Abstract
Currently, the use of small-scale energy sources is a rapidly developing approach that solves the problems of quality and guaranteed power supply for local communities. The use of combined heat and power production in the sources of small energy is the most effective way to save fuel in the municipal sector and in industry. In this regard, studies that are focused on a solution to the problem of implementing small-scale energy approaches are promising and relevant. In this article, an exergy analysis is performed on the scheme of a low-capacity coal-based power plant that produces electrical and thermal energy and sulfur as a by-product. The exergetic dependences describing the basic processes are cited. The proposed indicators of efficiency allow the estimation of the work of a low-capacity power plant to produce by-products in addition to energy. The results of the exergetic efficiency of individual processes and of the entire scheme are obtained. The exergetic efficiency of a low-capacity coal-firing thermal power plant with the production of sulfur was 26 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, small-scale (autonomous, local) energy technologies (electric power <25 MW) are being used in increasing numbers in developed and developing countries and under different natural and climatic conditions. This increasing use is explained by quite natural facts. Small-scale energy provides users independence from centralized power sources and allows the use of fuel and energy resources that are optimal for specific local conditions. In addition, small-scale energy improves the stability and reliability of the power system of the state or region. This improvement is achieved by the generation of additional capacities, improvement of the energy system at different levels, and reduction of the losses of energy by bringing generating facilities directly to the consumer.
In some European countries, the share of the total power production from small-scale energy sources is 40 %. This share for all industrialized countries is 10–15 % on average (Vagin et al. 2005). For example, in Denmark, the development of a decentralized energy supply was announced as a state program (IEA 2011). Some states in the USA have laws that exempt owners of small power plants from taxes and allow them to receive compensation from part of the capital cost; also, utilities must buy the excess energy from the owners of low-capacity power plants (IEA 2007). In Germany, the number of existing, under construction, and planned small power plants are approximately 2,000 (BMWi 2014). In Japan, each new construction project is required to be equipped with its own low-capacity power plant (Kotler 2004; Novoselova 2013). In Italy (Cau et al. 2012), in 2009, the distributed generation sector included 74,348 power generation units (each with less than 10 MW). The share of cogeneration plants should increase to 65 % in China by 2015 (Liao et al. 2013).
Although Russia has a huge potential demand for solutions of small-scale energy, such energy sources represent a small fraction of the total energy balance. Currently in Russia, the approximately 50,000 small-scale energy plants represent the total capacity of approximately 17 million kW. The small-scale energy sites in Russia provide approximately 7 % of the total annual electricity production in the country (Filippov 2009), which is significantly lower than the world average.
The small-scale energy capacity in Russia on 96.4 % of the power is provided by thermal power plants (see Table 1).
Diesel power plants represent approximately 55 % of the installed capacity of small-scale power plants and 31 % of the electricity generated by small-scale power plants. Steam-turbine power plants account for 23 % of the small-scale installed capacity and 37 % of the electricity generated by small-scale power plants. The largest amount of heat generated is from steam-turbine units—65 % of the thermal energy generated by small-scale power plants (Filippov 2009).
The primary obstacles to the development of small-scale energy in Russia are the following:
-
No laws for the sale of energy;
-
The absence of effectively operating economic mechanisms and measures that stimulate the development of autonomous power;
-
The complexity of connecting small-scale generating units to a centralized system;
-
The lack of tariff support for the application of small-scale energy.
Overcoming these obstacles will enable small-scale energy development to play a significant role during the period of a smooth increase in energy consumption, during the stage of improving energy reorientation on the market, in the absence of major investors, and during the growth of small and medium businesses.
The solutions to the problems of small-scale energy should be based on a systemic and comprehensive approach. The solutions will determine the conditions for the organizational, financial, scientific, technological, teaching and methodological, normative, informational, and staffing resources of small-scale energy. State support of small-scale generation is necessary to overcome the existing barriers. Specifically, the state should create additional incentives that will contribute to small-scale energy development in Russia.
