Abstract
This study reports the chemical composition and isotopic signatures of snow and ice over a Himalayan Glacier in India. An observational campaign was carried out from September 22, 2016, to October 2, 2016, over Satopanth in central Himalaya. The pH value of ice and snow, respectively, was 5.6 ± 0.4 and 5.9 ± 0.35 over the glacier, indicating moderate acidity of the glacier components. Calcium (Ca2+) was the dominant component in snow (35.2%), while sulfate (SO42−) was dominant in ice samples (52.7%). The neutralization factor was estimated to find the extent of neutralization of acidic fractions by basic components. It is found that Ca2+ was the prominent neutralizing factor both in snow and ice over the region. Oxygen and hydrogen isotopic analyses of snow, surface layer ice and debris-covered ice suggest that the moisture source is common for all three components. δD and d-excess values of snow at Satopanth are different than that of those for Chorabari, Dokriani and Tiprabank Glacier, indicating the plausibility of different sources of moisture for these glaciers. Limited observations suggest that the interaction of ice with the debris has no impact on the isotopic signatures of the ice over the region; such non-alteration of isotopic signatures makes the region important for ice core-based paleoclimatic studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The melting and retreat of glaciers due to global warming have been a major issue in the Himalayan region. Even though major portion of the glaciers in Himalaya are retreating, some portion are remaining stationary or advancing [3, 27, 39]. The dust particles present over glaciers have significant effect in controlling the glacier retreat. It is shown that the high loading of dust works as an insulator and moderates solar heating of the underlying surface [9]. The chemical composition of snow and ice over glaciers indicates the essential minerals and salts present in the glacier, which can account for the climatic and environmental changes [4]. The ionic deposition over glaciers are mainly contributed by circulation patterns and activities in the local environments [24]. Many glaciers in central Himalaya are featured with the covering of soil and stone debris over the ice surface. The climate responses of debris-covered glaciers are poorly understood [3]. It is suggested that debris-covered glaciers can withstand glacier retreat by 45–50% more as compared to the bare glaciers [3]. Since the last century, a number of attempts have been made by several investigators to study the various glaciological aspects of the Himalayan Glaciers (e.g., [6, 12, 17, 20, 23, 27,28,29], etc). However studies on ionic composition of snow/ice observations are limited, as most of the studies are oriented in the geological perspective over central Himalaya. Hence a campaign mode study was conducted over debris-covered Satopanth Glacier during September 22, 2016, to October 2, 2016, in central Himalaya to cover the chemical aspects. The exploration was mainly focused on the measurements of aerosols and chemical composition of ice and snow over the glaciers. Isotopic analysis also has been carried out with the collected samples of ice and snow over the region. Stable isotopes of water are widely used in understanding the hydrological and paleoclimatic features (e.g., [36, 37]). Stable isotopic composition of ice/snow mainly depends on precipitation isotopic composition and gets modified with the base temperature of glacier. Further melting, percolation, snow drifting, evaporation and sublimation also alter the isotopic composition of ice. Despite such complications, δD and δ18O contents of ice represent the climatic history of the region [2]. In the Indian Himalayan region, no altitude effect was observed in δ18O of fresh snow collected from Changme-Khangpu, Nehnar, Chhota Shigri Glaciers [30, 31, 33]. It is reported that the glacial melt contribution from Gangotri Glacier at Devprayag is 11% [17]. The recent isotopic studies, reported dominance of westerly and monsoon sources during winter and summer, respectively, in the Chorabari and Dokriani Glaciers [21, 43]. Still, stable isotopic studies for the Himalayan Glaciers are limited [13, 31, 32, 34, 42]. This paper exclusively presents the chemical composition of snow and ice and its isotopic signatures over Satopanth Glacier. The sample collection and data are explained in Sect. 2. Results in detail are discussed in Sect. 3 and summary is provided in Sect. 4.
