Abstract
A simple, efficient and environmentally benign protocol has been developed for the synthesis of substituted benzo[4,5]imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one by a reaction of 4-hydroxycoumarin, aldehydes and 2-aminobenzimidazole using silane@TiO2 nanoparticles as heterogeneous catalyst under reflux condition in ethanol. The surface modification of TiO2 nanoparticles was confirmed by using FT-IR, FE-SEM, EDX, XRD and TEM analyses. Furthermore, the stability of the catalyst was evaluated by thermal gravimetric analysis (TGA). Some advantages of this method are high yield of products, short reaction time; recyclability of the catalyst and column chromatography-free protocol. The synthesized compounds were screened for their in vitro antioxidant activity and most of the compounds exhibited remarkable antioxidant activity.

A simple, efficient and environmentally friendly synthesis of substituted benzo[4,5]-imidazo-[1,2-a]-chromeno-[4,3-d]-pyrimidin-6-one is described using silane@TiO2 nanoparticles as heterogeneous catalyst under reflux conditions in ethanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, nano-catalysis has been utilized as an alternative approach for the improvement in rates, yields and workability of many significant organic reactions. This also leads to a search for or design of new efficient affordable catalysts for specific applications in synthetic chemistry.[1] Nanoparticles have large surface area and due to this they get strongly agglomerated and hence their surface modification is an alternative way to produce new hybrid nanoparticles having virtuous properties.[2,3]The covalent attachment of functionalities on to the surface of nanoparticles enhances their newer properties which either increases the interaction or decrease the aggregation of nanoparticles which lead to various purposes to wider applications.[4–7]Nano materials have wide applications in science and technology such as medicinal, cosmetics and environmental purification, etc. Titanium dioxide belongs to the family of transition metal oxides and is considered as an ideal photocatalyst because of its merits such as low cost, nontoxicity, high stability, optical and electronic properties, etc. Furthermore, its band gap is sufficient to initiate a variety of organic reactions.[8,9]
Multi-component reactions are becoming the most significant synthetic tool for synthesis and design of new libraries of molecules of pharmaceutical interest through single step reactions.[10–12]In modern organic synthesis, combination of green chemistry and multi-component reactions will lead to generation of molecules with structural complexity in a single step from three or more reactants with greater efficiency and atom economy.[13–16]Therefore, the design of efficient multi-component reactions offers an enduring challenge for synthetic organic chemists.
In the development of several new drugs, the fused heterocyclic scaffolds with nitrogen and oxygen atoms play an important role in medicinal chemistry. Benzimidazole derivatives are important pharmacophores and considered as privileged structures in medicinal chemistry.[17,18]Benzimidazole derivatives have been reported to possess diverse biological activities such as antihistamine, antitumor, antibacterial, antifungal and antioxidant,[19] etc. Various substituted benzimidazoles are known to have diverse biological activities and among them 2-substituted benzimidazoles are found to be more potent.[20] Furthermore, the chromene[2,3-d]pyrimidine derivatives occupy an important place in the realm of natural and synthetic organic chemistry because of their biological and pharmacological activities such as antioxidant, antimicrobial,[21] antitumor,[22] antitubercular,[23]analgesic,[24] inflammatory,[25] etc.
To the best of our knowledge only Heravi et al., and Mazhukina et al., have reported the synthesis of benzo[4,5]imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one derivatives.[26]However, there are no other reports on the synthesis of these benzo[4,5]imidazo[1,2-a] chromeno[4,3-d]pyrimidin-6-ones. This inspired us to continue our work on the multi-component synthesis under environmentally benign approach. In this article, we wish to report the synthesis of benzo[4,5] imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one derivatives by a reaction of 4-hydroxycoumarin (1) with aromatic aldehydes (2a-q) and 2-aminobenzimidazole (3), in the presence of catalytic amount of silane@TiO2 nanoparticles as an efficient catalyst (scheme 2). All synthesized compounds were screened for their antioxidant activities and it was found that most of the compounds exhibit excellent antioxidant properties.
