Abstract
The snow and ice in Mt. Yulong offer a unique opportunity to investigate changes in climate and large scale atmospheric circulations over Asia. During February and April 2012, surface snow samples were collected from the Baishui Glacier No. 1 at different altitudes along the eastern slope of Mt. Yulong. Two snowpits were also excavated from Mt. Yulong at altitudes of 4780 and 4730 m a.s.l. in February 2012. The concentrations of inorganic ions were higher at an elevation of 4506 m a.s.l. in the glacier with significant contribution of anthropogenic (mainly NH\(_{4}^{\mathrm {+}}\), SO\(_{4}^{\mathrm {2-}}\), NO\(_{3}^{\mathrm {-}})\) and crustal (mainly Ca 2+) constituents. Concentration of HCOO − in surface snow exhibited large variability, ranging from 0.04 to 6.8 μeq L −1, attributed to dominant contribution from biomass burning emissions. Ion balance (ΔC) and Na +/Cl − calculations indicated an excess of cations (particularly higher Ca 2+ concentrations) and Cl − in snow, considering the sea-salt ratio, respectively. Monsoon season (June–September) ion concentrations in snowpit samples were generally two-fold lower than in other seasons. Principal component analysis was used to identify different sources of ions. Three main factors, accounting for more than 80% of the total variance, were related to different sources, including agricultural activities, biomass burning, and crustal aerosols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ice cores retrieved from the polar ice sheets and high mountain glaciers arguably provide the highest resolution and most direct view of Earth’s paleoatmosphere (e.g., Legrand and Mayewski 1997; Yalcin et al. 2006a). In the last few years, a great deal of research has been devoted in evaluating the impact of anthropogenic pollution on remote areas. The Himalayas and surrounding areas represent an ideal environment for studying the chemistry of remote areas, since they are located far from industrialized zones and also strongly influence the global atmospheric circulation (Marinoni et al. 2001; Kang et al. 2002b, 2007). The glaciochemical record contained in glaciers located within the Himalayan area represent a valuable resource that can be used to document the atmospheric deposition of this region and reconstruct its past climate (Seko and Takahashi 1991; Nijampukar et al. 1993; Kang et al. 1999; Marinoni et al. 2001). High-elevation glaciers are sensitive indicators of climate change and are natural archives of variations in atmospheric circulation processes and ion burdens. Chemical and physical analyses of ice cores or snowpits recovered from carefully selected accumulation zones of Asian glaciers or ice caps not only hold great potential for the development of detailed, high-resolution paleoclimate and environment records (Mayewski et al. 1984; Thompson et al. 1989, 1997, 2000; Yao and Thompson 1992; Yao et al. 1995; Kang et al. 2001a,2001b, 2002a), but also can provide information on changes to atmospheric circulations in response to climatic change and changes in the strength of the summer Indian monsoon (Mayewski et al. 1984, 1986; Qin et al. 2000), as well as the strength of the westerly jet. Snowpit studies assessing the spatial representativeness of summit ice-core records have reported greater spatial variability in snow chemistry for species present as coarse-mode particles than for species found in the accumulation mode or gas phase (e.g., Dibb and Jaffrezo 1997; Li et al. 2011). Snowpit research can also help improve ice-core dating via a multi-proxy approach identifying seasonal variations in glaciochemical species (e.g., Kreutz et al. 1999; Yalcin et al. 2006b).
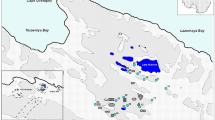
Studies of glaciochemistry in the central Himalayas have been performed, with a key focus on defining the spatial and temporal distribution of snow and ice chemistry to identify different sources of ions in high-elevation areas (Williams et al. 1992; Thamban et al. 2010). These studies concluded that snow chemistry over the Tibetan Plateau was dominated by desert dust from the frequent dust events in the region and transported from the vast arid regions of central Asia by the prevailing winds (Williams et al. 1992; Liu et al. 2010). The meteorological and pluviometric regime of the Asian continent is mainly controlled by polar air masses from the Arctic, continental air masses from central Asia, and maritime air masses from the Pacific and Indian Ocean (Bryson 1986; Kang et al. 2004). The location of the Baishui Glacier No. 1 on the eastern slope of Mt. Yulong (27\(^{\circ }10^{\prime }-27^{\circ }40^{\prime }\)N; 100\(^{\circ }07^{\prime }-100^{\circ }10^{\prime }\)E) (figure 1) at the boundary of the South Asia monsoon (Indian monsoon) and the continental climate of central Asia, combined with high elevation of the site (the highest elevation of Mt. Yulong is 5596 m a.s.l.), offers a unique opportunity to better understand changes in climate and aerosol composition of large-scale atmospheric circulations over Asia.
In this study, we present chemical data of surface snow, ice, and snowpits samples collected on the eastern slope of Mt. Yulong, China, during February–April, 2012. The main purpose of the present work is to expand the snow chemistry dataset for the high mountainous regions of Mt. Yulong, and to improve our understanding of snow chemistry by investigating its variations with elevation. In particular, we focus on the seasonal differences of snow chemistry reconstructed from the snowpits formed over recent years.
2 Sampling methods
Fresh snow samples were collected immediately after a major snowfall event along the eastern slope of Mt. Yulong in March, 2012. Surface snow samples were collected in April, 2012. We selected the sample sites on the flat area of Baishui Glacier No. 1 (figure 1) with the elevation range between 4436 and 4798 m a.s.l.