According to the Australian Association of Cogeneration, the fraction of natural gas among all the fuels that are used in cogeneration systems is approximately 55 %; 25 % is the fraction of the other types of gaseous fuel (associated gas, coke, pyrolysis, biogas), 14 % is the fraction for solid fuel, and 6 % is the fraction for liquid fuel.
The traditional fuel for small-scale power plants is natural gas (Badami & Mura 2010; Kanoglu & Dincer 2009). Only a limited number of works in the scientific literature are related to research in the field of coal-based small-scale energy (Blohin et al. 2005; Cau et al. 2012). However, there is currently a tendency to increase the use of solid fuel, which is to expand range of fuel base for these objects. Using of solid fuel allows to provide autonomy of small-scale power plants (do not need laying of pipelines), the fuel can be delivered with the various types of transport.
This research paper describes the study of small-scale energy that is based on the use of solid fuels. The present theoretical study focuses on the processes of conversion of solid fuel for gasification, the cleaning of the generator gas to produce sulfur as a by-product, and the process of production of both heat and power in a low-capacity coal-based power plant. Exergy analysis is used as an instrument for the theoretical study.
The main target of this study is a comprehensive analysis the efficiency of the technological scheme of low-capacity power plant which produced not only energy but also by-product as sulfur.
The use of coal in small-scale energy sources
Coal is one of the most important natural resources, and its consumption in many countries is quite high. In the fuel-energy complex of Russia, coal usage is rather low (approximately 17 %), underutilizing the existing potential. The dynamics of the indicators of the coal industry in Russia from 1994 to 2010 are listed in Table 2.
According to the forecast of the International Energy Agency, the share of coal in the global energy balance will increase to 29 % by 2030. Coal consumption will grow at a faster rate than all other primary energy sources, at an average growth rate of 1.9 %, to reach a consumption of 6,980 million tons of coal equivalent in 2030 (Energy Strategy of Russia up to 2030 year 2010).
According to the Energy Strategy up to 2030 in Russia, the share of coal in the fuel balance will rise to 34–36 % in 2030 by reducing the share of gas from 70 to 60–62 % (IEA 2013).
At the present time in Russia, power plants use coals that vary greatly in quality: over 25 % of the total coal consumed has ash content of over 40 %, 18.8 % of the coal consumed has a lower heating value (LHV) of 12,500 kJ/kg, and 6.8 million tons of coal have a sulfur content of over 3.0 %.
Insufficient application of the enrichment and standardization of steam coal and the use of an intermediate product (the enrichment of coal) in power generation lead to the fact that each plant in Russia is tied to a particular type of coal. In this case, the complete or partial replacement of other ranks of coal is difficult and expensive.
For a small-scale independent energy provider, this problem should be avoided. The unification of fuel should be incorporated in the planning of these low-capacity power plants so that the plants can use different types of solid fuel, ranging from various ranks of coal to peat and shale, without significant structural changes in the technology. This unification of fuel can be achieved by implementing an effective technology of fuel reprocessing and by varying the operating parameters of the main processes (Shamsutdinov et al. 2011).
To ensure the competitiveness of the solid fuels in a small-scale energy plant, two major problems must be solved: reducing the ecological impact on the environment and minimizing the cost of its preparation for the burning of solid fuel.
It is known that the use of coal produces emissions of NOx, CO2, ash and slag wastes, and H2S which are a serious problem for the environment. But that problem is solved by the use of processing technologies for solid fuel: gasification, the combustion of the coal-water slurries, in a fluidized bed. Solving the second problem requires a detailed consideration of the processes for the transformation of solid fuels in their preparation for combustion and the determination of the processes that can reduce the cost.