2 Experimental
2.1 Material and Methods
All chemicals purchased from Sigma-Aldrich and S. D. Fine chemicals Ltd. were used without further purification. All solvents and chemicals obtained from S. D. fine chemicals used were of reagent grade. Melting points of all synthesized compounds were taken in open capillary tubes and are uncorrected. Reactions were monitored by thin-layer chromatography (TLC) on 0.2 mm precoated silica-gel 60 F254 plates (E. Merck). The spots were detected under UV light. The IR spectra (KBr) were recorded on Shimadzu IR Affinity-1 FT-IR spectrophotometer. The 1H NMR and 13C NMR spectra were recorded on a FT-NMR Cryo-magnet Bruker Avance-II spectrometer at 400 and 100 MHz, respectively, using DMSO-d6 as solvent and the chemical shift values are recorded on the δ scale and coupling constant (J) values are in hertz (Hz). Mass spectra were recorded on Waters, Q-Tof Micromass (LCMS) spectrometer. TGA data were obtained with Perkin Elmer instrument at the temperature range of 28–800 ∘C at a constant heating rate of 10 ∘C/min in the nitrogen atmosphere (20 mL/min). X-ray diffractograms (XRD) of the catalyst were recorded in the range of 10–80 ∘ on a Bruker X-ray diffractometer with Ni-filtered Cu K α radiation at a wavelength of 1.54060 Å. The FE-SEM analysis of the catalyst was performed on a Hitachi S-4800 scanning electron microscope (Japan) with accelerating voltage of 30 KV. The EDX analysis of the catalyst was performed by using Bruker EDX flash detector mode 5030. The Transmission Electron Microscopy of the catalyst was performed on PHILIPS CM200 of operating voltages of 20–200 kV, Resolution 2.4 Å. DPPH radical scavenging assay was performed on Shimadzu UV mini-1240 UV-Vis Spectrophotometer.
2.2 Preparation of Silane@TiO2 nanoparticles
The TiO2 nanoparticles were synthesized by sol-gel method[27] and then treated for surface modification. The surface modification of TiO2 was achieved by reacting TiO2 nanoparticles with vinyltriethoxysilane.[28] Typically, TiO2 nanoparticles (1 g) were dispersed in acetone (10 mL) and the mixture was sonicated for 5–6 min. Simultaneously, vinyltriethoxysilane (0.5 mL) solution was prepared in acetone (10 mL) and then added slowly into TiO2 solution under vigorous stirring provided by a high speed disperser and the mixture was stirred for an additional 20 min. After the modification of TiO2, acetone was removed by evaporation. The silane@TiO2 nanoparticles were washed with ethanol three times for the removal of physically adsorbed vinyltriethoxysilane. Finally, the silane@TiO2 powder was dried at 110 ∘C for 2 h in a oven. The schematic representation for the synthesis of silane@TiO2 is summarized in scheme 1.
2.3 General procedure for synthesis of benzo[4,5] imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one
In a 50 mL round-bottom flask, a mixture of 4-hydroxycoumarin 1 (2 mmol, 0.32 g), aldehyde 2a-q (2 mmol), 2-aminobenzimidazole 3 (2 mmol, 0.27 g) and 10 mol% of silane@TiO2 catalyst in ethanol (5 mL) was heated at reflux for specified time (table 3). After completion of the reaction (monitored by TLC), the reaction mixture was filtered and then the solid residue was dissolved in DMF (5 mL), followed by centrifugation of the mixture for 5–10 min at 2000–3000 rpm to remove the catalyst. The organic solution was then poured into cold water (15 mL), filtered, washed with ethanol and dried to afford the pure products. (The residual catalyst was washed with ethanol and dried in oven at 110 ∘C for 2–3 h and reused) (scheme 2).
2.4 DPPH free radical scavenging assay
The scavenging of α, α-diphenyl- β-picrylhydrazyl (DPPH) radical by chemically synthesized seventeen compounds were analysed. One millilitre of 0.2 mM DPPH reagent prepared in methanol were added in tubes containing 0.8 mL of each compound (1 mg mL −1 in DMSO) and the mixture was allowed to stand for 30 min in the dark at room temperature. Similarly, same protocol was performed for L-ascorbic acid as a standard antioxidant. The absorbance of the resulting mixture was measured at 517 nm using UV-Vis spectrophotometer. The control was prepared by adding only DMSO to DPPH reagent and the analysis followed as described above. The % scavenging activity was determined as follows.
