro evaluation for the development of liver organoids. Artif Organs 1998;22(10):837–846
Lee KH, Shin SJ, Kim CB, Kim JK, Cho YW, Chung BG, et al. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010;10(10):1328–1334
Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009;30(14):2753–2763
Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng 2003;9(4):757–766
Glicklis R, Shapiro L, Agbaria R, Merchuk JC, Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol Bioeng 2000;67(3):344–353
Miranda JP, Rodrigues A, Tostoes RM, Leite S, Zimmerman H, Carrondo MJ, et al. Extending hepatocyte functionality for drug-testing applications using high-viscosity alginate-encapsulated three-dimensional cultures in bioreactors. Tissue Eng C 2010;16(6):1223–1232
Seo SJ, Kim IY, Choi YJ, Akaike T, Cho CS. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold. Biomaterials 2006;27(8):1487–1495
Wang X, Yan Y, Lin F, Xiong Z, Wu R, Zhang R, et al. Preparation and characterization of a collagen/chitosan/heparin matrix for an implantable bioartificial liver. J Biomater Sci Polym Ed 2005;16(9):1063–1080
Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, et al. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 2003;24(19):3213–3220
Catapano G, De Bartolo L, Vico V, Ambrosio L. Morphology and metabolism of hepatocytes cultured in Petri dishes on films and in non-woven fabrics of hyaluronic acid esters. Biomaterials 2001;22(7):659–665
Zavan B, Brun P, Vindigni V, Amadori A, Habeler W, Pontisso P, et al. Extracellular matrix-enriched polymeric scaffolds as a substrate for hepatocyte cultures: in vitro and in vivo studies. Biomaterials 2005;26(34):7038–7045
Li YS, Harn HJ, Hsieh DK, Wen TC, Subeq YM, Sun LY, et al. Cells and materials for liver tissue engineering. Cell Transpl 2013;22(4):685–700
Kumashiro Y, Fukumori K, Takahashi H, Nakayama M, Akiyama Y, Yamato M, et al. Modulation of cell adhesion and detachment on thermo-responsive polymeric surfaces through the observation of surface dynamics. Colloids Surf B 2013;106:198–207
Huang H, Hanada S, Kojima N, Sakai Y. Enhanced functional maturation of fetal porcine hepatocytes in three-dimensional poly-L-lactic acid scaffolds: a culture condition suitable for engineered liver tissues in large-scale animal studies. Cell Transpl 2006;15(8–9):799–809
Jiang J, Kojima N, Guo L, Naruse K, Makuuchi M, Miyajima A, et al. Efficacy of engineered liver tissue based on poly-L-lactic acid scaffolds and fetal mouse liver cells cultured with oncostatin M, nicotinamide, and dimethyl sulfoxide. Tissue Eng 2004;10(9–10):1577–1586
Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, et al. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res 1997;37(3):413–420
Ting Wang Z-QF, Leach MK, Wud JH, Jianga Q. Nanoporous fibers of type-I collagen coated poly(l-lactic acid) for enhancing primary hepatocyte growth and function. J Mater Chem B 2013;1:339–347
Torok E, Lutgehetmann M, Bierwolf J, Melbeck S, Dullmann J, Nashan B, et al. Primary human hepatocytes on biodegradable poly(l-lactic acid) matrices: a promising model for improving transplantation efficiency with tissue engineering. Liver Transpl 2011;17(2):104–114
Tamai M, Adachi E, Tagawa YI. Characterization of a liver organoid tissue composed of hepatocytes and fibroblasts in dense collagen fibrils. Tissue Eng A 2013;19(21–22):2527–2535
Otsuka H, Hirano A, Nagasaki Y, Okano T, Horiike Y, Kataoka K. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. ChemBioChem 2004;5(6):850–855
Underhill GH, Chen AA, Albrecht DR, Bhatia SN. Assessment of hepatocellular function within PEG hydrogels. Biomaterials 2007;28(2):256–270
Huang H, Oizumi S, Kojima N, Niino T, Sakai Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials 2007;28(26):3815–3823
Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv Drug Deliv Rev 2010;62(15):1479–1485
Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med 2007;13(7):880–885
Tsuda Y, Kikuchi A, Yamato M, Nakao A, Sakurai Y, Umezu M, et al. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials 2005;26(14):1885–1893
Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010;31(13):3596–3603
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011;53(2):604–617.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010;16(7):814–820
Uygun BE, Yarmush ML. Engineered liver for transplantation. Curr Opin Biotechnol 2013;24(5):893–899