The use of coal in low-capacity power plants usually does not involve direct combustion in the boilers; instead, the thermal processing of coal to produce gas fuels is used, such as in gasification. Gasification is a thermochemical process that causes the interaction of the fuel with an oxidant. In gasification, almost all the organic mass is converted into fuel gas, and the mineral fraction of the mass remains in the solid or liquid state. Currently, gasification is widely represented in the literature (Kantorovich 1958; Shabbar & Janajreh 2013). The gasification of coal can provide a clean energy fuel, i.e., the generator gas, and will expand the range and scale of the use of low-grade solid fuels. Gasification also simplifies the preparation of fuel, as there is no need for pulverization (gasification of lump fuel) and purification of the combustion gases from fly ash, which is instead used for cleaning the generator gas.
During the thermal processing of solid fuels in gas, 30–40 % of the sulfur content in the coal is processed. Moreover 95–98 % of all sulfur compounds that are present in the gas phase of the generator gas are from H2S. The need to remove sulfur compounds, particularly H2S, from the generator gas arises from ecological considerations. Another reason is the possibility of utilizing H2S to obtain by-products.
The disposal of hydrogen sulfide is possible with the production of elemental S or H2SO4. The choice of this method for small-scale power plants is determined by the following criteria:
-
Simplicity of design for the production of by-products;
-
Low capital cost;
-
The possibility of selling power and by-products in the nearby regions.
In the case considered in this article, a low-capacity power plant provides the most appropriate utilization of H2S to produce S.
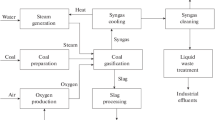
The technological scheme of a low-capacity coal-based power plant and the main parameters of its operation
The proposed scheme for the operation of a low-capacity power plant using generator gas is shown in Fig. 1. Coal feeds into a hammer mill, where it is simultaneously crushed and dried by the heat of the drying agent entering the mill. Next, coal is sent to the gasifier, where, in the presence of the oxidizing agents water vapor and air, which is waste air that was used as a drying agent from the mill, it is gasified to produce generator gas and slag.
The generator gas, after cooling to 400 °C in the heat exchanger, enters the purification unit. Cleaning is performed in two stages: the larger particles are captured in the cyclone, and the smaller particles are captured in the bag filter. For removal of H2S, the generator gas is cooled to 40 °C under the terms of the process and fed to the adsorber. Removal of H2S from the generator gas is performed using activated carbon.
Different methods for the purification of H2S are presented in the scientific literature. However, a low-capacity power plant can only use a few of these methods. Most of the methods for the regeneration of the adsorbent require additional equipment and large areas, which will result in additional capital cost for a low-capacity power plant. In the purification process, the adsorption of hydrogen sulfide and the regeneration of the adsorbent are performed in the same apparatus, which is determined by the choice of the method. Another consideration is the allocation of pure S, which can be used as a by-product of the H2S removal process.
The cleaning process is the catalytic oxidation of H2S to elemental sulfur with the air on the surface of activated carbon (Goihrah & Piniagin 1954):
NH3 is added to the generator gas for passing this reaction with sufficient speed under normal temperature.
The rate of passage of the generator gas through the activated carbon is 75–100 mm/s. The activated carbon layer in the adsorber is 750–1,200 mm, which confirms its compactness. Activated carbon adsorbs other sulfur compounds contained in the generator gas in addition to H2S. The generator gas is passed through the unit from the top to the bottom. The reaction passes through the reaction zone, the height of which depends on the amount of H2S in the gas and the gas velocity. In the reaction zone, the temperature rises and is judged by the intensity of the process. The optimum temperature of the process is 35–50 °C.