2 Sample collection and data
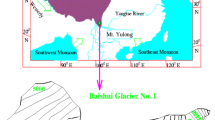
The observations were carried out over Satopanth Glacier (30.7456°N, 79.3437°E; 3858 m ASL) in central Himalayas. Major portion of the Satopanth Glaciers are debris covered. The glacier is approximately 13 km long and 750 m wide. River Alaknanda, which is one of the major streams of river Ganges, originates from Satopanth Glacier. The location map of the observation site is shown in Fig. 1. The meteorological features of different central Himalayan Glaciers are described elsewhere [22]. The monsoonal time mean temperatures were found to range between 2.5 and 9.5 °C in different glaciers over the region. While maximum temperature was up to 14.7 °C, minimum temperatures ranged between − 3.4 and 6.1 °C. The relative humidity varied between 68 and 91% during monsoon seasons, and the wind speed was 2.3 to 10 km/h over the region. Thermally driven valley wind system was found to influence the intensity of winds and transport of pollutants over the region [22]. The sample collection of ice and snow was carried out on September 24 and 26 and October 1, 2016, in three different locations over the glacier. Due to the adverse weather conditions and time constraints, sample collection was possible only in these 3 days and could not separate fresh and old snow in the samples. Further, regarding isotopic data, this is a preliminary study for the Satopanth Glacier and showing limited isotopic data for a very short time period, collected randomly at glaciated region with the aim of exploring the region for paleoclimatic and moisture source studies. pH of the samples were measured using a pH meter (Eigenbrodt Gmbh Inc.). The details of pH meter are available elsewhere [38]. We have used Metrohm 850 professional ion chromatography (IC) system for the ion analysis. A sample processor is used to introduce samples for inline sample preparation. After the preparation of sample, the sample solution is introduced through a sample loop on the injector in to the device system. The sample is pumped on to the column by the eluent, and ions in the samples are attracted by stationary phase of the column [40]. The solution then go through the conductivity detector and interprets peak in the chromatogram for respective cations and anions. A detailed description of ion chromatograph and its working principle is available elsewhere [26, 38].
Oxygen and hydrogen isotopic analyses of the snow and ice samples were carried out using a LGR isotopic water analyzer (Model: TIWA-45-EP; [7]). These analyses yielded simultaneous δD and δ18O isotopic data through high-resolution laser spectroscopy that quantifies concentrations of individual isotopologues of H2O and calculates isotopic ratios [16, 25]. Using the LASER absorption property, the stable isotope ratios are measured directly based on the molecular mass dependency of the individual absorption lines. The autosampler is used to inject water sample from the sample vial using 1.2 µL syringe to an injector block (vaporization chamber) heated at 70 °C which is connected to the analyzer. A Teflon tube is connected to the pre-evacuated (using pump) optical cavity. For each sample and calibrated standard, nine injections are measured and average values are reported. The precision of the hydrogen and oxygen isotopic measurements was better than 1.0‰ and 0.1‰, respectively. Accuracy of the analyses was monitored through isotopic measurement of International (USGS-46 and USGS-47) and our in-house lab standard (IITM-A) standards. The conventional δ notations are presented with reference to V-SMOW (Vienna standard ocean water), in this study, the d-excess values for the samples are estimated as (δD-8 × δ18O) [8].
