Abstract
Purpose
Patients with hepatitis B e antigen (HBeAg)-positive chronic hepatitis B, who achieve HBeAg seroconversion 6 months after completing 48 weeks of peginterferon alfa-2a therapy, have an increased chance of clearing hepatitis B surface antigen (HBsAg) during long-term treatment-free follow-up. This analysis aimed to determine whether HBsAg quantification during treatment could be used to identify posttreatment response.
Methods
Patients (n = 399) treated with peginterferon alfa-2a (180 μg/week) alone or in combination with lamivudine (100 mg/day) for 48 weeks during a large, randomized study were included in this retrospective analysis. Receiver-operating characteristic analyses were used to identify baseline and on-treatment HBsAg levels associated with response (HBeAg seroconversion 6 months posttreatment).
Results
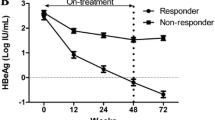
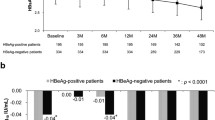
Baseline HBsAg levels were lower in patients achieving posttreatment response than in nonresponders (3.97 and 4.21 IU/mL, respectively, p = 0.039). Two baseline HBsAg cutoff levels (5,000 and 50,000 IU/mL) provided a positive predictive value of 42% and a negative predictive value of 77%. HBsAg decline was significantly greater during and posttreatment in responders than in nonresponders (p < 0.0001). HBeAg seroconversion rates 6 months posttreatment were significantly higher in patients with HBsAg < 1,500 IU/mL at weeks 12 and 24 (56.7 and 54.4%, respectively) versus patients with HBsAg 1,500–20,000 IU/mL (32.3 and 26.1%, respectively) or HBsAg < 20,000 IU/mL (16.3 and 15.4%, respectively) (all p < 0.0001 and <0.0001).
Conclusions
HBsAg levels at baseline strongly associated with posttreatment response were not identified. Low HBsAg levels during peginterferon alfa-2a therapy were associated with high rates of posttreatment response. On-treatment HBsAg quantification may, therefore, help guide patient management in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic hepatitis B (CHB) affects 350–400 million people worldwide, which makes it one of the most common chronic liver diseases [1]. It leads to high rates of morbidity and mortality as a result of the development of cirrhosis and hepatocellular carcinoma (HCC). As CHB treatment options that result in clearance of hepatitis B surface antigen (HBsAg) have been shown to improve liver histology, reduce rates of cirrhosis and HCC and increase patient survival [2, 3], HBsAg clearance has become an important critical end point in the management of CHB. However, HBsAg clearance is not the most appropriate short-term efficacy end point as rates are generally low in the immediate posttreatment period. Recent international guidelines highlight that durable hepatitis B e antigen (HBeAg) seroconversion is a satisfactory end point in HBeAg-positive patients, because it is associated with improved prognosis [4–6]. The recognized sustained posttreatment response to finite course of peginterferon alfa has made it one of the recommended first-line treatment options for HBeAg-positive CHB in all international guidelines [4–8].
Approximately, one-third of HBeAg-positive CHB patients achieve seroconversion 6 months after completing pegylated interferon therapy [7, 8]. Importantly, patients who achieve a sustained posttreatment response to interferon-based therapy have an increased chance of clearing HBsAg during long-term follow-up [3, 9]. Early identification of responders either before or during treatment would be of considerable benefit to clinicians, as it would allow therapy to be initiated only in patients likely to achieve a response, and to be modified in those patients unlikely to respond to the standard duration (48 weeks) of pegylated interferon monotherapy. Clinical experience with pegylated interferon has shown that adverse events are associated with therapy; however, these occur less frequently in hepatitis B than in hepatitis C and do not result in reduced treatment compliance [1, 10]. Early identification of nonresponders would prevent patients who are unlikely to gain clinical benefit from having to deal with potential side effects from the entire course of treatment.