In addition, in February 2012, two snowpits were excavated on Baishui Glacier No. 1. The snowpit samples were collected continuously at 10 cm depth intervals, at elevations 4780 (Snowpit 1) and 4730 m a.s.l. (Snowpit 2). Maximum snow depths of the two snowpits are 3.4 m (Snowpit 1) and 2.9 m (Snowpit 2) respectively. The snowpit samples were collected from the bottom to the top. The sampling methods for both the surface and snowpit samples were based on Mayewski et al. (1986), Wake et al. (1993, 1994), Qin et al. (2002) and Kang et al. (2000, 2002b, 2004).
During snowpit excavation, we recorded the snow physical properties of the snowpits associated with snow accumulation and dust deposition (figure 2). The mean snow density is 0.44 g cm −3 for snowpit samples. Snow from the snowpits were divided into fresh snow, fine firn (grain size d< 1 mm), medium firn (grain size: 1 <d< 2 mm), coarse firn (grain size d> 2 mm), and ice pieces (Paterson 1981; Hou and Qin 1999). The field records of the excavated snowpits show clear boundary marks in the snowpits. These different snow types (including dusts or impurities) comprise the entire profile of each snowpit. The impurities deposited from the atmosphere were documented from the snow cover in time sequence. They can significantly accelerate glacier melt.
Extreme care and stringent sampling protocols were used at all times during sample collection and handling to assure samples were not contaminated at the μeq L −1 level. All samples were collected wearing clean suits, polyethylene gloves, and masks at all times during sampling campaign. The polyethylene bottles used to load the snow samples were pre-cleaned with ultrapure water in the laboratory, and the small shovels were carefully disinfected before their use in sampling. A total of 190 surface and snowpit snow samples were collected for major ion analyses. Stable oxygen isotope and deuterium were analyzed by a Finnigan Delta-plus mass spectrometer (accuracy ± 0.05%). The cations (Na +, K +, NH\(_{4}^{\mathrm {+}}\), Ca 2+, Mg 2+) and anions (F −, HCOO −, SO\(_{4}^{\mathrm {2-}}\), Cl −, NO\(_{3}^{\mathrm {-}}\), NO\(_{2}^{\mathrm {-}})\) were analyzed on a Dionex 300 and a Dionex 600 ion chromatograph, respectively. Each sample was analyzed twice and results were averaged, yielding an estimated error of 8% or less on ion concentrations.
Analyses of snow and blank samples demonstrate that sample contamination during sample transfer, transport, and subsequent analytical procedures are negligible (Kang et al. 2004; Niu et al. 2013). Especially, the concentrations of NH\(_{4}^{\mathrm {+}}\) and NO\(_{2}^{\mathrm {-}}\) are accurate through our multiple measurements, though they are quite difficult to measure because of contamination problems. Snow samples were packed into insulated and cleaned polyethylene bottles and transported frozen into a −15 ∘C chamber in the laboratory at Lanzhou until measurements were performed in a class 100 clean room.
3 Results
3.1 Surface snow chemical compositions
Table 1 presents surface snow chemical concentrations of major ions. Of the detected 11 ions, the highest ion concentration was of Ca 2+, followed by NH\(_{4}^{\mathrm {+}}\) and SO\(_{4}^{\mathrm {2-}}\), while the lowest ion concentration was of NO\(_{2}^{\mathrm {-}}\). The ΔC (sum of the cation ion concentrations minus anion concentrations) has been reported to reflect the CO\(_{3}^{2}\)/HCO\(_{3}^{\mathrm {-}}\) value (Wake et al. 1992), which is thought to be important during periods with high input of crust-derived alkaline impurities (i.e., CaCO 3 and MgCO 3) (Hansson 1994). Ion balance calculations (ΔC) indicated that there was an excess of cations, and they varied by a huge range of 22.2–47.1 μeq L −1. The large excess of cations in surface snow is due to high Ca 2+ concentrations, which are related to the abundant/extensive distribution of limestone around Mt. Yulong (Zhang et al. 2010; Niu et al. 2014). The large excess of Ca 2+ in the surface snow was balanced by the alkalinity anions (HCO\(_{3}^{\mathrm {-}}\) and CO\(_{3}^{\mathrm {2-}})\) (Mitamura et al. 2003; Murakami et al. 2007), and the deficiency of anions may be attributed to a carbonate concentration (because of its unstable features and easy reaction with CO 2 in the atmosphere, we generally do not directly measure HCO\(_{3}^{\mathrm {-}}\)/CO\(_{3}^{\mathrm {2-}}\) ions, and in this case the concentrations of alkalinity anions were calculated from the ion balance). The calculated results indicated that the concentration of CO\(_{3}^{\mathrm {2-}}\) is 35.1 μeq L −1 on average in the surface snow. Particularly, when few rainfall events occur during the dry season, more dust can be brought to the air by the prevailing winds, thus enhancing the concentration of snow Ca 2+.
The Na + /Cl − ratios displayed an absolute excess of Cl − concentration in comparison with the Na + or standard sea-salt ratio (0.86) (table 1). The excess Cl − indicates an additional source, which could be due to enrichment of Cl − in the snow from the precipitation scavenging of gas-phase HCl in the atmosphere (Legrand and Delmas 1988; Toom-Sauntry and Barrie 2002; Niu et al. 2013). From the perspective of ionic concentrations, there were large variations of HCOO − concentrations over higher altitudes, with the maximum value 6.8 μeq L −1 and minimum levels below the detection limit of the Dionex 600 ion chromatograph. Biomass burning is likely to be the main contributor to these distinct variations. Total variations of snow chemistry with elevation (figure 3) showed that ion concentrations displayed a slight unimodal type over different elevations on the eastern slope of Mt. Yulong.