The released sulfur fills the pores of the activated carbon, gradually reducing its activity. Regeneration of the activated carbon is performed by treatment with a solution of (NH4)2S, which extracts sulfur from the coal through the formation of polysulfides:
Upon heating under pressure, polysulfides allocate elemental sulfur:
NH3 and H2S are released during heating, and the water vapor is cooled to form (NH4)2S, which is sent to the extraction equipment:
Regenerated carbon, after removal of the adsorbed sulfur and blowing with steam, is used again for the purification of gas. Sulfur, which falls out of the polysulfide solution when it is heated, is melted and released into molds (forms) or released as a coarse sediment (which is subjected to filtration and washing). The recovery of elemental sulfur in the regeneration of carbon is 95 % efficient. When cleaning the gas using activated carbon, H2S is removed almost completely and 25–30 % of organic sulfur compounds is removed. The advantages of this method are the high H2S removal with the simultaneous extraction of the organic sulfur compounds and the possibility of obtaining high-quality marketable sulfur.
The purified generator gas enters the combustion chamber of the gas turbine, which is the working actuating medium for generating electric power. The combustion products after passing the gas turbine are sent to a heat recovery boiler to produce heat energy in the form of steam.
The characteristics of the initial fuel (coal) and the generator gas are given in Table 3.
The main indicators of gasification of solid fuels, i.e., composition, the calorific value of the gas, and consumption of the oxidants (steam and air), are determined in the generalized method using known relationships (Beloselsky 2005; Kantorovich 1958). The composition of the generator gas was determined for the equilibrium state and based on the partial pressure of the components (Higman & van der Burgh 2003).
The characteristics of the primary flows of the scheme that was used for the calculations are listed in Table 4. All indicators in the table are for 1 kg of fuel source.
The initial environmental parameters used were T 0 = 293.7 K and p 0 = 101,325 Pa. The power of the low-capacity coal-based power plant was 16 MW, with a coal consumption rate of 4.04 kg/s.
Description of the basic processes in the scheme using exergy analysis
The exergy method is the most common approach for the thermodynamic research of various energy conversion systems. Exergy analysis allows one to visually determine the degree of perfection and power losses in systems and to determine ways to improve them.
The exergy method is widely represented in works related to the study of combined heat and power systems (Badami & Mura 2010; Dincer & Rosen 2007; Ozkan et al. 2012), but it is rarely used for small-scale coal-fired energy sources (Rosen 2001; Afanasyeva & Mingaleeva 2011).
The important components of exergy, physical Δ 0 E and chemical E ch, together determine the thermal exergy E t:
Physical exergy Δ 0 E is that part of exergy that is a result of the mismatch of the temperature and pressure considered pertinent to the temperature and environmental pressure. The exergy that arises from the difference in composition is the chemical exergy E ch.
There are also a kinetic exergy (kinetic energy that is calculated using the rate of movement relative to the environment) and potential exergy (potential energy relative to the zero level connected to the environment), but according to (Szargut & Petela 1967), they can be disregard in the calculations.
The scientific and methodological basis of the concept of exergy, which is based on the second law of thermodynamics, and the principles of the exergy analysis of processes and technical systems are developed and presented in the fundamental works. However, current requirements for the optimization of the technical systems require the basic provisions of the exergy analysis to be performed with new representations.
The primary processes that occur in the production of heat and electricity from coal in small-scale power plants are as follows: drying, grinding coal, gasification, cleaning the gas, combustion of generator gas in the combustion chamber of the gas turbine unit, and heat production in the recovery boiler.
This work describes in detail the processes of the preparation of the fuel for gasification, including its crushing and drying, as well as the process of gasification to produce the product gas and to clean it.
One of the key processes in the preparation of the fuel is mechanical grinding, which is primarily performed in two stages: crushing and pulverizing. Advanced exergy analysis of the process has not been conducted, although the process increases the reactivity of coal during its subsequent use due to the increase in the surface area and due to the mechanical activation for breaking the connection between the components of the organic mass of coal. If crushing is performed without heat transfer, the exergy balance is given by the following:
where E ′c and E ″c are the exergy of coal before and after grinding, respectively, L mill is the electric power consumed to drive the mill, А mill is the work spent on crushing coal, Е los.mill is the external exergy loss in crushing related to heat exchange with the environment and the loss of coal from dusting, and ΔЕ mill is the internal exergy losses during grinding.
