Abstract
Deafness is commonest curable childhood handicap. Most remedies and programmes don’t address this issue at childhood level leading to detrimental impact on development of newborns. Aims and objectives are (A) screen all newborns for deafness and detect prevalence of deafness in children less than 2 years of age. and (B) assess efficacy of multi-staged OAE/ABR protocol for hearing screening. Methodology: Non-randomized, prospective study from August 2008 to August 2011. All infants underwent a series of oto-acoustic emission (OAE) and final confirmatory auditory brainstem evoked response (ABR) audiometry. Finally, out of 1,101 children, 1,069 children passed the test while 12 children had impaired hearing after final testing, confirmed by ABR. Positive predictive value of OAE after multiple test increased to 100 %. OAE–ABR test series is effective in screening neonates and multiple tests reduce economic burden. High risk screening will miss nearly 50 % deaf children, thus universal screening is indispensable in picking early deafness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hearing is the deepest, most humanizing philosophical sense man possesses. It is the soul of knowledge. Hearing loss and deafness are global issues that affect at least 278 million people worldwide. Two-thirds of these people live in developing countries [1]. Losses in either partial or total hearing may lead to poor language and speech development and thereby affects the comprehensive development of the individual and his productivity. As otologists, the greater responsibility is to diagnose the condition timely and provide appropriate mode of correction. For the same, it is essential to screen all newborns to detect deafness irrespective of predisposition to risk factors. Otoacoustic emissions (OAE) and brainstem evoked response audiometry (BERA/ABR) are tests that effectively assess the type and degree of hearing loss. But it is need of the hour to devise protocols that will not only effectively screen all neonates but also reduce false positive results thereby reducing the time and money invested on test like ABR by the patients.
Work done in similar direction by audiologists and otologists is indicative of the fact that early diagnosis and treatment is the key that offers effective hearing to the individual. This also avoids the setting in of neural plasticity which may be refractory to any mode of treatment. A study done on universal newborn hearing screening at Bulgaria advocates that intervention should begin ideally by the age of 6 months [2]. The same study states that early intervention has significantly higher levels of receptive and expressive language, personal-social development, vocabulary etc. P. Nagapoornima et al. reports that though incidence per 1,000 is higher among high risk infants, focusing only at high risk may miss 50 % of newborns with hearing impairment [3]. American Academy of Pediatrics (AAP) in 1999 advocated universal newborn hearing screening and remedial intervention which is now being practiced in most of the developed countries [4, 5]. In our country, most of the work done in this direction is at treatment level. Even the Indian National Programme for Prevention and Control of Deafness (NPPCD) does not address the issue at neonatal and infant level. Also most of the protocols developed for screening purposes are two staged protocols. A study at Cochin adopted a two stage OAE/ABR protocol but also suggests that this may not be very practical in our set up where cost effectiveness is a major issue [6]. The goal of any neonatal hearing screening programme (NHSP) is to perform hearing screening in all newborns prior to hospital discharge. There is no single model of NHSP. Each programme must carefully consider what type and severity of hearing loss it wishes to identify. Based on available screening tools, programme philosophy, prior experience, maternity length of stay etc., an appropriate protocol must be developed. In a set-up like ours, there is a need to tailor a protocol that will address these issues without compromising the compliance of patient families.
This study aims at filling these lacunae by screening all newborns and children below 2 years of age in rural based tertiary care center to evaluate the burden of hearing loss in the given age group and to assess the efficacy of multi-staged OAE–ABR test protocol as screening tool of this life modulating impairment. The aims of this study are to:
-
A)
screen all newborns for deafness and detect prevalence of deafness in children less than 2 years of age.
-
B)
assess efficacy of multi-staged OAE/ABR protocol for hearing screening.
Materials and Methods
The study was conducted in the department of ENT of Shree Krishna Hospital, Anand, Gujarat. It was a prospective study conducted between August 2008 and August 2011. A total of 1,101 babies including newborns, neonates, infants and children less than 2 years who attended Shree Krishna hospital during the study period were screened for hearing status. All babies were screened with Distortion Product Otoacoustic Emission (DPOAE) testing, and those who failed to pass OAEs test series were then confirmed with Auditory Brainstem evoked Response Audiometry (ABR).