3 Results and discussion
3.1 Chemical composition of snow and ice
The samples of ice and snow were analyzed for chemical composition using an IC as explained in Sect. 2. The average pH of ice samples was found to be 5.6 ± 0.4, and the snow samples showed a pH of 5.9 ± 0.35. This indicates the moderate acidic nature of both snow and ice over the region. However, mild alkalinity was reported over another central Himalayan region [20, 23]. A pH of 6.1 for snow was reported over Antarctica [1]. The chemical composition of ice is shown in Fig. 2. Sulfates were found to be the major contributor in ice samples (52.71%). The respective contribution of available components in ice was SO42− > Ca2+ > K+ > Na+ > Mg2+ > Cl− > F− > NO3− > NO23− > NH4+. The lower pH in ice may be attributed to the higher presence of SO42−. Ca2+ was found to be the second major component in the glacier ice. Ca2+ (31.6%), K+ (5.32%), Na+ (3.79%) and Mg2+ (3.29%) were other major contributors to ice. However, it may be noted that Ca2+ was the major component contributing up to 35.2% of snow samples (Fig. 3). SO42− showed the second highest contribution to snow (13.1%). The respective contribution of chemical components in the snow was Ca2+ > SO42− > Cl− > Na+ > K+ > Mg2+ > NO23− > NO3− > NH4+ > F−. Presence of more Ca2+ might have led to comparatively less acidity of snow compared to ice. The major reason for sulfate contribution was reported to be industrialization [10]. The natural reasons for excessive sulfate in ice could be due to the transported sulfur and its oxidation and to sulfate, which gets deposited by precipitation over the region [41]. Other natural sources of sulfates in the soil debris could be the sulfate bearing minerals like gypsum and oxidation of sulfide mineral like pyrite present over the region [11]. The anthropogenic contribution could be due to the deposition of long range transported sulfate aerosols over the region [44]. As natural sources for sulfates such as marine air mass and vegetation emission hardly contribute over the region, the higher sulfates could be transported from adjacent areas of Mana or Badrinath due to anthropogenic activities such as cooking or vehicular transport. The larger concentration of SO42− and Ca2+ in Himalayan region are attributed due to vehicular traffic and biomass burning over the region [38]. Other reason for sulfate concentration could be explained by aqueous-phase oxidation of SO22− in clouds and prominent gas to particle conversion processes [35]. Na+ (9.09%), K+ (8.87%) and Mg2+ (11.91%) were found to be the other major contributors in snow over the region. Soil is reported as the major sources of the above components [35]. Like sulfates, one major source for excess calcium in snow could be the dust present in debris. It is reported that dust is a major contributor of Ca2+ [35]. Calcium in these areas is mainly of crustal origin and Ca2+ could be found in lime /calcium carbonate, dolomite, etc. of subsiding soil [14, 41]. The mass of sulfates and calcium in ice was almost 10–45 folds higher than that over snow. It suggests that the long time mixing of debris with ice is a more prominent source of the minerals in ice compared to deposits of relatively new snow. Another central Himalayan chemical composition study showed the concentration order as follows Ca2+ > Cl− > Na+ > SO42− > HCO3− > NH4+ > NO3− > Mg2+ > K+ > F− in the fresh snow [20, 23]. Chemical composition data over Chhota Shigri glacier of central Himalayas is well documented [34]. It showed that K+ and Ca2+ have mostly terrestrial sources, but Mg2+ has both marine and terrestrial source connection. It is found that southwest monsoon rain increases weathering of glacial debris and produce more sulfate ions (SO42−) in snow and ice [15].
3.2 Neutralization factors
The lower pH of ice (5.6) and snow (5.9) suggests that the glacier environment is moderately acidic. To reduce the escalation of acidity, basic components plays vital role. To estimate the neutralization of such acidic components such as SO42− and NO3− by basic components, neutralization factors were estimated (mainly for Ca2+, Mg2+ and K+) over Satopanth. Another major alkaline component NH4+ was not considered due to its very meager contribution to the total composition. To avoid the contribution of potential transported less neutralizing sea salt fractions, we calculated
The non-sea salt fraction (sea salt fractions of anions could be transported to the area by monsoon winds and can be deposited on glacier surface). The neutralization factors are estimated according to the methodology suggested elsewhere [19]. Non-sea salt fraction (nss) was computed considering Na as reference component for sea source and using standard seawater ratios [18]. Accordingly, NF values for Ca, Mg and K were calculated as:
The neutralization factors are tabulated in Table 1. It is found that Ca2+ is the prominent neutralizing agent of acidity and keeping the escalation of acidity in limits. The neutralization was found to be of the order Ca2+ > K+ > Mg2+ for both snow and ice. The NF was 0.05 for Mg2+, 0.09 for K+ and 0.6 for Ca2+ in ice. NF, respectively, was 0.795, 0.63 and 2.57 for Mg2+, K+ and Ca2+ in snow. The lower acidity in snow could be attributed due to the higher neutralization by Ca2+. However, comparatively lower NF in ice could be reason for enhanced acidic nature of ice samples.