The value of А mill can be indirectly estimated from the grinding parameters defined according to the theory of P.A. Rebinder by the formula (Rebinder 1979):
where σ is the specific surface energy consumed by the formation of new fracture surfaces in the solid, ΔF is the surface formed at failure, k is the work of the elastic and plastic deformation per unit volume of a solid, and ΔV is the part of the solid affected by deformation.
In the case of a large crushing product, kΔV is much higher than σΔF and the power consumption is approximately proportional to the fragmentation of the volume of a solid. When a member of the fine grinding σΔF becomes prevalent, the power consumption is proportional to the grinding surface of the crushed particles.
The chemical exergy of coal can be defined in various ways (Eisermann & Conger 1980). In this paper, the chemical exergy of coal is determined by the ratio proposed by Stepanov (Stepanov 1990):
where O is the oxygen content of the coal in terms of the working mass, %; W is the moisture content in terms of the working masses, %; А is the ash content of coal in terms of the working masses, %; and LHV is the lower heating value of coal, kJ/kg.
The most common preparation technique for solid fuel is drying combined with crushing in a pulverizing mill. All the components of the exergy balance of drying and grinding can be determined from the known dependence on exergy efficiency using the formula:
where E ′d. a and E ″d. a are the exergy of the drying agent at the inlet and outlet, respectively, of the mill; E ′c and E ″c are the exergy of the coal supplied to the mill and the pulverized coal, respectively; ∑ L g. d is the electrical power used by the equipment for drying and grinding; E ″evap is the exergy expended on evaporation; and E mech is the exergy used for the operation of the mill.
The most difficult process in the preparation of the fuel is thermochemical conversion (pyrolysis and gasification) with the production of energy and gas, liquid, and solid products. The efficiency of the gasification process is determined by the relation:
where E ″g. g is the exergy of the generator gas; E ′c is the exergy of the fuel; E ′ox is the exergy of the oxidants (water vapor and the drying agent from the mill); ∑ L gas is the electrical power used to drive auxiliary devices; and E chem. r is the chemical exergy of the heat gasification reaction.
Szargut and Petela (Szargut & Petela 1967) calculate the chemical exergy E t of the generator and pyrolysis gases produced in the process of gasification of coal according to the formula:
where G i is the quantity of the substance of a component of a solution, mol; e ni is the normal chemical exergy of the pure substances of a solution, kJ/mol; d ni is the enthalpy of devaluation of the pure substances of a solution, kJ/mol; T 0 is an environmental temperature, K; T is the valid temperature, K; \( \varDelta {i}_i\left|{}_{T_0}^T\right. \) is the change of enthalpy component in the range from an environmental temperature to the valid temperature, kJ/mol; \( {n}_{{\mathrm{H}}_2\mathrm{O}} \) is the quantity in kilomoles of the water steam participating in the reaction of the devaluation in the quality of the resultant substance of readout count, kmol; n i is the quantity in kilomoles of a component, kmol; n is the total quantity in kilomoles of the substances the solution, kmol; \( {p}_{0{\mathrm{H}}_2\mathrm{O}} \) is the effective partial pressure of steam water in the environment, МPа; \( {p}_{0{\mathrm{H}}_2\mathrm{O} n} \) is the normal partial pressure of steam water in the environment, МPа; p is the pressure of the substance, МPа; \( \varDelta {s}_{pi}\left|{}_{T_0}^T\right. \) is the isobaric change of the entropy component in the range from an environmental temperature to the valid temperature, kJ/kmol · K; and R is the universal gas constant, J/mol · K.
The exergy of the air and water vapor acting as oxidants is determined according to the dependences given in a previous work (Afanasyeva & Mingaleeva 2009).