Inclusion Criteria
All newborns delivered in our hospital, neonates admitted in Neonatal ICU and children less than 2 years attended our hospital.
Exclusion Criteria
Meatal atresia, anomalies of external ear where probe insertion was not possible. Otitis media, otitis externa, discharge and wax in external auditory canal were included in the study only after the condition was treated.
For this study children were divided into three groups according to the age.
-
Group A—Less than 6 months
-
Group B—6 months to 1 year
-
Group C—1–2 years.
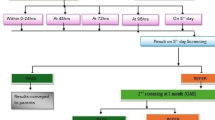
Following five staged protocol was employed for screening of the children:

Observations
Total 1,101 children were tested between August 2008 and August 2011. All these children were divided into three groups and tested on five stage protocol basis as described above.
Out of 1,101 children, 1,069 children passed the test and had normal hearing while 12 children had impaired hearing after multi-staged test series, which was confirmed by ABR. This account for 1 % refer rate. 20 children were lost in follow up from all three groups. Of the total 1,101 children, 1,049 belonged to group A, 28 in group B and 24 in group C (Table 1). The refer rate in group A, B and C was 5, 3, and 4 respectively (Fig. 1). Majority of these refer cases belonged to group A. 629 children tested were males while 472 were females. Out of 1,101 children, 126 had positive history predisposing them to be at high risk for deafness. 5 % of these were diagnosed to have impaired hearing after complete test series which is ten times higher than the refer rate in children having no documented risk for deafness (Fig. 2). According to the group specific protocols, 122 children had impaired hearing in initial OAE test which reduced to 3 after 4th test. All the three children had poor wave morphology on ABR testing suggesting that the positive predictive value of OAE increased from <10–100 % after multi-stage test series (Figs. 3–4). Of 28 children in group B, the final refer rate was 10 % (Fig. 5). While out of ten children referred in initial OAE test in group C, six children came for follow up and four were confirmed to have hearing impairment on ABR (Fig. 6).
Discussion
Hearing assessment in children is one of the dark areas in spite of the fact that two out of every 1,000 children have permanent bilateral hearing loss above 60 dB [7]. According to a recent survey data reported by the World Health Organization (WHO), 278 million people worldwide have moderate to profound hearing loss (HL) in both ears. Most of the people who have hearing disabilities live in developing countries [1]. Four to six out of every 1,000 children born in India are found to have severe to profound hearing loss [8]. Hearing impairment has a devastating, detrimental and an invariably adverse impact on the development of newborns and psychological well-being of their families [9] and these children are often out-casted as deaf and dumb rather than deaf and mute.
It is a well known fact that childhood deafness can have a severe impact on speech and language development. Language—in our society, oral language—is the prime means through which socialization and learning occurs. Speech and language develops rapidly in the first year of life. When communicative interactions between a child and his or her family are disrupted during these early critical years, serious delay in cognitive development is likely to occur. If the deprivation goes on for a long time, the child may never make up for the lost learning, even after extensive rehabilitation [10]. Thus, severe to profound hearing loss has the potential to adversely affect crucial aspects of development, including social, cognitive, and academic abilities, mostly because of a delay in oral language. Hearing loss directly affects a child’s ability to develop normal language skills, impairs his ability to communicate with others in the environment, and has been shown to correlate with poor academic performance. Screening is only the beginning of a successful path for infants who are deaf or hard of hearing.
W K Low et al. [11], had shown in their study in 2005, prevalence of hearing loss in newborns is 0.4 %, while Downs et al. [12] had shown prevalence of hearing loss in newborns is 0.32 %. In present study the prevalence of the hearing loss in children less than 2 years is nearly 1 %. High prevalence of hearing loss in present study is because along with newborns, children up to 2 years were screened for hearing loss and there were selective children between 9 months and 2 years who had taken visit of our hospital as their parents had suspected their hearing ability, and rate of hearing loss in those specific children was high which has made the all over prevalence of hearing loss high in present series.