3.2 Snowpits chemical compositions
Major ion concentrations versus depth for the two snowpits are presented in figures 4 and 5. Snowpit 1 taken from elevation of 4780 m a.s.l. showed distinct seasonal variations of major ions. Ion concentrations were relatively high from the top to a depth of 1.2 m, but lower from 1.2 to 3.0 m, and higher once more from 3.0 m to the bottom of the pit (figure 4). Previous glaciochemical studies of the snow and ice in the Himalayan regions have revealed that the concentrations of major ions (e.g., Ca 2+ and Mg 2+) in the monsoon season (June–September) are generally lower than those in other months, presenting striking seasonal variations (Shrestha et al. 2000; Kang et al. 2004; Liu et al. 2010; Niu et al. 2013, 2014). Lower ion concentrations from 1.2 m to a depth of 3.0 m represented monsoon snow. Thus, we divided Snowpit 1 into three seasons marked by coloured areas (figure 4). It is clear that ion concentrations in non-monsoon are much higher than those in monsoon season (figure 4). However, there are striking ion peaks from 1.7 to 2.0 m and from 2.4 to 2.8 m for Ca 2+ and Mg 2+ respectively, during the monsoon season. This may reflect heavy monsoon precipitation events with high coarse ion loadings from local or regional crustal aerosol inputs related to monsoon circulation (Zhang et al. 2002; Kang et al. 2002a; Liu et al. 2010; Niu et al. 2013). Higher ion concentrations in non-monsoon seasons are due to heavy ion loadings/deposition and less washed out by precipitation events.
Similar methods were applied to identify the monsoon and non-monsoon snow layers for Snowpit 2. High ion concentrations occurring in the upper 1.9 m snow represent non-monsoon season, and lower concentrations from a depth of 1.9 m to the bottom of the pit represent monsoon season snow (figure 5). The physical records indicate that distinct dust layers are present within the upper 1.90 m snow (figure 2). The striking spikes of Ca 2+ concentrations appear in non-monsoon snow layers with values as high as 187.6 μeq L −1. Detailed examination reveals that Na + and Cl − have more similar spikes (figure 5), as do NH\(_{4}^{\mathrm {+}}\), K +, NO\(_{3}^{\mathrm {-}}\) and SO\(_{4}^{\mathrm {2-}}\), reflecting close source region or transport way for both species (Kang et al. 2004) or similar contributors to these species. The concentrations of major ions show general high values (or value peaks) during the non-monsoon season in snow layers corresponding to the spring and/or winter dust deposition. In addition, a lack of precipitation in the non-monsoon season has been associated with a gradual build up of pollutants (Shrestha et al. 2000). Seasonal information of the snow chemistry can also be confirmed from the variations of isotope and deuterium concentrations in the snowpit samples (figure 6). It is clear that there are distinct differences in both the isotope and deuterium concentrations at different depths of the snowpits. The higher values of these two indicators of snowpit chemistry correspond to the non-monsoon seasons, and lower values indicate monsoon seasons, which supports our previous definitions (figures 4 and 5). We calculated the mean chemical composition of monsoon and non-monsoon snow from the two snowpits (figure 7). Both cations and anions show higher concentrations in the non-monsoon season than in the monsoon season. Of the detected eight ions, the highest ion concentration was Ca 2+ in both the monsoon and non-monsoon seasons, followed by SO\(_{4}^{\mathrm {2- } }\) and NO\(_{3}^{\mathrm {-}}\), while Na + had the lowest concentrations (with concentrations 3.4 and 0.8 μeq L −1 in non-monsoon and monsoon seasons, respectively). Non-monsoon Ca 2+ concentration is one order of magnitude higher than that of monsoon Ca 2+ (figure 7), consistent with the surface snow samples.
In contrast to results from the East Ruongbuk Glacier (Kang et al. 2004), there is a striking seasonal variation in snowpit SO\(_{4}^{\mathrm {2-}}\) shown in the snow samples collected from Mt. Yulong, with non-monsoon season snow SO\(_{4}^{\mathrm {2-}}\) concentration more than two fold higher than that of the monsoon SO\(_{4}^{\mathrm {2-}}\). Similarly, non-monsoon Na +, NH\(_{4}^{\mathrm {+}}\), NO\(_{3}^{\mathrm {-}}\), Cl − concentrations from snowpits are nearly two times higher than monsoon values (figure 7), which agrees well with surface snow samples. In addition, non-monsoon Mg 2+ concentrations (6.6 μeq L −1 on average) are also nearly two fold higher than the monsoon values (3.7 μeq L −1) in snowpit samples. This result supports the conclusions that the highest values of ion concentrations in snow occur in spring (Wake et al. 1993; Kang et al. 2000), resulting from dust deposition during the peak in the dust-storm activity–mainly April and May over Asia (Parrington et al. 1983; Gao et al. 1992; Qian et al. 1997).
The mean ion concentrations of snowpit samples collected from the Baishui Glacier No. 1 on the eastern slope of Mt. Yulong are summarized in table 2. Despite the different sampling dates and snowpit depths, as well as the different altitudes of the two snowpits, major ion concentrations from the two snowpits are consistent. A key feature of snowpit chemistry is that Ca 2+ concentrations are much higher than other ions (cf. Kang et al. 2004). In addition, snowpit ions balance (C) and Na +/Cl − ratios are in accordance with the results of surface snow samples.