3.3 Isotopic analysis
The isotopic analysis of the samples has been carried out as described in Sect. 2 for surface layer ice, debris-covered ice and also for snow samples. As stated earlier, this is a preliminary study with limited isotopic data for a very short time period collected randomly at glaciated region. Here, we present δD and δ18O data (Table 2) for fresh snow, accumulated surface ice and ice from debris collected over the Satopanth Glacier. Surface ice isotopic values δ18O (δD) − 14.5‰ (− 94.2‰) from the Satopanth Glacier are very close to that of reported [δ18O (δD) − 13.51‰ (− 91.2‰)] for similar altitude 3905 m a.s.l. of Tiprabank Glacier [34]. However, different d-excess values (21.4 for Satopanth Glacier versus 16.9 for Tiprabank Glacier) indicate the different moisture sources at both the glaciers. It is shown that the westerlies are the main source of moisture of snow in Chorabari and Dokriani Glaciers during post-monsoon and winter [21, 43]. δ18O (δD and d-excess) values of the present study are similar to that of snow collected during September–October from Chorabari and Dokriani Glaciers, suggest the similar source of moisture, i.e., westerlies at Satopanth as well. The surface ice isotopic signatures are slightly depleted with respect to the fresh snow at the Satopanth Glacier. The depletion in the surface ice indicates slightly cooler temperature conditions during the time of accumulation of ice. However, similar d-excess values of snow, ice and ice from debris suggest that the moisture source is common for all. Evaporation and sublimation processes are plausible reasons of the enriched isotopic values of ice collected from debris. However, no alteration of d-excess suggests that the interaction of ice with the debris has no impact on the isotopic signatures of the ice. Presence of carbonate minerals influence isotopic values of ice by interacting ice through physical–chemical reactions and hence change isotopic signature of accumulated ice. However, similar isotopic values of surface ice and ice associated with debris do not alter isotopic values of surface ice. This is because the region is dominated by granite-granodiorite and low in carbonate [5]. Further it could also be concluded that evaporative effect is minimum once ice gets accumulated in the region as the region remains under very low temperature throughout the year. Such non-alteration of isotopic signals in snow and ice of the Satopanth Glacier shows the importance of the region for paleoclimatic studies related to past temperature reconstructions, variability in moisture sources, glacier moments, etc.
4 Summary and conclusion
A campaign was carried out over Satopanth Glacier in Himalaya during September 22–October 3, 2016, periods to collect snow/ice samples for its chemical characterization and also to analyze its isotopic signatures. The pH of glacier ice was found to be 5.6 ± 0.4, and deposited snow was found to be 5.9 ± 0.35 over Satopanth Glacier. This indicates toward more acidity in ice. The SO42− was the major contributor to ice (52.7%), whereas Ca2+ dominated in snow (35.2%) over Satopanth Glacier. Debris cover over glacier could be major source of calcium than long range transport of the minerals. Estimates of neutralization factors revealed that Ca2+ component is able to neutralize and keep the acidity of ice and snow under check over the region. Oxygen and hydrogen isotopic analyses of snow, surface layer ice and debris-covered ice suggest that the moisture source is common for all three components in the glacier. It is also identified that the interaction of ice with the debris has no impact on the isotopic signatures of the ice. δD and d-excess values of snow at Satopanth are different than that of those for Chorabari, Dokriani Glaciers and Tiprabank Glacier, which indicates the plausibility of different sources of moisture for these glaciers.