Age wise distribution of the children showed that majority were less than 6 months of age. This is the target population. In our institute, we started universal neonatal hearing screening and therefore most of the children screened were below 6 months of the age. The US Joint Committee on Infant Hearing position statement in 2,000 proposed the golden rule of “1-3-6” [13]. This stated that all the children must be screened for deafness by 1 month, diagnosed with deafness by 3 months and necessary intervention must be undertaken by as early as 6 months. This is so because after that neural plasticity starts to set in. Neural plasticity is the ability of the nervous system to modify its organization and function based on changing external or internal demand and once it sets in, the occurs irreversible damage to the nerve cells which subsequently atrophy and the child is rendered permanently deaf.
The refer rate is higher in children belonging to group B and group C. This can be explained on the basis that after 6 months of age, as the child grows and achieves milestones, the parents, readily identify this handicap. And so, only the suspected deaf and mute children or who are lagging behind their peers are brought to the hospital for screening. Before 6 months of age, identification of deafness if difficult. This fact also supports universal neonatal hearing screening.
The male: female ratio was nearly 3:2.5. Although hearing loss of not a sex-linked disorder and has no gender predisposition, such data is essential for epidemiological purposes.
Nearly 1 % of the total children screened were at high risk of developing deafness. In a study by Albert I. Mehl At et al., out of 126 hearing impaired babies, 63 (50 %) were high risk babies [15]. In present study out of 12 hearing impaired babies from 1,101 babies screened, 07 (60 %) babies were high risk babies. These results showing proportion of high risk in hearing loss is comparable.
According to John et al., 2009, low birth weight was the most common risk factor in high risk babies, which accounts for 26 (56.52 %) out of 46 high risk babies [16]. In present study, low birth weight babies/preterm babies account for 54 %. Proportion of very low birth weight babies is increased in recent times because of better and advanced neonatal intensive care units (NICU) provided to preterm and low birth weight babies now a days, which has improved the survival rate in them. The risk factors for hearing loss are well established now. The US Joint Committee on Infant Hearing position statement in 2000 [13] enumerates three major risk factors:
-
(a)
History of treatment in NICU for >48 h
-
(b)
Family history of early childhood deafness
-
(c)
Cranio-facial anomalies associated with hearing impairment.
Babies admitted to a neonatal intensive care unit for more than 48 h are 10.2 times more likely to have a permanent hearing loss than those who did not undergo Meningitis—most common cause of acquired hearing loss is childhood meningitis. Chan had shown proportion of high risk babies was 309 (5.04 %) out of 6,127 babies screened [14]. In present series proportion of high risk babies was 126 out of 1,101 babies screened. So according to this data in high risk babies screening programme, we need to screen only 5–8 % of total babies, but with it we definitely miss nearly 50 % hearing impaired babies from not at high risk groups. Chan had also shown 20 (6.47 %) hearing impaired babies were identified out of 309 high risk babies screened [14]. In present study 07 (6 %) hearing impaired babies were identified out of 126 high risk babies screened, which is consistent with Chan’s data. Data of this table suggest that the high risk babies have much higher rate of hearing impairment then normal babies. Only 0.5 % (five out of 975) of children without any high risk had confirmed hearing impairment. Therefore, meticulous screening as well as ensured follow up is a must for children at high risk for developing deafness.
In W K Low et al. series, after initial test refer out of 220 babies, 18 (8.18 %) babies were lost in follow up test [11]. In present series out of 145 initial refer babies, 20 (14 %) babies were lost in follow up test. According to both series, these dropouts are because of change in the phone numbers, addresses and sometimes refusal from parents’ side for further testing of their babies.
John et al. had shown refer rate of initial test of 6.4 % was reduced to 1.6 % on subsequent tests [16]. In present study refer rate of initial study of 10 % was reduced to 1 % on subsequent follow up tests. Data of this table suggest importance of retesting of refer babies to reduce false positive rate. With each retest, the positive predictive value of OAE increases and children with true deafness are screened out. This warrants a multi step protocol for hearing assessment. Due to the limitations of the technical fallacies of the test and inability to detect hearing loss in infants clinically, newborns, infants and preschool children are difficult to assess by routine investigations which apply to patients aged above 5 years. A five tier test battery approach in which a thorough history taking, clinical tests and multiple testing are combined together is suggested in these groups of children.