A number of studies have found that HBeAg or hepatitis B virus (HBV) DNA levels either before or during treatment with pegylated interferon may be associated with posttreatment response [11–13]. In addition, there is increasing interest in the association between HBsAg levels before or during therapy and posttreatment response. In HBeAg-negative patients, a significant association between end of treatment HBsAg levels and response ≤3 years posttreatment has been demonstrated [14], while in HBeAg-positive patients, there was a trend toward lower baseline HBsAg levels in those achieving a sustained response to peginterferon alfa-2b 5 years posttreatment than in patients not achieving a virologic response (4,402 and 9,127 IU/mL, respectively; p = 0.064) [15].
The current analysis investigated the potential value of HBsAg quantification in identifying HBeAg-positive patients likely to achieve a sustained response following treatment with peginterferon alfa-2a.
Methods
Study design
This was a retrospective analysis of data from a large, randomized study in HBeAg-positive patients treated with peginterferon alfa-2a (180 μg/week) alone or in combination with lamivudine (100 mg/day) for 48 weeks [7]. The primary efficacy parameter was HBeAg seroconversion 6 months posttreatment. Other parameters assessed included HBV DNA ≤2,000 IU/mL (~10,000 copies/mL) and HBsAg clearance 6 months posttreatment.
HBsAg and HBV DNA levels were analyzed retrospectively from stored samples collected at baseline, during therapy at weeks 12, 24 and 48 (end of treatment), and 6 months posttreatment (week 72). HBsAg levels were quantified using the Abbott Architect HBsAg assay (Abbott Laboratories, IL, USA; dynamic range 0.05–250.0 IU/mL) after 1:100 dilution. Samples with HBsAg >250 IU/mL at this dilution were retested at a final dilution of 1:1,000. Samples with HBsAg levels <0.05 IU/mL at 1:100 dilution were retested at the same dilution [16]. HBV DNA levels were quantified using the Amplicor HBV test (Roche Molecular Diagnostics, Pleasanton, CA, USA; range 71–35,714 IU/mL); samples with HBV DNA >35,714 IU/mL were retested after a 1:100 dilution, according to the manufacturer’s recommendations. Only those patients with HBsAg values available at baseline and at all other time points (i.e., weeks 12, 24, 48, and 72) were included in the analysis.
Statistical analysis
Receiver-operating characteristic (ROC) analyses at baseline, week 12, and week 24 were used to identify an absolute HBsAg level associated with high rates of response posttreatment. The target was to identify a cutoff value that would provide a negative predictive value (NPV) ≥95%. If a single cutoff value did not achieve this level of NPV, a second, higher cutoff level could also be investigated to increase NPV. Logistic regression was used to analyze the chance of a response. Missing samples were treated as nonresponse. All statistical tests were considered exploratory and no adjustment for multiple testing was performed. Cochran Armitage Trend and Wald chi-square (WCχ²) tests were used as appropriate. The statistical analysis software SAS version 8.0 (SAS Institute, Cary, NC) was used.
Results
Data from 399 HBeAg-positive patients treated with peginterferon alfa-2a alone (n = 204) or in combination with lamivudine (n = 195), and with HBsAg values available at all time points (i.e., baseline and weeks 12, 24, 48 and 72) were included in the following analyses. The majority of patients were infected with HBV genotype B (32.6%, 130/399) or HBV genotype C (58.4%, 233/399).
Response to peginterferon alfa-2a alone or in combination with lamivudine
Baseline characteristics of the 399 patients treated with peginterferon alfa-2a alone or in combination with lamivudine (74% of the patients treated with peginterferon alfa-2a alone or in combination with lamivudine included in the initial study) were similar to those of the initial study population (Table 1).
In total, 34% (137/399) of peginterferon alfa-2a alone or in combination with lamivudine patients achieved HBeAg seroconversion 6 months posttreatment, 28% (112/399) achieved HBV DNA ≤2,000 IU/mL 6 months posttreatment, and 4% (17/399) achieved HBsAg clearance 6 months posttreatment compared with 30% (161/542), 24% (130/542) and 4% (20/542), respectively, in the initial study population. The response rates were similar in the peginterferon alfa-2a plus lamivudine and peginterferon alfa-2a monotherapy groups.