The observations over Satopanth Glacier suggest that the snow samples present over the glacier are less acidic compared to ice plausibly due to more neutralization of acidic fraction by dominance of Ca2+ in snow. Limited observations over the glacier suggest that the interaction of ice with the debris has no impact on the isotopic signatures of the ice over the region such non-alteration of isotopic signatures makes the region important for ice core-based paleoclimatic studies. Hence, more exploration is necessary in Satopanth Glacier to unravel its changes in chemical and paleoclimatic aspects in the context of climate change.
References
Ali K, Budhavant KB, Safai PD, Rao PSP (2012) Seasonal factors influencing in chemical composition of total suspended particles at pune, India. Sci Total Environ 414:257–267. https://doi.org/10.1016/j.scitotenv.2011.09.011
Arnason B (1969) The exchange of hydrogen isotopes between ice and water in temperate glaciers. Earth Planet Sci Lett 6(6):423–430
Banerjee A, Shankar R (2013) On the response of Himalayan glaciers to climate change. J Glaciol 59:480–490. https://doi.org/10.3189/2013JoG12J130
Barrie LA (1985) Atmospheric particles : their physical and chemical characteristics and deposition processes relevant to the chemical composition of glacier. Ann Glaciol 7:100–108
Bsssi UK (2004) Geology of the Gangothri-Gaumukh area. In: Srivastava D, Gupta KR, Mukerji S (ed) Geological survey of India, Spl. Publ, Uttarkashi District, Uttaranchal, 80, pp 235–239
Bose RN, Dutta NP, Lahiri SM (2017) Refraction seismic investigation at Zemu Glacier, Sikkim. J Glaciol 10:113–119. https://doi.org/10.1017/s0022143000013046
Chakraborty S, Sinha N, Chattopadhyay R, Sengupta S, Mohan PM, Datye A (2016) Atmospheric controls on the precipitation isotopes over the Andaman Islands, Bay of Bengal. Sci Rep 6:1–11. https://doi.org/10.1038/srep19555
Dansgaard W et al (1973) Stable isotope glaciology. In: Dansgaard W, Johnsen SJ, Clausen HB, Gundestrup N (eds) Meddelelser om Grenland, Bd. 197, Nr. 2
Dragosics M, Meinander O, Jónsdóttír T, Dürig T, De Leeuw G, Pálsson F, Dagsson-Waldhauserová P, Thorsteinsson T (2016) Insulation effects of Icelandic dust and volcanic ash on snow and ice. Arab J Geosci 9:1–10. https://doi.org/10.1007/s12517-015-2224-6
Duan K, Thompson LG, Yao T, Davis ME, Mosley-Thompson E (2007) A 1000 year history of atmospheric sulfate concentrations in southern Asia as recorded by a Himalayan ice core. Geophys Res Lett 34:10–13. https://doi.org/10.1029/2006GL027456
Fennell J, Bentley LR (1998) Distribution of sulfate and organic carbon in a prairie till setting: natural versus industrial sources. Water Resour Res 34:1781–1794. https://doi.org/10.1029/98WR00827
Ganjoo RK, Koul MN (2009) Is the Siachen glacier melting? Curr Sci 97:309–310. https://doi.org/10.2307/24104556
Grabczak J, Niewodnicza J, Różański K (1983) Isotope stratification in high mountain glaciers: examples from the Peruvian Andes and Himalaya. J Glacial 29(103):417–424
Gupta GP, Kumar B (2015) Snow chemistry at Mukteshwar in Central Himalayan region of India. Mod Chem Appl. https://doi.org/10.4172/2329-6798.1000160
Hasnain SI, Thayyen RJ (1999) Controls on the major-ion chemistry of the Dokriani glacier meltwaters, Ganga basin, Garhwal Himalaya, India. J Glaciol 45(149):87–92