Du Y, Han R, Wen F, San Ng, San S, Xia L, Wohland T, et al. Synthetic sandwich culture of 3D hepatocyte monolayer. Biomaterials 2008;29(3):290–301
Xia L, Sakban RB, Qu Y, Hong X, Zhang W, Nugraha B, et al. Tethered spheroids as an in vitro hepatocyte model for drug safety screening. Biomaterials 2012;33(7):2165–2176
Carlisle ES, Mariappan MR, Nelson KD, Thomes BE, Timmons RB, Constantinescu A, et al. Enhancing hepatocyte adhesion by pulsed plasma deposition and polyethylene glycol coupling. Tissue Eng 2000;6(1):45–52
Pricer WE Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem 1971;246(15):4825–4833
Weigel PH, Schmell E, Lee YC, Roseman S. Specific adhesion of rat hepatocytes to beta-galactosides linked to polyacrylamide gels. J Biol Chem 1978;253(2):330–333
Cho CS, Seo SJ, Park IK, Kim SH, Kim TH, Hoshiba T, et al. Galactose-carrying polymers as extracellular matrices for liver tissue engineering. Biomaterials 2006;27(4):576–585
Kim SH, Hoshiba T, Akaike T. Effect of carbohydrates attached to polystyrene on hepatocyte morphology on sugar-derivatized polystyrene matrices. J Biomed Mater Res A 2003;67(4):1351–1359
Kobayashi A, Goto M, Kobayashi K, Akaike T. Receptor-mediated regulation of differentiation and proliferation of hepatocytes by synthetic polymer model of asialoglycoprotein. J Biomater Sci Polym Ed 1994;6(4):325–342
Lopina ST, Wu G, Merrill EW, Griffith-Cima L. Hepatocyte culture on carbohydrate-modified star polyethylene oxide hydrogels. Biomaterials 1996;17(6):559–569
Donati I, Gamini A, Vetere A, Campa C, Paoletti S. Synthesis, characterization, and preliminary biological study of glycoconjugates of poly(styrene-co-maleic acid). Biomacromolecules 2002;3(4):805–812
Yoon JJ, Nam YS, Kim JH, Park TG. Surface immobilization of galactose onto aliphatic biodegradable polymers for hepatocyte culture. Biotechnol Bioeng 2002;78(1):1–10
Yin C, Ying L, Zhang PC, Zhuo RX, Kang ET, Leong KW, et al. High density of immobilized galactose ligand enhances hepatocyte attachment and function. J Biomed Mater Res A 2003;67(4):1093–1104
Higashiyama S, Noda M, Kawase M, Yagi K. Mixed-ligand modification of polyamidoamine dendrimers to develop an effective scaffold for maintenance of hepatocyte spheroids. J Biomed Mater Res A 2003;64(3):475–482
Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53(3):1035–1045
Semler EJ, Lancin PA, Dasgupta A, Moghe PV. Engineering hepatocellular morphogenesis and function via ligand-presenting hydrogels with graded mechanical compliance. Biotechnol Bioeng 2005;89(3):296–307
You J, Park SA, Shin DS, Patel D, Raghunathan V, Kim M, et al. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng Part A 2013;19(23–24):2655–2663
Ranucci CS, Kumar A, Batra SP, Moghe PV. Control of hepatocyte function on collagen foams: sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials 2000;21(8):783–793
Zmora S, Glicklis R, Cohen S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002;23(20):4087–4094
Mishra R, Kumar A. Inorganic/organic biocomposite cryogels for regeneration of bony tissues. J Biomater Sci Polym Ed 2011;22(16):2107–2126
Bhat S, Tripathi A, Kumar A. Supermacroprous chitosan-agarose-gelatin cryogels: in vitro characterization and in vivo assessment for cartilage tissue engineering. J R Soc Interface 2011;8(57):540–554
Vishnoi T, Kumar A. Conducting cryogel scaffold as a potential biomaterial for cell stimulation and proliferation. J Mater Sci 2013;24(2):447–459
Langenbach R, Malick L, Tompa A, Kuszynski C, Freed H, Huberman E. Maintenance of adult rat hepatocytes on C3H/10T1/2 cells. Cancer Res 1979;39(9):3509–3514
Guguen-Guillouzo C, Clement B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, et al. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res 1983;143(1):47–54
Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, et al. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 2012;33(33):8304–8315
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499(7459):481–484
Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA 2009;106(37):15714–15719
Simoni J. Artificial oxygen carriers: scientific and biotechnological points of view. Artif Organs 2009;33(2):92–96
Sullivan JP, Gordon JE, Palmer AF. Simulation of oxygen carrier mediated oxygen transport to C3A hepatoma cells housed within a hollow fiber bioreactor. Biotechnol Bioeng 2006;93(2):306–317
Matsui H, Osada T, Moroshita Y, Sekijima M, Fujii T, Takeuchi S, et al. Rapid and enhanced repolarization in sandwich-cultured hepatocytes on an oxygen-permeable membrane. Biochem Eng J 2010;52(2–3):255–262