Development of speech and hearing is essentially a complex learning process. The development of speech and language is an ongoing process, beginning at birth. The period from 0 to 5 years is recognized as important for all aspects of development in a child, including hearing, language and speech. Every day and every month, the baby matures in each of these three interwoven areas, and gradually learns the skills to communicate with the help of those around him. Thus, early detection and timely intervention can not only help prevent this silent handicap of deafness but also contribute to social and economic productivity of a community.
Conclusion
Hearing loss is commonest childhood handicap that is curable and with a large quantum of its burden in developing countries like India, there is need to address this issue at national forum. Universal neonatal hearing screening is indispensable in picking up early deafness in order to intervene timely. Targeted screening i.e., of high risk babies only will miss out nearly 50 % of deaf children who do not present with any known high risk. OAE–ABR test series is an effective way to screen the neonates and multiple stage protocol is essential to reduce false positive results and increase the positive predictive value to OAE from less than 10–100 %. This also reduces the community economic burden considerably by reducing the number of ABR test. Thus, universal hearing screening of neonates must be mandatory and multi-staged protocol based screening should be tailored as per the epidemiology of the set-up.
References
Tucci DL, Merson MH, Wilson BS (2010) A summary of the literature on global hearing impairment: current status and priorities for action. Otol Neurotol 31(1):31–41
Roclev P, Mumdzhiev H, Spiridonova J, Dimov P (2004) Universal newborn hearing screening in Bulgaria. Int J Pediatr Otorhinolaryngol 3354:1–6
Nagapoornima P, Ramesh A, Srilakshmi, Rao S, Patricia PL, Gore M, Dominic M, Swarnarekha (2007) Universal hearing screening. Indian J Pediatr 74:545–549
Report of collective study on prevention and etiology of hearing impairment (1983) ICMR and Department of Science, New Delhi
Kacker SK (1997) The scope of pediatric audiology in India. Otorhinolaryngology Research Society of AIIMS, New Delhi, p 20
Paul AK (2011) Early identification of hearing loss and centralized newborn hearing screening facility—the Cochin experience. Indian Pediatr 48:355–359
Biswas A (2002) Clinical audio-vestibulometry for otologists and neurologists, Assessing the deaf child, 3rd edn. Bhalani Publishing House, Mumbai, p 97
Harvey C (2003) New born hearing screening. Aust Prescr 26(4):82
Mangla S, Kaushal R (2009) Importance of new born hearing screening. Indian J Otolaryngol Head Neck Surg 61:157–159
Margaret AK (2003) Neonatal hearing screening. Pediatr Clin N Am 50:301–313
Low WK, Pang KY (2005) Universal newborn hearing screening. Ann Acad Med Singap 34:301–306
Downs SM, Kemper A (2000) A cost-effectiveness analysis of new born hearing screening strategies. Arch Pediatr Adolesc Med 154:484–488
Joint committee on Infant Hearing (2000) Position statement; principles and guidelines for early hearing detection and intervention programmes. Pediatrics 106:798–817
Chan KY, Lee F, Chow CB, Shek CC, Mak R (1998) Early screening and identification of deafness of high risk neonates. HK J Paediatr 3:131–135
Mehl AL, Thomson V (1998) Newborn hearing screening: the great omission. Pediatrics 101:1–6
John M, Balraj A, Kurien M (2009) Neonatal screening for hearing loss: pilot study from a tertiary care centre. Indian J Otolaryngol Head Neck Surg 61:33–36
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, G., Sharma, Y., Mehta, K. et al. Efficacy of Distortion Product Oto-Acoustic Emission (OAE)/Auditory Brainstem Evoked Response (ABR) Protocols in Universal Neonatal Hearing Screening and Detecting Hearing Loss in Children <2 Years of Age. Indian J Otolaryngol Head Neck Surg 65, 105–110 (2013). https://doi.org/10.1007/s12070-012-0553-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-012-0553-2