Abstract
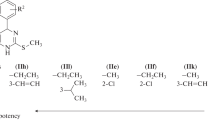
A novel series of 2-amino-4-(coumarin-3-yl)-6-substituted phenyl pyrimidines (5a–h) were synthesized from 3-acetylcoumarin (3). The structures of the synthesized compounds were elucidated by I.R., 1H NMR, 13C NMR, and Mass spectroscopic techniques. The synthesized compounds were screened for in vivo analgesic activities at a dose of 20 mg/kg body weight (b.w). Among them, compounds 5b and 5h exhibited significant analgesic activity comparable with control as well as standard drug diclofenac sodium using acetic acid-induced writhing model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a complex defensive mechanism of the body to any noxious stimulus; this process may vary from a localized to a generalized response characterized by the accumulation of fluids and leukocytes leading to edema and pain (Black, 2005). This inflammatory response seems to be mediated by different physiological and immunological mediators that play a role in acute and chronic inflammation. The acute inflammation occurs as the initial response to tissue injury, being mediated by the release of autocoids, for example, histamine, bradykinin, prostaglandins, and leukotrienes (Black, 2005; Sherwood and Toliver-Kinsky, 2004). On the other hand, the chronic inflammatory process involves the release of diverse mediators, as interleukins, interferon and tumor necrosis factor a (TNF-α), a cytokine that plays a major role in this kind of inflammatory process and whose production is associated with some inflammatory diseases such as rheumatoid arthritis (Black, 2005; Sherwood and Toliver-Kinsky, 2004). Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of pain and inflammation associated with different diseases particularly rheumatoid arthritis (Sorbera et al., 2001).
Coumarins have a variety of bioactivities including analgesic, antimicrobial (Radulovic et al., 2006), anticoagulant, vasodilator, anthelmintic, sedative and hypnotics, and antipyretic activity (Soine, 1964; O’Kennedy and Thornes, 1997). In addition to this, studies are performed on coumarin derivatives for their therapeutic role in the treatment of cancer (Lacy and O’Kennedy, 2004). Various pyrimidine derivatives have been reported as anti-microbial (El-Sayed Ali, 2009), analgesic and anti-inflammatory (Amr et al., 2007; Abu-Hashem et al., 2010; El-Gazzar et al., 2009; Chikhale et al., 2009), anticonvulsants (Gupta et al., 2009), anti-HIV (Fujiwara et al., 2008), antiviral (Hockov et al., 2004), anti-tubercular (Ballell et al., 2007), anti-tumor (Wagner et al., 2008), anti-neoplastic (Xie et al., 2009), antioxidant, and radioprotective agents (Ghorab et al., 2010).
Both the pyrimidines and coumarins exhibit diverse biological properties (Kulkarni et al., 2006; Shafiee et al., 2010). It was observed that linking of these two active pharmacophores would generate novel molecular templates which are likely to exhibit interesting biological properties in animal models (Keri et al., 2010). Owing to the importance, we wish to describe the synthesis of new pyrimidine derivatives linked with coumarin moiety from 3-acetylcoumarins (Reaction Scheme 1). The compounds were screened for their in vivo analgesic activity.
Chemistry
For the synthesis of target compounds, the reaction sequence outline in Scheme 1, were followed. Salicylaldehyde (1) is reflux with ethyl acetoacetate (2) in the presence of piperidine which gave 3-acetyl coumarin (3). This synthesized compound condensed with different substituted benzaldehydes using piperidine as a catalyst yielded 3-substituted phenyl-1-(coumarin-3-yl) prop-2-ene-1-ones (4a–h). 2-amino-4-(coumarin-3-yl)-6-substituted phenyl pyrimidines (5a–h) were prepared by treating compounds (4a–h) with guanidine HCl. The structures of newly synthesized compounds were confirmed by their spectral analysis. The physical data of these compounds are summarized in Tables 1 and 2.
When salicylaldehyde (1) is treated with ethyl acetoacetate (2) in refluxing ethanol in the presence of piperidine, it afforded a single precipitated product that was analyzed correctly for C11H8O3. The reaction takes 2–3 h for completion. The structure of the precipitated product was identified as 3-acetyl coumarin (3) based on its spectral data. For example, IR spectrum of the isolated compound showed the characteristic band at 1,275 cm−1, 13C NMR spectrum revealed the presence of 11 carbon atoms in the form of 11 peak signal.
In the second step, treatment of 3-acetyl coumarin (3) with different types of benzaldehydes in the presence of piperidine for 8–10 h using ethanol as a solvent yielded one precipitated product (4a–h) respective of their benzaldehydes. The isolated product was identified as 3-substituted phenyl-1-(coumarin-3-yl) prop-2-ene-1-ones (4a–h). The IR spectrum of the isolated product revealed in each case bands due to carbonyl function and aryl ethers functions. Moreover, the 1H NMR spectrum of compound (4a–h), taken as an example, revealed two doublet signals between δ 6–8 ppm, and one multiplet at δ 7–8 ppm due to methylene protons and aromatic protons, respectively.