Evenou F, Hamon M, Fujii T, Takeuchi S, Sakai Y. Gas-permeable membranes and co-culture with fibroblasts enable high-density hepatocyte culture as multilayered liver tissues. Biotechnol Prog 2011;27(4):1146–1153
Tilles AW, Berthiaume F, Yarmush ML, Tompkins RG, Toner M. Bioengineering of liver assist devices. J Hepatobiliary Pancreat Surg. 2002;9(6):686–696
Park J, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnol Bioeng 2005;90(5):632–644
Lawrence BJ, Devarapalli M, Madihally SV. Flow dynamics in bioreactors containing tissue engineering scaffolds. Biotechnol Bioeng 2009;102(3):935–947
Leclerc E, Sakai Y, Fujii T. Microfluidic PDMS (polydimethylsiloxane) bioreactor for large-scale culture of hepatocytes. Biotechnol Prog 2004;20(3):750–755
Hoque ME, Mao HQ, Ramakrishna S. Hybrid braided 3-D scaffold for bioartificial liver assist devices. J Biomater Sci Polym Ed 2007;18(1):45–58
Lu JN, Wang CC, Young TH. The behaviors of long-term cryopreserved human hepatocytes on different biomaterials. Artif Organs 2011;35(3):E65–E72
Kim SS, Utsunomiya H, Koski JA, Wu BM, Cima MJ, Sohn J, et al. Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann Surg 1998;228(1):8–13
Li J, Tao R, Wu W, Cao H, Xin J, Guo J, et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev 2010;19(9):1427–1436
Tan GD, Toh GW, Birgersson E, Robens J, van Noort D, Leo HL. A thin-walled polydimethylsiloxane bioreactor for high-density hepatocyte sandwich culture. Biotechnol Bioeng 2013;110(6):1663–1673
Provin C, Takano K, Yoshida T, Sakai Y, Fujii T, Shirakashi R. Low O2 metabolism of HepG2 cells cultured at high density in a 3D microstructured scaffold. Biomed Microdevices 2009;11(2):485–494
Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol 2006;24(11):1412–1419
Kazemnejad S, Allameh A, Soleimani M, Gharehbaghian A, Mohammadi Y, Amirizadeh N, et al. Biochemical and molecular characterization of hepatocyte-like cells derived from human bone marrow mesenchymal stem cells on a novel three-dimensional biocompatible nanofibrous scaffold. J Gastroenterol Hepatol 2009;24(2):278–287
Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J 2007;21(3):790–801
Scott EA, Nichols MD, Kuntz-Willits R, Elbert DL. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater 2010;6(1):29–38
Lee J, Cuddihy MJ, Cater GM, Kotov NA. Engineering liver tissue spheroids with inverted colloidal crystal scaffolds. Biomaterials 2009;30(27):4687–4694
Bruns H, Kneser U, Holzhuter S, Roth B, Kluth J, Kaufmann PM, et al. Injectable liver: a novel approach using fibrin gel as a matrix for culture and intrahepatic transplantation of hepatocytes. Tissue Eng 2005;11(11–12):1718–1726
Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med 1977;145(1):204–220
Schuetz EG, Li D, Omiecinski CJ, Muller-Eberhard U, Kleinman HK, Elswick B, et al. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol 1988;134(3):309–323
Bierwolf J, Lutgehetmann M, Feng K, Erbes J, Deichmann S, Toronyi E, et al. Primary rat hepatocyte culture on 3D nanofibrous polymer scaffolds for toxicology and pharmaceutical research. Biotechnol Bioeng 2011;108(1):141–150
Sosnik A, Sefton MV. Semi-synthetic collagen/poloxamine matrices for tissue engineering. Biomaterials 2005;26(35):7425–7435
Miranda JP, Leite SB, Muller-Vieira U, Rodrigues A, Carrondo MJ, Alves PM. Towards an extended functional hepatocyte in vitro culture. Tissue Eng C 2009;15(2):157–167
Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, et al. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng A 2010;16(6):2007–2016
Turner WS, Schmelzer E, McClelland R, Wauthier E, Chen W, Reid LM. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J Biomed Mater Res B 2007;82(1):156–168
Acknowledgements
The authors would like to acknowledge the Department of Biotechnology (DBT), Government of India, for financially supporting the project in this area. The authors would also like to acknowledge all those whose works are cited here.
Compliance with ethical requirements and Conflict of interest
The authors declare that the studies reviewed in this article have been in compliance with ethical requirements. Era Jain, Apeksha Damania and Ashok Kumar declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, E., Damania, A. & Kumar, A. Biomaterials for liver tissue engineering. Hepatol Int 8, 185–197 (2014). https://doi.org/10.1007/s12072-013-9503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-013-9503-7