Rates of HBeAg seroconversion 6 months posttreatment were similar to the overall population in patients infected with genotype B (34%, 44/130) or genotype C (34%, 80/233).
Association between baseline HBsAg levels and response 6 months posttreatment
Baseline HBsAg levels were similar in the peginterferon alfa-2a (4.11 ± 0.70 log10 IU/mL) and peginterferon alfa-2a plus lamivudine (4.14 ± 0.63 log10 IU/mL) groups, and the groups were, therefore, pooled for the baseline analysis. Baseline HBsAg levels were lower in patients achieving HBeAg seroconversion 6 months posttreatment (3.97 ± 0.72 log10 IU/mL) than in nonresponders (4.21 ± 0.63 log10 IU/mL; WCχ 2 test p = 0.0390).
ROC analysis was used to identify baseline HBsAg levels associated with HBeAg seroconversion 6 months posttreatment. A lower level of 5,000 IU/mL was identified that resulted in a positive predictive value (PPV) for HBeAg seroconversion 6 months posttreatment of 42% and an NPV of 68%. Applying an upper cutoff at baseline of 50,000 IU/mL increased the NPV to 77%.
Changes in quantitative HBsAg and HBV DNA levels over time
Decline in HBsAg level from baseline during therapy was similar in both peginterferon alfa-2a and peginterferon alfa-2a plus lamivudine groups (Fig. 1). As rates of response in both groups were also similar, data were pooled for all further analyses. However, decline in HBV DNA level from baseline to weeks 12 and 24 was substantially greater in peginterferon alfa-2a plus lamivudine group compared with the peginterferon alfa-2a monotherapy group (Fig. 2).
HBsAg decline according to posttreatment response (HBeAg seroconversion)
HBsAg decline was significantly higher at weeks 12, 24, 48 and 72 in patients with HBeAg seroconversion 6 months posttreatment than in patients without HBeAg seroconversion (WCχ 2 test p = 0.0036, <0.0001, <0.0001 and <0.0001, respectively; Fig. 3a).
HBV DNA decline according to posttreatment response (HBeAg seroconversion)
As seen previously [13], HBV DNA decline was significantly greater at all on-treatment and posttreatment time points in responders to peginterferon alfa-2a monotherapy compared with nonresponders (WCχ 2 test p = 0.0019, 0.0002, <0.0001, and <0.0001 at weeks 12, 24, 48 and 72, respectively; Fig. 3b). However, in the peginterferon alfa-2a plus lamivudine group, a significant difference between responders and nonresponders was observed only at week 72 (Fig. 3c).
Association between on-treatment HBsAg level and response 6 months posttreatment
ROC analyses identified HBsAg levels <1,500 IU/mL at weeks 12 and 24 that generated PPVs of ~55% for HBeAg seroconversion 6 months posttreatment (57 and 54%, respectively). However, as NPVs were <95% (72 and 76%, respectively) using a single cutoff, an upper cutoff value of 20,000 IU/mL was introduced, leading to NPVs of 84 and 85%, respectively. Response rates in the three groups generated using these cutoff values are given in Fig. 4.
At week 12, 22.6% of patients treated with peginterferon alfa-2a alone or in combination with lamivudine had HBsAg <1,500 IU/mL, and this proportion increased to 34.1% at week 24. These patients achieved significantly higher rates of HBeAg seroconversion than patients with HBsAg >1,500 IU/mL (Fig. 4). Approximately, one-half of patients had HBsAg levels of 1,500–20,000 IU/mL at weeks 12 and 24. Rates of HBeAg seroconversion 6 months posttreatment were 32.3 and 26.1% in these patients according to HBsAg levels at weeks 12 and 24, respectively.