Table 2 also summarizes mean ion concentrations, ion balance and Na +/Cl − ratio for snowpit, surface/fresh snow, and ice samples. The average total ionic burden (total cations plus total anions) ranged from 86.6 to 172.7 μeq L −1 and averaged 139.7 μeq L −1, which suggests a relatively high ion loading in snow/ice of this region, compared with the other areas. The mean value of ion balance ΔC (114.1 μeq L −1) indicates that there is an absolute excess of cations, mainly due to higher concentrations of Ca 2+ in snow and ice samples. Higher Ca 2+ and Mg 2+ probably originated from natural sources (soil and dust) and contributed to the neutralization reactions that occur in the rainfall (Saxena et al. 1996; Migliavacca et al. 2004) or precipitation. With respect to sea-salt ratio (0.86), the ratio of Na +/Cl − of surface snow is very low, with a mean value of 0.61 (table 1), whereas the ratio of Na +/Cl − is relatively high (1.8) in ice samples.
4 Discussion
Many of the observed dust layers in the snowpit profiles were the result of dust particles or impurities, deposited and accumulated in the snow during the dry season (i.e., winter and spring seasons). This resulted in more micro-particles (crustal or mineral particles) deposited in snow cover in the form of dry deposition. Frequent storm events in winter and spring can enrich and enhance the amount of dust deposited in the snow cover. Therefore, snow cover contains a substantial amount of climate information. Snow layers containing dust or impurities (4 in Snowpit 1 and 3 in Snowpit 2) probably indicate that they occurred during similar seasons with nearly identical climatic features (figure 2).
The interesting unimodal phenomenon (figure 3) can be explained by the pollutants introduced by climbers or tourists to the glacier. The upper ropeway station where tourists gather to enjoy the glacial scenery is at around 4506 m a.s.l., and may be related to the single concentration peak of major ions observed in surface snow at this elevation.
In addition, strong winds occur during spring and winter over the high elevation regions of Mt. Yulong, which could redistribute the surface snow and move crustal aerosols short distances from nearby rock areas (bare rock areas) onto the snow. Thus, surface snow redistribution and local crustal aerosol inputs may change the vertical profile of chemical species in the free atmosphere (Warneck 1988; Kang et al. 2007). It is plausible that wind re-distribution resulted in single modal pattern distribution of ionic concentrations over elevations within the current studied elevation range. In addition, the elevated Ca 2+ concentrations at higher elevation areas could be influenced by very local inputs of crustal dust, since the study area is close to bare rock. The elevated Ca 2+ concentrations clearly indicated towards the crustal sources, which were deposited and accumulated in the higher regions of Mt. Yulong under the upward transportation of the prevailing atmospheric circulation, while external weak effects on the distribution of Ca 2+ concentration over elevations were masked.
The main sources of major ionic components can be identified by a principal component analysis (PCA) of chemical data (including two snowpits and surface snow samples data). PCA allows a robust assessment of the behaviour of the eleven variants. PCA decomposition or factor loading provides objective representations of multivariate data through the analysis of the covariance structure of its variants (e.g., Meeker et al. 1995; Kang et al. 2002a; Niu et al. 2013; 2014). In this study, the PCA involved eleven initial variables (NH\(_{4}^{\mathrm {+}}\), K +, NO\(_{3}^{\mathrm {-}}\), SO\(_{4}^{\mathrm {2-}}\), Ca 2+, Mg 2+, Cl −, Na +, F −, and HCOO −). Three factors, which explained over 80% of the total variance (F3 only accounting for 10% of the total variance), are presented in table 3. The first factor (F1) loaded with NO\(_{3}^{\mathrm {-}}\), NO\(_{2}^{\mathrm {-}}\), Cl −, NH\(_{4}^{\mathrm {+}}\), and K +, can be attributed to agricultural activities and biomass burning (NH\(_{4}^{\mathrm {+}}\), NO\(_{2}^{\mathrm {-}}\), K +) and it controls the acidity of snow (NO\(_{3}^{\mathrm {-}}\), Cl −). NO\(_{3}^{\mathrm {-}}\) and NH\(_{4}^{\mathrm {+}}\), SO\(_{4}^{\mathrm {2-}}\) and NH\(_{4}^{\mathrm {+}}\) were highly correlated, suggesting that these three ions presented in the accumulation mode as NH 4 NO 3 and (NH 4)2 SO 4 aerosols, which suggested an agricultural pollutant source, arising from fertilizer use in Lijiang city and the surrounding areas. Moreover, most of the K + and some of the NH\(_{4}^{\mathrm {+}}\) originated from biomass burning, since majority of villagers in Lijiang and the surrounding areas use wood and crop straw to heating and cooking. Based on the scatter plot (figure 8) and crustal ratio of K/Na (table 4), it is clear that there is excess Na + concentration in snow cover in terms of K +. The correlation between K + and Na + is [K +] = 0.102 [Na +] + 0.488, r 2= 0.55. The excess Na + may originate from the deposition of Na-rich mineral particles in the snow/ice in Mt. Yulong transported from central Asia (Niu et al. 2014). A previous study also showed that some of the excess Na + may be attributed to the Na +-rich rock dust present within the monsoon circulations over the
study region (Niu et al. 2013). In addition, strong relationships exist between K + and NO\(_{3}^{\mathrm {-}}\), NO\(_{2}^{\mathrm {-}}\) and NH\(_{4}^{\mathrm {+}}\), NO\(_{2}^{\mathrm {-}}\) and NO\(_{3}^{\mathrm {-}}\), for which the correlation coefficients are 0.88 (p< 0.01), 0.58 (p< 0.05), and 0.62 (p< 0.05), respectively. This further confirms the common sources of those ions. Agricultural activities and anthropogenic emissions substantially contributed to the origins of these chemical ions in snow and ice. Moreover, based on the crustal ratio of Ca/Mg shown in table 4, the concentration of Ca 2+ is abundant. Ca 2+ and Mg 2+ concentrations balanced with total alkalinity, are presented in the form of Ca(OH) 2 and Mg(OH) 2, which represents the alkali in snow. The second factor (F2) was loading with F − and HCOO −; the high correlation between them (r=0.79, p< 0.01) indicated similar sources between these two anions and also the contribution of human activities to their deposition in snow and ice. In addition, strong correlation between Ca 2+ and Mg 2+ (r=0.67, p< 0.01), can allow us to relate the third factor (F3) to a substantial continental/crustal contribution. Generally, the Tibetan Plateau is considered one of the key dust source regions for atmospheric dust deposition on glaciers (Fang et al. 2004; Li et al. 2012), especially because of the high frequency of dust storms during the winter–spring non-monsoon seasons. Moreover, the anomalous high values of Ca 2+ and Mg 2+ on the Baishui Glacier, considerably higher than those of other sites, are very likely to be related to strong weathering of the local limestone rocks (Niu et al. 2014).