For the determination of the chemical exergy of complex substances, they can be considered as a mechanical mixture (sum of included compounds). Then, knowing the unit values of all chemical exergy included compounds, the chemical exergy of the slag will be determined by the following equation:
where v z is the share of z chemical compound in a unit of the substance under consideration and e z is the specific chemical exergy of the z compound.
The exergy of slag is not included in the equation of the efficiency of the gasification process, but it is present as an expense in the exergy balance. The slag composition in this example is as follows, %: SiO2—58, Al2O3—25, Fe2O3—14.5, CaO—1.8, and MnO—0.7. The specific chemical exergy of each compound has been adopted in accordance with the values in the literature (Stepanov 1990). The slag in the low-capacity power plant after treatment can also be used as a by-product. Such technological solutions exist, but the issue is not considered here.
Further use of the product gas in power plants requires the removal of H2S. The exergetic efficiency of the cleaning unit used to remove H2S is calculated by the following equation:
where E ′g. g and E ″g. g are the exergy crude and refined generator gas, respectively; E ″s is the exergy of the by-product sulfur; E ′а. c is the exergy of the activated carbon-absorbent entering the treatment unit; \( {E}_{{\mathrm{NH}}_3}^{\prime } \) is the exergy of NH3 for the adsorption process; \( {E}_{{\left({\mathrm{NH}}_4\right)}_2\mathrm{S}}^{\prime } \) is the exergy of (NH4)2S for regeneration of activated carbon; E ′a is the exergy of air; and E ′w. v is the exergy of steam for the regeneration of activated carbon.
The exergy of activated carbon that is used for cleaning the generator gas is calculated by analogy with the formula for the slag (8) with the corresponding component composition and the specific chemical exergy components. The composition of the activated carbon is the following, %: C—94, H—0.7, and O—5.3. The exergetic efficiency of the primary generating equipment, the gas turbine, is determined by the following relationship:
where N g. t. p is the capacity of the power equipment—the gas turbine unit; E ″comb is the exergy of the combustion products generated in the combustion chamber of the combustion generator gas; E ′g. g is the exergy of the generator gas supplied to the gas turbine combustor.
The exergetic efficiency of the waste heat boiler is calculated by the relation:
where E ′s. w is the exergy of feed water supplied to the heat recovery boiler; E ″w. v is the exergy of steam produced in the recovery boiler; and E ′comb is the exergy of the combustion products entering the waste heat boiler.
For a complete evaluation of the effectiveness of the low-capacity power plant, which operates on solid fuel and produces heat, electricity, and useful by-products, we propose a new indicator that includes the exergy of all the useful components.
The exergetic efficiency of the entire scheme of a low-capacity coal-fired power plant is determined by the ratio of the generated electrical power N g. t. p, the water vapor produced in the recovery boiler E ″w. v , the exergy of sulfur E ″s to the exergy of fuel E ′c , the exergy of air ∑ E a required for the basic processes (refining and combustion of the generator gas in the combustion chamber of the gas turbine unit), the exergy values of the cooling water and feed water in the heat recovery boiler ∑ E w, ammonia \( {E}_{{\mathrm{NH}}_3}^{\prime } \), water vapor ∑ E ′w. v , sulfur ammonium \( {E}_{{\left({\mathrm{NH}}_4\right)}_2\mathrm{S}}^{\prime } \), activated carbon E ′a. c. , and electric power of main and auxiliary equipment ∑ L:
The proposed performance indicator includes the exergy of the produced by-product in addition to the thermal and electrical energy. This approach greatly increases the possibility of complex assessing the efficiency of technical objects, the results of which are the energy and chemical products.
Results
Using the above relations for the considered low-capacity coal-fired power plant, we determined its exergy efficiency. The relevant exergy values of the major flows are listed in Table 5.
The results of the calculations of the exergy efficiency are listed in Table 6.