Rates of HBV DNA ≤2,000 IU/mL and HBsAg clearance were also substantially higher in patients with HBsAg <1,500 IU/mL at weeks 12 and 24 than in patients with HBsAg >1,500 IU/mL (Fig. 4).
High rates of HBsAg clearance 6 months posttreatment were achieved by patients with HBsAg <1,500 at week 12 and HBeAg seroconversion 6 months posttreatment (17.6%, 9/51). Similarly, 20.3% (15/74) of patients with HBsAg <1,500 at week 24 and HBeAg seroconversion 6 months posttreatment achieved HBsAg clearance 6 months posttreatment.
Association between on-treatment HBsAg level and response 6 months posttreatment in patients infected with genotypes B and C
At week 12, 26% of genotype B, patients treated with peginterferon alfa-2a alone or in combination with lamivudine had HBsAg <1,500 IU/mL, and this proportion increased to 37% at week 24. HBeAg seroconversion 6 months posttreatment was achieved by 50 and 50% of patients with HBsAg <1,500 IU/mL at weeks 12 or 24, respectively. Rates of response in the 22 and 12% of genotype B patients with HBsAg >20,000 IU/mL at weeks 12 and 24, respectively, were 10 and 13% [Cochran Armitage Trend Test (2-sided) p = 0.0018 and 0.039, respectively].
At week 12, 23% of genotype C patients treated with peginterferon alfa-2a alone or in combination with lamivudine had HBsAg <1,500 IU/mL, and this proportion increased to 34% at week 24. HBeAg seroconversion 6 months posttreatment was achieved by 59 and 55% of patients with HBsAg <1,500 at weeks 12 or 24, respectively. Rates of response in the 17 and 10% of genotype C patients with HBsAg >20,000 IU/mL at weeks 12 and 24, respectively, were 15 and 10% [Cochran Armitage Trend Test (2-sided) p = 0.0024 and 0.0021, respectively].
Discussion
Several parameters have been investigated as potential markers of sustained response to interferon-based therapy. In HBeAg-positive CHB, the importance of both HBV DNA and HBeAg has been investigated. Fried et al. [13] showed that HBV DNA levels decreased consistently during peginterferon alfa-2a treatment and that the kinetics of HBV DNA varied between responders and nonresponders. However, the HBV DNA kinetics during peginterferon alfa-2a plus lamivudine therapy differed from the response to peginterferon alfa-2a monotherapy, and as HBV DNA quantification is technically challenging, costly and subject to variability, assessing HBV DNA levels quantitatively in clinical practice may be difficult [17–19]. Fried et al. [13] also investigated the value of HBeAg quantification in monitoring pegylated interferon treatment success and demonstrated that this was a more appropriate marker than HBV DNA. However, practical difficulties exist with assessing HBeAg levels, as a commercial assay is not currently available and more readily available markers are still required.
Interest in HBsAg as a marker of sustained response to interferon-based therapy has been based upon studies that showed a positive association between HBsAg and covalently closed circular DNA (cccDNA) [20–22]. cccDNA reflects the number of hepatocytes infected with the virus and acts as a template for transcription of viral genes [22–24]. The ongoing presence of cccDNA in hepatocytes even when serum HBV DNA levels are undetectable is responsible for the persistence and potential recurrence of HBV infection [25]. The immunomodulatory activity of interferon results in activation of cytotoxic T cells [26]. These cells are important in clearing infected hepatocytes and thereby act to reduce levels of cccDNA [27]. Taken together, these data suggest that HBsAg quantification is an appropriate way to monitor the ongoing immune clearance of infected hepatocytes and elimination of cccDNA mediated by pegylated interferon.
The focus of the current analysis is on-treatment prediction of response, but there is also considerable interest in trying to identify, before treatment is initiated, those patients likely to respond to peginterferon alfa-2a. Recently, data from the Phase 3 peginterferon alfa-2a study used in the current analysis were analyzed together with data from a similar study in patients treated with pegylated interferon alfa-2b [12]. The results identified that high baseline alanine aminotransferase (ALT) levels and low HBV DNA levels were significant predictors of response. However, some patients with low baseline ALT levels and/or high HBV DNA levels have been shown to respond to interferon-based therapy. Accordingly, therefore, electing not to start treatment based on these characteristics may prevent potential responders from achieving posttreatment benefits.