The main results of the snow chemical composition at Mt. Yulong (table 2) are consistent with results from snowpits and fresh snow over East Rongbuk Glacier on the eastern slope of Mt. Everest (Kang et al. 2004) and fresh snow from the Cho Oyu Range (Balerna et al. 2003), Mt. Logan Massif (Yalcin et al. 2006a), and ice core chemical records from Sentik Glacier (Mayewski et al. 1984) and Dasuopu Glacier (Kang et al. 2000). The excess Cl − can be explained by assuming an enrichment of Cl − in the snow from the scavenging of gas-phase HCl (produced by acidification of sea-salt particles) in the atmosphere (Legrand and Delmas 1988; Toom-Sauntry and Barrie 2002; Niu et al. 2013). This is supported by comparison and interrelation analyses between ion concentrations in snow and aerosol at Yukon, Canada (Yalcin et al. 2006a) and over the Hidden Valley (Shrestha et al. 1997, 2002) and Mt. Qomolangma (Everest) (Ming et al. 2007). Conversely, the differences in Na +/Cl − ratios can also be interpreted by different sources of the Na + and Cl −.
The snow chemistry compositions from the eastern Mt. Yulong are comparable with concentrations of monsoon snow samples collected on both northern and southern slopes of the Himalayas (figure 9). For these areas (e.g., Mt. Qomolangma, Mt. Cho Oyu, the Khumu-Himal region), ion concentrations of pre-monsoon (March–May) (Niu et al. 2013) samples are far higher than those of monsoon samples, especially for NH\(_{4}^{\mathrm {+}}\) and Cl − concentrations. A distinct seasonality of major ions was also evident for the Dasuopu firn core, with maximum concentrations of Ca 2+, Mg 2+, NH\(_{4}^{\mathrm {+}}\), and NO\(_{3}^{\mathrm {-}}\) during the pre-monsoon and minimum concentrations during late summer (Liu et al. 2010). The ion chemistry in the snow collected from both northern and southern slopes of Everest clearly demonstrates seasonal variations and pre-monsoon ion concentrations are higher than those monsoon ones, particularly, each ion in both snow samples displayed distinct seasonal variations. The higher ion concentrations that occurred during the non-monsoon periods were mainly due to dust storm activities (Kang et al. 2010; Zhang et al. 2012, 2015; Niu et al. 2013, 2014) and dry deposition. The lowest values of ion concentrations occur in monsoon/late summer season (Wake et al. 1994; Kang et al. 2000), reflecting not only decreased dust deposition (Wake et al. 1994; Shrestha et al. 1997; Kang et al. 2002b; Liu et al. 2010), but also the effect of increased precipitation (from the southwest and southeast monsoons) washing out aerosols (Kang et al. 2004; Niu et al. 2014). Thus, it is interesting that the seasonality of snow chemistry is a common climatic scenario, important at both the temporal and spatial scales, reflecting large scale atmospheric circulation and intrinsic chemical aerosols deposition conditions.
Various contributions of chemical ions to the total ionic concentrations in snow can be further determined by ternary plot. The ternary plot can reflect digital information image of many geographical elements, which use percentage (%) to show the structural proportion of the part and the whole of a specific geographical object. Three sides denote three different elements in the ternary plot. The position of Ca 2+ nearly at the apex of the cation ternary plot (or accounted for the exactly high percentage of the cation in snow) (figure 10a), reflects that the crust-derived Ca 2+ accounts absolutely high composition of the cation in snow. SO\(_{4}^{\mathrm {2-}}\) and NO\(_{3}^{\mathrm {- } }+\) NO\(_{2}^{\mathrm {- } }\) composited/constituted a great part of the anion composition in snow, which can be deduced from the anion ternary plot (figure 10b). The phenomenon robustly indicates that local human activities or exhaust gas emissions have greatly promoted the S or N-contained atmospheric dust deposition in the study area, though we also cannot exclude the contribution of dust particles from remote transport, from other regions such as South Asia (Cong et al. 2015).