Where, A0 is the absorption of the control (blank, only DMSO) and A1 is the absorption of the compound.
3 Results and Discussion
3.1 FT-IR analysis of the catalyst
The surface modification of TiO2 nanoparticles was evaluated by FT-IR spectroscopy. The figure 1 shows the FT-IR spectrum of TiO2 (a), silane@TiO2 (b) and vinyltriethoxysilane (c). In the spectrum 1a, the hydroxyl groups present on the surface of nano TiO2 exhibit absorption bands at 3348 and 3453 cm −1, respectively, which were found to be absent in the spectrum 1b. This indicates the absence of hydroxyl groups on the surface of silane@TiO2, as ethoxy groups of vinyltriethoxy silane get condensed with hydroxyl groups present on TiO2 surface. The bands at 1100 cm −1 and 760 cm −1 are assigned to the symmetrical and asymmetrical stretching of the Si-O bonds, respectively. The bands appearing at 1400 cm −1 also substantiate the presence of Si-C bond of vinyltriethoxy silane. The absorption band at 2990 cm −1 is attributed to symmetrical and asymmetrical stretchings of –CH2 and –CH3 groups. Thus, FT-IR spectra confirm the successful surface modification of TiO2 nanoparticles.
3.2 XRD spectral analysis
Figure 2 shows the XRD patterns of TiO2 and silane@TiO2 nanoparticles. The peaks appearing at 101, 111, 112, 210, 121, 212, 032, 114, 024 are for the crystalline portion of TiO2. The patterns allow the comparison with similar peaks that appeared in case of silane@TiO2 compared to the TiO2 nanoparticles, at 2θ values corresponding to the planes 101, 112, 200, 211, 204, 220, 301. Therefore, it is satisfying to observe that the modification of TiO2 does not change the crystalline structure of silane@TiO2. The XRD pattern of silane@TiO2 shows broad peak at 2θ = 15–25 ∘ which indicates the amorphous nature of silica.[29] The most intense peak of silane@TiO2 nanoparticles was used to determine the size of crystallites to be approximately 5.4 nm, using the half-width of the diffraction peak and the Debye-Scherrer equation. The Sherrer’s equation is D = K λ/β cos θ, where D is the crystallite size, λ is wavelength of the radiation, θ is the Bragg’s angle and β is the full width at half maximum.
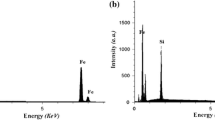
3.3 FE-SEM and EDX analysis of the catalyst
FE-SEM analysis is another useful tool for the analysis of the surface morphology of a catalyst. The FE-SEM images of TiO2 and silane@TiO2 nanoparticles are shown in figure 3 (a–b). Notably, the particles are seen to be in the nano range (<100 nm). Figure 3c shows the EDX spectrum of silane@TiO2 nanoparticles in which the characteristic peaks of Si and C are observed, which confirm the conversion of TiO2 into silane@TiO2 nanoparticles.
3.4 TG analysis of the catalyst
The thermal properties of TiO2 and silane@TiO2 nanoparticles were investigated using thermo gravimetric analysis (TGA) and the observed results are shown in figure 4. The TGA curve (a) shows slight weight loss (4.83%) probably due to vaporization of water or other volatile impurities that were physically adsorbed at the particle surface. The TiO2 nanoparticles were relatively stable in air and somewhat decomposed. The TGA curve (b) shows two step degradation in that the first weight loss started from 28–165 ∘C (10.69%) and second weight loss began in the range of 165–762 ∘C (7.61%). The silane@TiO2 nanoparticles showed two steps weight loss which is attributed to dehydration of the water absorbed by nanoparticles and oxidative thermal decomposition of the grafted vinyltriethoxy silane on the TiO2 nanoparticles.