Based on the current analysis, it seems unlikely that baseline HBsAg should be used as a sole criterion for deciding which patients may or may not respond to posttreatment. To identify patients who should not be started on therapy, an NPV >95% is required to ensure that potential responders are not missed. In this analysis, two cutoff values at baseline were required to provide a relatively high PPV; however, this methodology resulted in an NPV of only 77%, meaning that almost one-quarter of potential responders would not be benefitted if these levels were used as a reason not to initiate treatment.
The current investigation is the largest to date to analyze the association between on-treatment HBsAg levels with posttreatment response to peginterferon alfa-2a in HBeAg-positive CHB. Importantly, it supports observations reported previously, which showed that the kinetics of on-treatment HBsAg decline during pegylated interferon therapy were different in patients achieving sustained immune control posttreatment compared with those patients not achieving sustained immune control [28, 29]. Low HBsAg levels during pegylated interferon therapy were associated with higher rates of response than were observed in the overall population and highest rates of HBsAg clearance were achieved by patients with an early decline in HBsAg levels who went on to achieve sustained immune control posttreatment. The genotype subanalysis of patients demonstrated that the highest rates of response were also associated with low on-treatment HBsAg levels in patients infected with genotype B or genotype C. Motivating patients to remain on-treatment is often a concern for clinicians and an early positive response—as indicated by low HBsAg levels—may provide a way of encouraging patients to complete the 48 weeks of therapy.
This analysis did not identify a single on-treatment cutoff level that could generate a high NPV. Even applying the two absolute HBsAg level cutoffs in HBeAg-positive CHB did not result in an NPV >95%. As a result, the cutoff values identified cannot be used to define a stopping rule as, depending on the time of HBsAg quantification, 15–16% of HBeAg-positive patients who would have responded to posttreatment would have their treatment stopped. Two recent analyses have also investigated whether on-treatment HBsAg levels can be used to predict response to peginterferon in HBeAg-positive patients. Buster et al. [11] showed that lack of HBeAg clearance before week 32 of treatment could provide an NPV of 98% for HBsAg clearance. Although HBsAg clearance is accepted as being the closest outcome to cure of CHB, rates of HBsAg clearance are low in the first few months after treatment and recent guidelines from the European Association for the Study of the Liver suggest that a surrogate marker of response, such as HBeAg seroconversion, should be used to indicate response to therapy. In addition, week 32 of treatment is very late to assess response to therapy; identifying a parameter that predicts response earlier during treatment would be of greater value. Sonneveld et al. [29] demonstrated that when response was defined as HBeAg loss combined with HBV DNA <10,000 copies/mL, HBeAg-positive patients with no HBsAg decline at week 12 of therapy had a 97% chance of nonresponse (NPV 97%). However, this observation could not be repeated in the Phase 3 population described here using either the combined end point (NPV 82%) or the more stringent end point of HBeAg seroconversion (NPV 69%) [30]. It is possible that differences in study population could explain why the stopping rule proposed by Sonneveld could not be validated in this peginterferon alfa-2a analysis. Whereas the Sonneveld analysis was a European study with only 20% of Asian patients, the peginterferon alfa-2a population was predominately Asian (87%) and this influenced the genotype distribution: the Sonneveld study included patients mainly infected with genotypes A or D, while the peginterferon alfa-2a had a high proportion of genotype B and C patients.