Iannone RQ, Romanini D, Cattani O, Meijer HAJ, Kerstel ERT (2010) Water isotope ratio (δ 2 H and δ 18 O) measurements in atmospheric moisture using an optical feedback cavity enhanced absorption laser spectrometer. J Geophys Res 115:D10111. https://doi.org/10.1029/2009JD012895
Khan AA, Pant NC, Sarkar A, Tandon SK, Thamban M, Mahalinganathan K (2017) The Himalayan cryosphere: a critical assessment and evaluation of glacial melt fraction in the Bhagirathi basin. Geosci Front 8:107–115. https://doi.org/10.1016/j.gsf.2015.12.009
Khemani LT (1989) Physical and chemical characteristics of atmospheric aerosols. In: Air pollution control, vol 2. Gulf Publ. Co., Encyclopedia of Environmental Control Technology, pp 401–452
Kulshrestha UC, Kulshrestha MJ, Sekar R, Sastry GSR, Vairamani M (2003) Chemical characteristics of rainwater at an urban site of south-central India. Atmos Environ 37:3019–3026. https://doi.org/10.1016/S1352-2310(03)00266-8
Kumar P, Kotlarski S, Moseley C, Sieck K, Frey H, Stoffel M, Ménégoz M, Krinner G, Balkanski Y, Boucher O, Cozic A, Lim S, Ginot P, Laj P, Gallée H, Wagnon P, Marinoni A, Jacobi HW, Madhulatha A, Rajeevan M, Venkat Ratnam M, Bhate J, Naidu CV, Palazzi E, Von Hardenberg J, Provenzale A, Kumar P, Kotlarski S, Moseley C, Sieck K, Frey H, Stoffel M (2015) Response of Karakoram–Himalayan glaciers to climate variability and climatic change: a regional climate model assessment. J Geophys Res Atmos 118:1–8. https://doi.org/10.1002/2015GL063392
Kumar A, Tiwari SK, Verma A, Gupta AK (2018) Tracing isotopic signatures (δD and δ18O) in precipitation and glacier melt over Chorabari Glacier-Hydroclimatic inferences for the Upper Ganga Basin (UGB), Garhwal Himalaya. J Hydrol Reg Stud 15:68–89
Kumar A, Verma A, Gokhale AA, Bhambri R, Misra A, Sundriyal S, Dobhal DP, Kishore N (2018) Hydrometeorological assessments and suspended sediment delivery from a central Himalayan glacier in the upper Ganga basin. Int J Sedim Res 33:493–509. https://doi.org/10.1016/j.ijsrc.2018.03.004
Kumar B, Gupta GP, Singh S, Lone FA, Kulshrestha UC et al (2015) Snow chemistry at Mukteshwar in Central Himalayan Region of India. Mod Chem Appl 3:160. https://doi.org/10.4172/2329-6798.1000160
Li Z, Li H, Dong Z, Zhang M (2010) Chemical characteristics and environmental significance of fresh snow deposition on Urumqi Glacier No. 1 of Tianshan Mountains, China. Chin Geogr Sci 20:389–397. https://doi.org/10.1007/s11769-010-0412-6
Lis G, Wassenaar LI, Hendry MJ (2008) High-precision laser spectroscopy D/H and 18O measurements of microliter natural water samples. Anal Chem 80:287–293
Metrohm (2009) 850 Professional IC manual 1–146
Nainwal HC, Banerjee A, Shankar R, Semwal P, Sharma T (2015) Shrinkage of Satopanth and Bhagirath Kharak Glaciers, India, from 1936 to 2013. Ann Glaciol 57:131–139. https://doi.org/10.3189/2016aog71a015
Nainwal HC, Chaudhary M, Rana N, Negi BDS, Negi RS, Juyal N, Singhvi AK (2007) Chronology of the late quaternary glaciation around Badrinath (Upper Alaknanda Basin): preliminary observations. Curr Sci 93:90–96
Nainwal HC, Negi BDS, Chaudhary MS, Sajwan KS, Gaurav A (2008) Temporal changes in rate of recession: evidences from Satopanth and Bhagirathi Khark glaciers, Uttarakhand, using total station survey. Curr Sci 97(5):653–660
Nijampurkar VN, Rao DK (1992) Accumulation and past flow rates of ice on Chhota Shigri glacier, Central Himalaya. J Glaciol 38(128):43