5 Conclusions
Snow chemistry data from snow and ice samples collected on the eastern slope of Mt. Yulong presented in this paper expand the dataset of snow chemistry in the remote and high mountain regions. Ion balance calculations indicated that there was an excess of cations in snow and ice, mainly because of high Ca 2+ concentrations. Ion concentrations display a unimodal pattern over the elevations on the eastern slope, which we attribute to anthropogenic inputs. Both surface and snowpit samples chemistry presents unequivocally higher concentrations of Ca 2+ than other ions mainly because of the presence of limestone around Mt. Yulong. Seasonal differences of ion concentrations were demonstrated in each snowpit. Major ion concentrations from snowpit samples display absolutely higher concentrations during the non-monsoon season than those during the monsoon season. Non-monsoon Ca 2+ concentration is one order of magnitude higher than that of other ions, followed by SO\(_{4}^{\mathrm {2-}}\) concentrations.
The identification of the main sources of major ionic components confirms that observations can be statistically categorized into agricultural activities (mainly NO\(_{3}^{\mathrm {-}}\), NH\(_{4}^{\mathrm {+}}\), and SO\(_{4}^{\mathrm {2-}})\), biomass burning (mainly K +, and some of the NH\(_{4}^{\mathrm {+}})\), and soil sources or local rock weathering (Ca 2+ and Mg 2+), making substantial contribution to the corresponding ions. The observed spatial and seasonal distribution of snow chemistry in Mt. Yulong region arose from the different vapour sources due to atmospheric circulation patterns (the south limb of westerlies and monsoon) and different geographical features.
In conclusion, snow chemistry reveals that Mt. Yulong provides a unique character for the observation of atmospheric and environmental conditions in southwestern China, and is subject to seasonal and elevational variation. Continued studies on snow chemistry with more extensive spatial coverage are necessary to better understand the issues discussed in the present study.
References
Balerna A, Balerna E, Pecci M, Polesello S, Smiraglia C and Valsecchi S 2003 Chemical and radio-chemical composition of fresh snow samples from northern slope of Himalayas (Cho Oyu range, Tibet); Atmos. Environ. 37 (12) 1573–1581.
Bryson R A 1986 Airstream climatology of Asia; In: Proceedings of the International Symposium on the Qinghai-Xizang Plateau and Mountain Meteorology, Am. Meteor. Soc. Boston, MA, pp. 604–617.
Cong Z Y, Kawamura K, Kang S C and Fu P Q 2015 Penetration of biomass-burning emissions from South Asia through the Himalayas: New insights from atmospheric organic acids; Sci. Rep. 5 9580, doi: 10.1038/srep09580.
Dibb J E and Jaffrezo J L 1997 Air-snow exchange investigations at Summit, Greenland: An overview; J. Geophys. Res. 102 (C12) 26,795–26,807.
Fang X, Han Y, Ma J, Song L, Yang S and Zhang X 2004 Dust storms and loess accumulation on the Tibetan Plateau: A case study of dust event on 4 March 2003 in Lhasa; Chinese Sci. Bull. 49 953–960.
Gao Y, Arimoto R, Zhou M Y, Merrill J T and Duce R 1992 Relationships between the dust concentrations over eastern Asia and the remote north Pacific; J. Geophys. Res. 97 (D9) 9867–9872.
Hansson M E 1994 The Renland ice core. A Northern Hemisphere record of aerosol composition over 120,000 years; Tellus 46B 390–418.
Hou S G and Qin D H 1999 The ion elution effect on the main ion profiles of the glacier snowpacks; Sci. Geogr. Sinica 19 (6) 1–8 (in Chinese with English abstract).
Li C L, Kang S C, Zhang Q G and Gao S 2012 Geochemical evidence on the source regions of Tibetan Plateau dusts during non-monsoon period in 2008/09; Atmos. Environ. 59 382–388.
Kang S C, Qin D H, Yao T D, Wake C P and Mayewski P A 1999 Summer monsoon and dust signals recorded in the Dasuopu firn core, central Himalayas; Chinese Sci. Bull 44 2010–2015.
Kang S C, Wake C P, Qin D H, Mayewski P A and Yao Y D 2000 Monsoon and dust signals recorded in the Dasuopu Glacier, Tibetan Plateau; J. Glaciol. 46 (153) 222– 226.
Kang S C, Qin D H, Mayewski P A and Wake C P 2001a Recent 180 years oxalate (C\(_{2}\textit {O}_{4}^{\mathrm {2-}})\) records recovered from the Mount Everest ice core: Some environmental implications; J. Glaciol. 47 (156) 155–156.
Kang S C, Qin D H, Mayewski P A, Wake C P and Ren J W 2001b Climatic and environmental records from the far east Rongbuk ice core, Mt. Qomolangma (Mt. Everest); Episodes 24 (3) 176–181.
Kang S C, Mayewski P A, Qin D H, Yan Y, Hou S G, Zhang D Q, Ren J W and Kruetz K 2002a Glaciochemical records from a Mt. Everest ice core: Relationship to atmospheric circulation over Asia; Atmos. Environ. 36 3351–3361.
Kang S C, Qin D H, Mayewski P A, Sneed S B and Yao T D 2002b Chemical composition of fresh snow on Xixabangma peak, central Himalaya, during the summer monsoon season; J. Glaciol. 48 (161) 337–339.
Kang S C, Mayewski P A, Qin D H, Sneed S R, Ren J W and Zhang D Q 2004 Seasonal differences in snow chemistry from the vicinity of Mt. Everest, central Himalayas; Atmos. Environ. 38 2819–2829.
Kang S C, Zhang Q Q, Kaspari S S, Qin D H, Cong Z Y, Ren J W and Mayewski P A 2007 Spatial and seasonal variations of elements composition in Mt. Everest (Qomolangma) snow/firn; Atmos. Environ. 41 7208–7218.