In the current analysis, applying the two cutoff levels increased response rates from approximately one-third of patients to over one-half of patients. However, the need to apply two cutoff levels resulted in the majority of patients having on-treatment HBsAg levels between the two cutoff levels, and these patients achieved response rates similar to those in the overall population. It is interesting to speculate how response rates in this group could be increased. The need for individualized treatment of patients with CHB is an increasing focus of discussion, especially the possibility of using either combination therapy or an extended, but still finite, course of therapy in patients not achieving a full response early during treatment. Recent data in HBeAg-negative patients showed that a 96-week course of peginterferon alfa-2a therapy increased sustained response compared with a 48-week course of therapy [31]. Further studies are, therefore, required to determine the potential of extended therapy in HBeAg-positive patients with a suboptimal on-treatment response.
Quantification of HBsAg during therapy has a number of advantages over HBV DNA or HBeAg measurement. While HBV DNA quantification can be used in both HBeAg-positive and -negative patients, it is limited because it cannot distinguish between sustained responders and relapsers [32]; also, as shown previously [13] and in the current investigation, HBV DNA decline varies considerably between monotherapy- and combination therapy-treated patients. As described previously, HBeAg quantification is limited as a result of the lack of a commercial assay.
The present investigation has limitations. Only a proportion of patients included in the original randomized controlled trial had HBsAg levels determined during treatment and 6 months posttreatment. As only patients with data available at all on-treatment and posttreatment time points were included in this study, there is a potential for selection bias. However, comparison of response rates in the initial and current populations reveals only small differences, and it is important to note the conservative statistical methods employed classified missing samples as nonresponders.
There is considerable interest in predicting sustained response to pegylated interferon therapy during treatment and it appears that a single, easily quantified parameter is unlikely to provide enough information to justify stopping treatment in some patients. However, combining several on-treatment, or baseline and on-treatment, factors may provide sufficient information to identify nonresponders. By extending the baseline prediction model described by Buster et al. [12], Hansen et al. [33] demonstrated that including on-treatment HBV DNA decline in the prediction algorithm improved its ability to predict response to pegylated interferon therapy.
In conclusion, on-treatment quantification of HBsAg may be an appropriate tool for on-treatment monitoring of response to a finite course of peginterferon alfa-2a in HBeAg-positive patients. This should help identify patients who may benefit from modification of their treatment regimen, thereby helping to improve the overall response rates to a finite course of peginterferon alfa-2a. However, as the stopping rules identified previously could not be validated in the current population, it is clear that further study is required before a cutoff level that identifies the patients likely to benefit, or not benefit, from treatment is validated and can be used in clinical practice.
References
Marcellin P. Hepatitis B and hepatitis C in 2009. Liver Int 2009;29:1–8
Fattovich G, Giustina G, Sanchez-Tapias J, et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alfa therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP). Am J Gastroenterol 1998;93:896–900
Moucari R, Korevaar A, Lada O, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up. J Hepatol 2009;50:1084–1092
European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009;50:227–242
Lok A, McMahon B. Chronic hepatitis B: update 2009. Hepatology 2009;50:1–36.
Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008;2:263–283
Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–2695
Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alfa-2b. Gastroenterology 2008;135:459–467.
van Zonneveld M, Honkoop P, Hansen BE, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology 2004;39:804–810.
Liaw Y-F, Chu C-M. Hepatitis B virus infection. Lancet 2009;373:582–592
Buster EH, Flink HJ, Simsek H, et al. Early HBeAg loss during peginterferon alfa-2b predicts HBsAg loss: results of a long-term follow-up study in chronic hepatitis B patients. Am J Gastroenterol 2009;104:2449–2457
Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to pegylated interferon alfa. Gastroenterology 2009;137:2002–2009
Fried MW, Piratvisuth T, Lau GK, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology 2008;47:428–434
Brunetto MR, Moriconi F, Bonino F, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009;49:1141–1150
Wong VW, Wong GL, Yan KK, Chim AM, Chan HY, Tse CH, Choi PC, Chan AW, Sung JJ, Chan HL. Durability of peginterferon alfa-2b treatment at 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:1945–1953
Kuhns MC, Kleinman SH, McNamara AL, Rawal B, Glynn S, Busch MP. Lack of correlation between HBsAg and HBV DNA levels in blood donors who test positive for HBsAg and anti-HBc: implications for future HBV screening policy. Transfusion 2004;44:1332–1339.