The obtained values of exergy efficiency for this scheme are consistent with the estimates for other types of scheme. In the work Cau et al. 2012, the results of evaluation of the efficiency of the process producing electrical and thermal energy presented, which accounted for 27.1 and 59.0 %, respectively. In the work by Afanasyeva & Mingaleeva (2009) exergy efficiency low-capacity power plant, but with the production of activated carbon, was 35 %. According (Szargut & Petela 1967) value of exergy efficiency for large coal-fired plants is in the range 30–39 %.
To visualize the conversion of the flows and the losses, we use the exergy diagram (Fig. 2).
The diagram of the exergy flows for the low-capacity power plant. I mill, II gasifier, III block of purification and cooling, IV adsorber, V heat exchanger, VI gas turbine unit, VII heat recovery boiler, E ″ сf exergy of coarse fraction of coal, E ″ f exergy of fines coal, ∑ L pc electrical power used by the equipment for purification and cooling, E ″ c. p exergy of combustion products
In that diagram, the value of each flow and the exergy loss matches the width of the strip that characterizes this flow and loss of exergy. Thus, using a diagrammatic representation, it is possible to indicate not only their value but also their distribution inside the system.
In determining the efficiency of power plants, especially for small-scale power plants, it is necessary that the main performance indicators are reflected in the comprehensive nature of the processing of the solid fuels.
This scheme can be improved in the following ways. Thus, a fraction of the heat of combustion products can be sent to the mill for heating the drying agent; the coarse and fine fractions of coal after the cyclone and filter can be sent back to the gas generator; and, in addition, the production of sulfur on the low-capacity power plant can be produced by other products: slag and activated carbon.
However, in this case, we need the definition of the economic performance of these technologies to provide getting of the profit. The main criteria guiding the choices of the owner of a power plant are the low cost of the energy produced in conjunction with the high-energy efficiency of the plant.
Conclusion
In the work, the analysis of efficiency of the scheme low-capacity power plant with coal gasification and production sulfur as a by-product of using the exergy method was carried out. The basic processes in the scheme were considered; efficiency indicator of low-capacity power plant with the production of by-products was obtained. Exergy efficiency of the scheme was 26 %. That approach allowed to assess comprehensively taking into account the formation of a new surface at crushing of coal and the capability of production by-product. It certainly will enhance the possibility of assessing technological decisions for low-capacity power plant and their comparative analysis.
References
Afanasyeva, O., & Mingaleeva, G. (2009). Exergy efficiency of small coal-fired power plants as a criterion of their wide applicability. Solid Fuel Chemistry, 43(1), 55–59.
Afanasyeva, O. V., & Mingaleeva, G. R. (2011). Thermo-economic efficiency of low capacity coal-based power plants. International journal of Exergy, 8(2), 175–193.
Badami, M., & Mura, M. (2010). Exergetic analysis of an innovative small scale combined cycle cogeneration system. Energy, 35, 2535–2543.
Beloselsky, B.S. (2005) Technology of fuel and energy oils: Textbook for universities. - 2nd ed. rev. and add. - M.: Publishing MEI.
Blohin, A., Karev, A., Keneman, F., & Stelmah, G. (2005). Coal mini-thermal power plants with intracyclic pyrolysis of fuel. Electric Power Stations, 7, 25–32.
BMWi (2014) Second Monitoring Report Energy of the future. Summary, from: http://www.bmwi.de/English/Redaktion/Pdf/zweiter-monitoring-bericht-energie-der-zukunft-kurzfassung,property=pdf,bereich=bmwi2012,sprache=en,rwb=true.pdf.
Cau, G., Cocco, D., & Serra, F. (2012). Energy and cost analysis of small-size integrated coal gasification and syngas storage power plant. Energy Conversion and Management, 56, 121–129.
Churashev, V.N., Markov, V.M. (2011) Coal in the XXI century: from the dark past to a bright future. Russian energy, 4.
Dincer, I., & Rosen, M. A. (2007). Exergy: energy, environment and sustainable development. Oxford: Elsevier.