Kang S C, Zhang Y, Grigholm B, Kaspari S, Qin D H, Ren J W and Mayewski P A 2010 Variability of atmospheric dust loading over the central Tibetan Plateau based on ice core glaciochemistry; Atmos. Environ. 44 2980– 2989.
Kreutz K J et al. 1999 Seasonal variations of glaciochemical, isotopic and stratigraphic properties in Siple Dome (Antarctic) surface snow; Ann. Glaciol. 2 38–44.
Legrand M R and Delmas R J 1988 Formation of HCl in the Antarctic atmosphere; J. Geophys. Res. 93 7153–7168.
Legrand M R and Mayewski P A 1997 Glaciochemistry of polar ice cores: A review; Rev. Geophys. 35 219–243.
Li S Y, Lu X X, He M, Zhou Y, Bei R T, Li L and Ziegler A D 2011 Major element chemistry in the upper Yangtze River: A case study of the Longchuanjiang River; Geomorphology 129 29–42.
Liu Y P, Geng Z X and Hou S G 2010 Spatial and seasonal variation of major ions in Himalayan snow and ice: A source consideration; J. Asian Earth Sci. 37 195– 205.
Mayewski P A, Lyons W B, Ahmad N, Smith G and Pourchet M 1984 Interpretation of the chemical and physical time-series retrieved from Sentik Glacier, Ladakh Himalaya, India; J. Glaciol. 30 66–76.
Mayewski P A, Lyons W B, Spencer M J and Clayton J L 1986 Snow chemistry from Xixabangma Peak, Tibet ; J. Glaciol. 32 542–543.
Marinoni A B, Polesello S B, Smirraglia C and Valssecchi S 2001 Chemical composition of fresh snow samples from the southern slope of Mt. Everest region (Khumbu–Himal region, Nepal); Atmos. Environ. 35 3183–3190.
Meeker L D, Mayewski P A and Bloomfield P 1995 A new approach to glaciochemical time series analysis; In: Ice Core Studies of Biogeochemical Cycles (ed.) Delmas R J, NATOASI Series Springer, Berlin, 130 383–400.
Migliavacca D, Teixeira E C, Pires M and Fachel J 2004 Study of chemical elements in atmospheric precipitation in south Brazil; Atmos. Environ. 38 1641–1656.
Ming J, Zhang D Q, Kang S C and Tian W 2007 Aerosol and fresh snow chemistry in the East Rongbuk Glacier on the northern slope of Mt. Qomolangma (Everest); J. Geophys. Res. 11 (2) D15307, doi: 10.1029/2007JD008618.
Mitamura O, Selke Y and Kondo K 2003 First investigation of ultra-oligotrophic alpine lake Puma Yumco in the pre-Himalaya, China; Limnology 4 167–175.
Murakami T, Terai H and Yoshiyama Y 2007 The second investigation of lake Pum Yum Co located in the southern Tibetan Plateau, China; Limnology 8 331–335.
Nijampukar V N, Sarin M M and Rao D K 1993 Chemical composition of snow and ice from Chota Shigri glacier, central Himalaya; J. Hydrol. 151 19–34.
Niu H W, He Y Q, Zhu G F, Xin H J, Du J K, Pu T and Zhao G Y 2013 Environmental implications of the snow chemistry from Mt. Yulong, southeastern Tibetan Plateau; Quat. Int. 313–314 168–178.
Niu H W, He Y Q, Lu X X, Shen J, Du J K, Zhang T and Pu T 2014 Atmospheric precipitation and chemical composition of Mt. Yulong region, southwestern China; Atmos. Res. 144 195–206.
Qian Z G, He H J, Zhao Z Y and Chen M L 1997 Grading standard and case statistics of dust storm in northwest China; In: Study on the Dust Storm in China (eds) Fang Z et al., China Meteorological Press, Beijing, pp. 1–10 (in Chinese).
Qin D H, Mayewski P A, Wake C P, Kang S C, Ren J, Hou S G, Yao T D, Yang Q, Jing Z and Mi D 2000 Evidence for recent climate change from ice cores in the central Himalayas; Ann. Glaciol. 31 153–158.
Qin D H, Hou S G, Zhang D Q, Ren J W, Kang S C, Mayewski P A and Wake C P 2002 Preliminary results from the chemical records of an 80.4 m ice core from East Rongbuk Glacier, Qomolangma (Everest), Himalaya; Ann. Glaciol. 35 278–284.
Parrington J R, Zoller W H and Aras N K 1983 Asian dust: Seasonal transport to the Hawaiian Islands; Science 220 195–197.
Paterson W S B 1981 The physics of glaciers; Perman, New York.
Saxena A, Kulshrestha U C, Kumar N, Kumari K M and Srivastava S S 1996 Characterization of precipitation at Agra; Atmos. Environ. 30 (20) 3405–3412.
Seko K and Takahashi S 1991 Characteristics of winter precipitation and its effect on glaciers in the Nepal Himalaya; Bull. Glacier Res. 9 9–16.
Shrestha A B, Wake C P and Dibb J E 1997 Chemical composition of aerosol and snow in the high Himalaya during the summer monsoon season; Atmos. Environ. 31 (17) 2815–2826.
Shrestha A B, Wake C P, Dibb J E, Mayewski P and Whitlow S 2000 Seasonal variations in aerosol concentrations and compositions in the Nepal Himalaya; Atmos. Environ. 34 3349–3363.