Laperche S, Thibault V, Bouchardeau F, et al. Expertise of laboratories in viral load quantification, genotyping and precore mutation determination for hepatitis B virus in a multicenter study. J Clin Microbiol 2006;44:3600–3607
Pawlotsky JM, Bastie A, Hézode C, et al. Routine detection and quantification of hepatitis B virus DNA in clinical laboratories: performance of three commercial assays. J Virol Methods 2000;85:11–21
Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology 2002;122:1554–1568
Deguchi M, Yamashita N, Kagita M, et al. Quantitation of hepatitis B surface antigen by an automated chemiluminescent microparticle immunoassay. J Virol Methods 2004;115:217–222
Rodella A, Galli C, Terlenghi L, Perandin F, Bonfanti C, Manca N. Quantitative analysis of HBsAg, IgM anti-HBc and anti-HBc avidity in acute and chronic hepatitis B. J Clin Virol 2006;37:206–212
Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004;126:1750–1758
Chan HL, Wong VW, Tse AM, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol 2007;5:1462–1468
Volz T, Lutgehetmann M, Wachtler P, Jacob A, et al. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 2007;133:843–852
Zoulim F. Assessment of treatment efficacy in HBV infection and disease. J Hepatol 2006;44:S95–S99.
Peters M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology 1996;23:909–916
Thimme R, Wieland S, Steiger C, et al. CD8 (+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76
Tangkijvanich P, Komolmit P, Mahachai V, Sa-Nguanmoo P, Theamboonlers A, Poovorawan Y. Comparison between quantitative hepatitis surface antigen, hepatitis B e antigen and hepatitis B virus DNA levels for predicting virological response to pegylated interferon alfa-2b therapy in hepatitis B e-antigen positive chronic hepatitis B. Hepatol Res 2010;40:269–277
Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010;52:1251–1257
Piratvisuth T, Marcellin P. Further analysis is required to identify an early stopping rule for peginterferon therapy that is valid for all HBeAg-positive patients. Hepatology. doi:https://doi.org/10.1002/hep.24136.
Lampertico P, Vigano M, Bhoori S, et al. Extended (2 years) treatment with peginterferon alfa-2a [40 KD] improves sustained response rates in genotype D patients with HBeAg negative chronic hepatitis B. J Hepatol 2010;52:S45
Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 2009;49:1151–1157
Hansen BE, Buster EH, Steyerberg EW, Lesaffre E, Janssen HL. Prediction of the response to peg-interferon-alfa in patients with HBeAg-positive chronic hepatitis B using decline of HBV DNA during treatment. J Med Virol 2010;82:1135–1142
Acknowledgements
Research grant was provided by F. Hoffmann-La Roche, Basel, Switzerland. Editorial support was provided by Dr Liesje Thomas of Elements Communications Ltd., Westerham, UK.
Conflict of interest
TP has participated in advisory boards for Roche, Novartis, MSD and GSK, in Speaker’s Bureau for Roche, Novartis, MSD, GSK and BMS, and has received research grants from Roche and Novartis. PM has acted as a speaker for Roche, Schering-Plough, Gilead, BMS, Novartis, Tibotec, and Intermune; has acted as an expert for Roche, Schering-Plough, Gilead, BMS, Verex, Novartis, Pharmasset, Tibotec, MSD, Biolex, Zymogenetics and Intermune; as an investigator for Roche, Schering-Plough, Gilead, BMS, Verex, Novartis, Tibotec, MSD, Biolex and Intermune; and has received grant support from Roche, Schering-Plough and Gilead. EBM is an employee of Genentech. H-PK is an employee of Abbott GmbH and Company. All other authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piratvisuth, T., Marcellin, P., Popescu, M. et al. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int 7, 429–436 (2013). https://doi.org/10.1007/s12072-011-9280-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-011-9280-0