3.5 TEM analysis
The morphology of the nanoparticles of TiO2 and silane@TiO2 (figure 5a, b) was studied using transmission electron microscopy (TEM). From TEM images it is observed that the TiO2 nanoparticles have spherical morphology with an average particle size of about 8 nm, while the silane@TiO2 nanoparticles show distorted spherical morphology with average particle size of about 9 nm. The TEM images thus indicate that the synthesized nanoparticles have nearly same crystalline character as that of TiO2. The selected area electron diffraction (SAED) image of silane@TiO2 nanoparticles (figure 5c) show ring patterns, confirming the crystalline nature.
3.6 Optimization of reaction conditions
We began our study from the reaction of 4-hydroxycoumarin 1 (2 mmol) with 3-nitrobenzaldehyde 2 (2 mmol), and 2-aminobenzimidazole 3 (2 mmol) as the model starting material in the presence of 5 mol% silane@TiO2 nanoparticles in ethanol (5 mL) at 78–80 ∘C for 1 h to give the desired product 3b in 75% yield. Encouraged by this result, we further moved to study and optimize the best reaction conditions by using different amounts of silane@TiO2 nanoparticles. The model reaction was stirred under same reaction conditions in the absence of silane@TiO2 nanoparticles which yielded the target product 3b in 33% yield only after 8 h (table 1, entry 1). Moreover, an increase in the amount (0 to 10 mol%) of silane@TiO2 nanoparticles decreased the reaction time and increased the yield of the product (table 1, entries 1–4). However, the yield did not increase when excess amount (15 mol%) of silane@TiO2 nanoparticles was used under similar reaction conditions. Therefore, 10 mol% of silane@ TiO2 nanoparticles was efficiently utilized for this multi-component reaction. Again, the model reaction was chosen for examining the effect of solvent on rate of reaction (table 2). The low yield of the target product 3b was obtained when the mixture was refluxed for 6 h in the presence of 10 mol% of silane@TiO2 nanoparticles in acetonitrile, toluene, THF, DMF, etc. (table 2, entries 1, 2, 3, 7). The reaction using ethanol as the solvent gave the corresponding product 3b in high yield (81%) with short reaction time (table 2, entry 6). However, when the model reaction was carried at room temperature in ethanol (table 2, entry 5), even after 12 h only 30% yield was obtained. From these experimentations, it is clear that the reaction could not proceed smoothly at room temperature and hence refluxing under ethanol was preferred. Here, ethanol was chosen as the reaction medium for all further reactions since it is accepted as a green solvent. Therefore, the best and the most optimum reaction condition for the target reaction is 10 mol% of silane@TiO2 nanoparticles as the catalyst in ethanol at reflux temperature. To study the recyclability and catalytic activity of the catalyst, the silane@ TiO2 nanoparticles (10 mol%) were used for the model reaction (table 1). The silane@TiO2 nanoparticles can be recycled and reused as a catalyst for at least three times without significant loss in catalytic activity for this multi-component reaction (scheme 3).
To realize the efficiency and limitations of the catalyst in this multi-component reaction, we applied the optimized reaction conditions of 10 mol% silane@ TiO2 nanoparticles in ethanol at reflux temperature to a series of aldehydes (table 3). The aromatic and hetero-aromatic aldehydes could react smoothly to give the corresponding benzo[4,5]imidazo[1,2-a]chromeno [4,3-d]pyrimidin-6-one in good yields (table 3, entries 1–17). Most significantly, aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents including hydroxyl group and methoxy group could react efficiently to give the corresponding products.
Herein, we propose a mechanism for the synthesis of benzo[4,5]imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one (figure 6). It is assumed that silane@TiO2 nanoparticles activate the carbonyl carbon of aldehyde through hydrogen bonding and promotes the Knoevenagel condensation reaction between 4-hydroxycoumarin (1) and benzaldehyde (2) to form Schiff’s base as an intermediate. In the next step, 2-aminobenzimidazole (3) reacts with the intermediate through Michael addition followed by cyclization and dehydration, to give the corresponding desired products.