Shrestha A B, Wake C P, Dibb J E and Whitlow S I 2002 Aerosol and precipitation chemistry at a remote Himalayan site in Nepal; Aerosol Sci. Technol. 36 (4) 441–456.
Thamban M, Laluraj C M, Mahalinganathan K, Redkar B L, Naik S S and Shrivastava P K 2010 Glaciochemistry of surface snow from the Ingrid Christensen Coast, East Antarctica, and its environmental implications; Antarctic Science 22 435–441.
Thompson L G, Mosley-Thompson E, Davis M, Bolzan J, Dai J, Gundestrup N, Yao T D, Wu X and Xie Z C 1989 Holocene-Late Wisconsin Pleistocene climatic ice core records from Qinhai-Tibetan Plateau; Science 246 (4929) 474–477.
Thompson L G, Yao T D, Davis M, Henderson K A, Mosley-Thompson E, Lin P N, Beer J, Synal H A C D and Bolzan J 1997 Tropical climate instability: The last glacial cycle from a Qinghai–Tibetan ice core; Science 276 1821–1825.
Thompson L G, Yao T D, Mosley-Thompson E, Davis M E, Henderson K A and Lin P N 2000 A high-resolution millennial record of the south Asian monsoon from Himalayan ice cores; Science 289 1916–1919.
Toom-Sauntry D and Barrie L 2002 Chemical composition of snowfall in the high Arctic: 1990–1994; Atmos. Environ. 36 2683–2693.
Wake C P, Mayewski P A, Wang P, Yang Q Z, Han J K and Xie Z C 1992 Anthropogenic sulfate and Asian dust signals in snow from Tien Shan, northwest China; Ann. Glaciol. 16 45–52.
Wake C P, Mayewski P A, Xie Z C, Wang P and Li Z 1993 Regional variation of monsoon and desert dust signals record in Asian glaciers; Geophys. Res. Lett. 20 1411–1414.
Wake C P, Dibb J E, Mayewski P A, Xie Z, Li Z, Wang P and Qin D H 1994 The chemical composition of aerosols over eastern Himalaya and Tibetan Plateau during low dust periods; Atmos. Environ. 28 695–704.
Warneck P 1988 Chemistry of the natural atmosphere; In: International Geophysics Series; Academic Press, San Diego, CA.
Williams M W, Tonnesen K A, Melack J M and Yang D 1992 Sources and spatial variation of the chemical composition of snow in the Tien Shan, China; Ann. Glaciol. 16 25–32.
Yalcin K, Wake C P, Dibb J E and Whitlow S I 2006a Relationships between aerosol and snow chemistry at King Col, Mt. Logan Massif, Yukon, Canada; Atmos. Environ. 40 7152–7163.
Yalcin K, Wake C P, Kang S C, Kreutz K J and Whitlow S L 2006b Seasonal and spatial variability in snow chemistry at Eclipse Icefield, Yukon Territory, Canada; Ann. Glaciol. 43 1–9.
Yao T D and Thompson L G 1992 Trends and features of climatic changes in the past 5000 years recorded by the Dunde ice core; Ann. Glaciol. 16 21–24.
Yao T D, Thompson L G, Jiao K, Mosley-Thompson E and Yang Z 1995 Recent warming as recorded in the Qinghai–Tibetan Plateau cryosphere; Ann. Glaciol 21 196–200.
Zhang D Q, Qin D H, Hou S G, Ren J W and Kang S C 2002 Chemical characteristic study of snow and snow pit in Mount Qomolangma region; J. Lanzhou University (Nat. Sci.) 38 (4) 119–124. (in Chinese).
Zhang N N, He Y Q, Theakstone W H and Pang H X 2010 Chemical composition of aerosol and fresh snow and tourism influences at Baishui Glacier No. 1 from the Mt. Yulong, southeastern Tibetan Plateau; J. Earth Sci. 21 (2) 199–209.
Zhang Q G, Huang J, Wang F, Mark L, Xu J Z, Armstrong D, Li C L, Zhang Y L and Kang S C 2012 Mercury distribution and deposition in glacier snow over western China; Environ. Sci. Technol. 46 5404–5413.
Zhang Y L, Kang S C, Zhang Q G, Grigholm B, Kaspari S S, You Q L, Qin D H, Mayewski P A, Cong Z Y, Hang J, Sillanpää M and Chen F 2015 A 500 year atmospheric dust deposition retrieved from a Mt. Geladaindong ice core in the central Tibetan Plateau; Atmos. Res. 166 1–9.
Zhu G F, Pu T, He Y Q, Shi P J and Zhang T 2013 Seasonal variations of major ions in fresh snow at Baishui Glacier No. 1, Yulong Mountain, China; Environ. Earth Sci. 69 1–10.
Acknowledgements
This work was supported by the Innovative Research Group National Natural Science Foundation of China (41121001) and Youth Talent Program of CAREERI (Y551C11001, Y451181001), CAS (KJZD-EW-G03-04), the Foundation from the State Key Laboratory of Cryospheric Sciences (05SS011201), and National Natural Science Foundation (41273010). We would like to thank two anonymous reviewers and Prof. K Krishnamoorthy for their constructive comments which substantially improved the quality of the paper. We would also like to express our appreciation to Prof. Neng-Huei (George) Lin and Dr Robbie Hart for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, H., He, Y., Kang, S. et al. Chemical compositions of snow from Mt. Yulong, southeastern Tibetan Plateau. J Earth Syst Sci 125, 403–416 (2016). https://doi.org/10.1007/s12040-016-0670-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12040-016-0670-5