Nijampurkar VN, Bhandari N (1984) Oxygen isotopic ratios of some Himalayan glaciers. Tellus 36B:300–302
Nijampurkar VN, Bhandari N, Ramesh R, Bhattacharya SK (1986) Climatic significance of D/H ratios of a temperate glacier in Sikkim. Curr Sci 55(18):910–912
Nijampurkar VN, Rao DK (1992) Ice dynamics and climatic studies on Himalayan glaciers based on stable and radioactive isotopes. Int Symp Snow Glacier Hydrol 218:355–369
Nijampurkar VN, Sarin MM, Rao DK (1993) Chemical composition of snow and ice from Chhota Shigri glacier, Central Himalaya. J Hydrol 151(1):19–34. https://doi.org/10.1016/0022-1694(93)90246-6
Park SH, Panicker AS, Lee DI, Jung WS, Jang SM, Jang M, Kim D, Kim YW, Jeong H (2010) Characterization of chemical properties of atmospheric aerosols over anmyeon (South Korea), a super site under global atmosphere watch. J Atmos Chem 67:71–86. https://doi.org/10.1007/s10874-011-9205-2
Ramesh R, Sarin MM (1992) Stable isotope study of the Ganga (Ganges) river system. J Hydrol 139(1–4):49–62
Rai SP, Thayyen RJ, Purushothaman P, Kumar B (2016) Isotopic characteristics of cryospheric waters in parts of Western Himalayas, India. Environ Earth Sci 75(7):1–9
Safai PD, Rao PSP, Momin GA, Ali K, Tiwari S, Naik MS, Kuniyal JC (2002) Chemical composition of size separated aerosols at two rural locations in the Himalayan region. Indian J Radio Sp Phys 31(5):270–277
Scherler D, Bookhagen B, Strecker MR (2011) Spatially variable response of Himalayan glaciers to climate change affected by debris cover. Nat Geosci 4:156–159. https://doi.org/10.1038/ngeo1068
Scientific T (2011) Sample preparation for ion chromatography
Sharma P, Ramanathan AL, Pottakkal J (2013) Study of solute sources and evolution of hydrogeochemical processes of the Chhota Shigri Glacier meltwaters, Himachal Himalaya, India. Hydrol Sci J 58:1128–1143. https://doi.org/10.1080/02626667.2013.802092
Tiwari SK, Kumar A, Gupta AK, Verma A, Bhambri R, Sundriyal S, Yadav J (2018) Hydrochemistry of meltwater draining from Dokriani glacier during early and late ablation season, West Central Himalaya. Himal Geol 39(1):121–132
Verma A, Kumar A, Gupta AK, Tiwari SK, Bhambri R, Naithani S (2018) Hydroclimatic significance of stable isotopes in precipitation from glaciers of Garhwal Himalaya, Upper Ganga Basin (UGB), India. Hydrol Process 32(12):1874–1893
Zhang X, Xu J, Kang S, Liu Y, Zhang Q (2018) Chemical characterization of long-range transport biomass burning emissions to the Himalayas: insights from high-resolution aerosol mass spectrometry. Atmos Chem Phys 18:4617–4638. https://doi.org/10.5194/acp-18-4617-2018
Acknowledgements
Authors acknowledge Director IITM and Vice chancellor HNBGU for their constant encouragements. IITM is funded by MoES, Govt. of India, New Delhi. Author A.S.Gautam thanks SERB-DST Project for financial support (No. SB/EMEQ-043/2014 dated March 08, 2016). Authors A. S. Panicker and A. S .Gautam acknowledge for the Junior associateship program of the Abdus Salam International centre for theoretical Physics (ICTP), Italy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panicker, A.S., Sandeep, K., Gautam, A.S. et al. Chemical composition and isotopic signatures of ice and snow over a Himalayan Glacier (Satopanth) in India. SN Appl. Sci. 1, 1166 (2019). https://doi.org/10.1007/s42452-019-0966-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0966-6