Eisermann, W. J., & Conger, P. W. L. (1980). Estimating thermodynamic properties of coal, char, tar and ash. Fuel Processing Technology, 3, 39–53.
Energy Strategy of Russia until 2030 (2010) App. to journal “Energy Policy”. Moscow: SI IES.
Filippov, S. (2009). Small power in Russia. Heat and Power Engineering, 8, 38–44.
Goihrah, I. M, Piniagin, N.B. (1954) Chemistry and technology of synthetic liquid fuel.
Higman, C., & van der Burgh, M. (2003). Gasification. USA: Elsevier Science.
IEA (2007) The United States Energy 2007 review. Policies of IEA Countries, from: http://www.iea.org/publications/freepublications/publication/us2007.pdf.
IEA (2011) Denmark 2011 review. Energy police of IEA countries, from: http://www.iea.org/publications/freepublications/publication/Denmark2011_unsecured.pdf.
IEA (2013) Medium Term Coal Market Report 2013, from: http://www.iea.org/newsroomandevents/speeches/131216_MCMR13_launch_presentation_Sadamori.pdf.
Kanoglu, M., & Dincer, I. (2009). Performance assessment of cogeneration plants. Energy Conversion and Management, 50, 76–81.
Kantorovich, L. V. (1958). Fundamentals of the theory of combustion and gasification of solid fuels. USSR: Publishers of Academy of Sciences.
Kotler, B. (2004). Mini-thermal power plant: global experience. Aqua-Thermal, 6, 34–37.
Liao, C., Ertesvag, I. S., & Zhao, J. (2013). Energetic and exergetic efficiencies of coal-fired CHP (combined heat and power) plants used in district heating systems in China. Energy, 57, 671–681.
Novoselova, О.А. (2013) Small distributed power—a new paradigm in the power. VIII professional forum Russian energy traders, from: http://www.myshared.ru/slide/483879/.
Ozkan, D., Kiziler, O., & Bilge, D. (2012). Exergy analysis of a cogeneration plant. Engineering and Technology, 61, 774–778.
Rebinder, P. A. (1979). Surface effect in disperse system. Moscow: Nauka.
Rosen, M. A. (2001). Energy- and exergy-based comparison of coal-fired and nuclear steam power plants. Exergy, 1, 180–192.
Shabbar, S., & Janajreh, I. (2013). Thermodynamic equilibrium analysis of coal gasification using Gibbs energy minimization method. Energy Conversion and Management, 65, 755–763.
Shamsutdinov, E.V., Mingaleeva, G.R., Afanasieva, O.V., Vandysheva, S.S. (2011) Patent RU no 2408660. The way to maintain a given regime gasification of coal-water slurry.
Stepanov, V. S. (1990). The chemical energy and exergy of substances. Novosibirsk: Nauka.
Szargut, J., & Petela, R. (1967). Egzergia. Warszawa: Wydawnictwo naukowo-techniczne.
Vagin, G. Y., Loskutov, A. B., & Mamonov, A. M. (2005). Autonomous cogeneration plants (LCPP) of modular type. Izvestiya of Russian Academy Engineering Sciences, 15, 280–288.
Acknowledgments
The research was supported by the Ministry of Education and Science of the Russian Federation, the Agreement no 8712 “Development of energy efficient technology independent power supply based on the use of solid fossil fuels with the production of by-products”, grant no SP-1484.2012.1 for the scholarship of the President of the Russian Federation for young scientists and graduate students in 2012–2014, and the grant of Russian Fund for Fundamental Research no 12-08-97055.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afanasyeva, O.V., Mingaleeva, G.R. Comprehensive exergy analysis of the efficiency of a low-capacity power plant with coal gasification and obtaining sulfur. Energy Efficiency 8, 255–265 (2015). https://doi.org/10.1007/s12053-014-9290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12053-014-9290-6