3.7 Biological evaluation
3.7.1 DPPH Radical scavenging assay
Antioxidant activity of compounds is related to their electron or hydrogen atom donating ability to DPPH radical, so that they become stable diamagnetic molecules. The DPPH is a stable free radical and it accepts an electron or hydrogen atom to become a stable diamagnetic molecule.[30] The reduction competency of DPPH radicals was determined by a decrease in their absorbance at 517 nm induced by antioxidants. The absorption maximum of a stable DPPH radical in methanol was at 517 nm. The decrease in absorbance of DPPH radical was caused by antioxidants because of the reaction between antioxidant molecules and radical, which resulted in the scavenging of the DPPH radicals.
The electronic absorption spectra of solutions of compounds (3a-q) were measured in DMSO. All the synthesized compounds were screened for their antioxidant activity by measuring their scavenging ability towards DPPH radical. The disappearance of DPPH was measured spectrophotometrically at 517 nm using ascorbic acid as standard. The results are shown in figure 7. It can be concluded from figure 7 that four compounds 3i, 3n, 3o and 3q showed radical scavenging activity comparable to the standard ascorbic acid, whereas other compounds 3b, 3e, 3f, 3g, 3h, 3l and 3p showed lower scavenging activity.
4 Conclusion
In summary, we have developed an efficient and environmentally benign method for the synthesis of benzo[4,5]imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one using silane@TiO2 nanoparticles as heterogeneous catalyst. The method has advantages such as clean and mild reaction conditions, short reaction time and excellent yield of products. Further, it is noteworthy to mention that, amongst the synthesized compounds, four compounds showed antioxidant activity comparable to ascorbic acid. The present work adheres to the concept of green chemistry and provides a synthetic strategy for chemists to opt for and confront research on multi-component reactions via use of nanocatalysts.
References
(a) Gawande M B, Bonifacio V D B, Varma R S, Nogueira I D, Bundaleski N, Ghumman C A A, Teodoro O M N D and Branco P S 2013 Green Chem. 15 1226; (b) Khedkar M V, Shinde A R, Sasaki T and Bhanage B M 2014 J. Mol. Catal. A: Chem. 385 91; (c) Santra S, Rahman M, Roy A, Majee A and Hajra A 2014 Catal. Commun. 49 52; (d) Naeimi H and Nazifi Z S 2014 Appl. Catal. A 477 132
Mallakpour S and Nikkhoo E 2014 Adv. Powder Technol. 25 348
Zhao J, Milanova M, Warmoeskerken M M C G and Dutschk V 2012 Colloids Surf. A: Physicochem. Eng. Aspects 413 273
Zamani F, Hosseini S M and Kianpour S 2013 Solid State Sci. 26 139
Tomovska R, Daniloska V and Asua J M 2013 Appl. Surf. Sci. 264 670
Chen X and Mao S S 2007 Chem. Rev. 107 2891
Mohammadi R and Kassaee M Z 2013 J. Mol. Catal. A: Chem. 380 152
Yin Z F, Wu L, Yang H G and Su Y H 2013 Phys. Chem. Chem. Phys. 15 4844
(a) Gupta S M and Tripathi M 2011 Chin. Sci. Bull. 56 1639; (b) Ismail A A and Bahnemann D W 2011 J. Mater. Chem. 21 1686
Ramon D J and Yus M 2005 Angew. Chem. Int. Ed. 44 1602
Volla C M R, Atodiresei I and Rueping M 2014 Chem. Rev. 114 2390
Tietze L F 1996 Chem. Rev. 96 115
(a) Singh M S and Chowdhury S 2012 RSC Adv. 2 4547; (b) Reddy M V, Reddy G C S and Jeong Y T 2014 RSC Adv. 4 24089; (c) Reddy M V, Rani C R and Jeong Y T 2014 Tetrahedron 70 3762
Gu Y 2012 Green Chem. 14 2091
Anastas P T, Warner J C 1998 In Green Chemistry: Theory and practice (Oxford University Press: Oxford UK)
Xiao Z, Lei M and Hu L 2011 Tetrahedron Lett. 52 7099
Mahire V N and Mahulikar P P 2015 Chin. Chem. Lett. 26 983
(a) Bansal Y and Silakari O 2012 Bioorg. Med. Chem. 20 6208; (b) Shah N K, Shah N M, Patel M P and Patel RG 2013 J. Chem. Sci. 125 525
(a) Richards M L, Lio S C, Sinha A, Tieu K K and Sircar J C 2004 J. Med. Chem. 47 6451; (b) Craigo W A, LeSueur B W and Skibo E B 1999 J. Med. Chem. 42 3324; (c) Raut C N, Bagul S M, Janrao R A, Vaidya S D, Kumar B V S and Mahulikar P P 2010 J. Heterocycl. Chem. 47 582; (d) Raut C N, Bharambe S M, Pawar Y A and Mahulikar P P 2011 J. Heterocycl. Chem. 48 419; (e) Kucukbaya H, Durmaz R, Okyucu N and Gunald S 2003 Folia Microbiol. 48 679; (f) Kuş C, Ayhan-Kılcıgil G, Özbey S, Kaynak F B, Kaya M, Çoban T and Can-Eke B 2008 Bioorg. Med. Chem. 16 4294; (g) Ansari K F and Lal C 2009 J. Chem. Sci. 121 1017; (h) Kathrotiya H G and Patel M P 2013 J. Chem. Sci. 125 993
Preston P N 1981 In Benzimidazoles and congeneric tricyclic compounds (Interscience and John Wiley: New York)
Jaggavarapu S R, Kamalakaran A S, Jalli V P, Gangisetty S K, Ganesh M R and Gaddamanugu G 2014 J. Chem. Sci. 126 187
El-Agrody A M, Fouda A M and Al-Dies A. -A M 2014 Med. Chem. Res. 23 3187
Kamdar N R, Haveliwala D D, Mistry P T and Patel S K 2011 Med. Chem. Res. 20 854
Gupta J K, Sharma P K, Dudhe R, Chaudhary A, Singh A, Verma P K, Mondal S C, Yadav R K and Kashyap S 2012 Med. Chem. Res. 21 1625
Dinakaran V S, Jacob D and Mathew J E 2012 Med. Chem. Res. 21 3598
(a) Heravi M M, Saeedi M, Beheshtiha Y S and Oskooie H A 2011 Chem. Heterocycl. Compd. 47 737; (b) Mazhukina O A, Platonova A G, Fedotova O V and Vasin V A 2015 Russ. J. Org. Chem. 51 691
Mahshid S, Ghamsari M S, Askari M, Afshar N and Lahuti S 2006 Semicond. Phys. Quant. Electro. Optoelectro. 9 65
(a) Sabzi M, Mirabedini S, Zohuriaan J and Atai M 2009 Prog. Org. Coat. 65 222; (b) Gite V V, Chaudhari A B, Kulkarni R D and Hundiwale D G 2013 Pigm. Resin Technol. 42 353; (c) Chaudhari A B, Gite V V, Rajput S D, Mahulikar P P and Kulkarni R D 2013 Ind. Crop. Prod. 50 550
Dehghani F, Sardarian A R and Esmaeilpour M 2013 J. Organomet. Chem. 743 87
Soarey J R, Dinis T C P, Cunha A P and Almeida L M 1997 Free. Rad. Res. 26 469
Acknowledgements
Authors are thankful to UGC, New Delhi for financial assistance under One Time Grant [F. No. 19-110/2013 (BSR)] and Major Research Project [F. No. 41-302/2012 (SR)]; Authors also thankful to SAIF, Panjab University, Chandigarh, SAIF IIT Bombay and Central Instrumentation Facility Centre, UICT, NMU, Jalgaon for providing instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
Spectral data, 1H and 13C NMR Spectra and Mas spectra are shown in Supplementary Information, available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MAHIRE, V.N., PATEL, V.E., CHAUDHARI, A.B. et al. Silane@TiO 2 nanoparticles-driven expeditious synthesis of biologically active benzo[4,5]imidazo[1,2-a]chromeno[4,3-d]pyrimidin-6-one scaffolds: A green approach. J Chem Sci 128, 671–679 (2016). https://doi.org/10.1007/s12039-016-1060-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1060-x