The formation of compounds (4a–h) can be explained on the basis of “Claisen–Schmidt condensation”.
In the third step, compound (4a–h) is refluxed with guanidine HCl, in equimolar quantity in ethanol as a solvent for 8–10 h, which afforded the final compounds (5a–h). The structure of the final compounds was assigned based on their elemental analysis and spectral data. For example IR spectrum of the compounds (5a–h) revealed the broad band between 3,300 and 3,400 cm−1 due to amino group. In addition to this 1H NMR spectrum showed the singlet signal δ 4–5 ppm due to the amino group. These signals were absent in the spectral data of compounds (4a–h). Moreover, mass spectrum and elemental analysis data also supported the structure assigned to the final compounds (5a–h).
Result and discussion
Analgesic activity
Analgesic activity was performed according to Turner (1965) technique using Swiss albino mice. The results of analgesic activity indicated that all test compounds, except 5a and 5c, exhibited significant analgesic activity. Compounds 5b and 5h have approximately half of the analgesic activity as that of reference drug, and the remaining compounds had moderate analgesic activity (Table 3).
Conclusion
A novel series of 2-amino-4-(coumarin-3-yl)-6-(substituted phenyl) pyrimidines analogues were synthesized and characterized. The synthesized compounds screened for their in vivo analgesic activity. Some of the synthesized compounds viz., 5b and 5h exhibited good analgesic activity in comparison to that of standard drug diclofenac sodium in the acetic acid-induced writhing response model at 20 mg/kg body weights of the animals.
Experimental section
Chemistry
All reagents and solvents were used as obtained from the supplier or recrystallized/redistilled as necessary. The melting points of the products were determined by open capillary method and are uncorrected. I.R. Spectra (KBr) were recorded on FTIR Spectrophotometer (Shimadzu FTIR 84005, 4,000–400 cm−1). 1H NMR and 13C NMR spectra were recorded on a JEOL AL300 FTNMR 300 MHz spectrometer in CDCl3 using TMS as an internal standard, with 1H resonance frequency of 300 MHz and 13C resonance frequency of 75 MHz. Chemical shift values are expressed in δ ppm. Mass spectra were recorded on a 70 eV EI-MS-QP 1000 EX (Schimadzu). The elemental analysis was carried out using Heraus CHN rapid analyzer. The homogeneity of the compounds was determined by TLC on alumina silica gel 60 F254 (Merck) detected by UV light (254 nm) and iodine vapors. The in vivo analgesic activity was performed at Meerut Institute of Engineering and Technology, Meerut, India.
General procedures for the preparation of compounds
Synthesis of 3-acetyl coumarin (3): general procedure
A mixture of salicylaldehyde (1) (0.02 mol) and ethyl acetoacetate (2) (0.03 mol) in ethanol were taken in round bottom flask. To this mixture, few drops of piperidine were added and refluxed for 2–3 h. After completion of reaction, the content was poured on crushed ice. The solid separated was filtered, dried, and recrystallized from ethanol. The purity of compound was established on the basis of TLC. M. P. 111–113°C, I. R. (KBr, cm−1): 1741.60 and 1677.95 (C=O), 1275 (aryl ethers, C–O–C); 1H NMR (CDCl3-d 6, δ, ppm): 2.58 (s, 3H, CH3), 7.25–7.98 (m, 5H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 35.50, 120.9, 123.8, 126.6, 127.3, 130.5, 132.5, 139.8, 155.7, 163, 200.6; MS, [M+], m/z 188 (100%), [M+ +2], m/z 190 (15%), [M+ +4], m/z 192 (2%); Anal. Calcd for C11H8O3 (188.18): C, 70.21; H, 4.29. Found: C, 70.15; H, 4.25.
Synthesis of 3-substituted phenyl-1-(coumarin-3-yl) prop-2-ene-1-ones (4a–h): general procedure
Equimolar quantities of 3-acetyl coumarin (3) and different substituted benzaldehydes were refluxed in absolute ethanol using piperidine as a catalyst for 8–10 h. The solution mixture was concentrated and poured on to crushed ice. The precipitate was filtered, dried, and recrystallized from ethanol to give pure crystalline solid.
Synthesis of 3-(2-chlorophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4a)
It was obtained from reaction of compound (3) with 2-chlorobenzaldehyde. IR (KBr, cm−1): 1724.24 and 1662.52 (C=O), 1184.21 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 6.02 (d, 1H, CH), 7.11–7.93 (m, 9H, Ar-H), 8.03 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 120.3, 124.2, 125.3, 125.9, 129.1, 129.9, 130, 131.9, 132.5, 133, 138.9, 142.6, 143.9, 145.2, 147.6, 157.8, 159.6, 180.5; MS, [M+], m/z 310 (100%), [M+ +2], m/z 312 (35%), [M+ +4], m/z 314 (10%); Anal. Calcd for C18H11ClO3 (310.73): C, 69.58; H, 3.57. Found: C, 69.52; H, 3.52.
Synthesis of 3-(3-chlorophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4b)
It was obtained from reaction of compound (3) with 3-chlorobenzaldehyde. IR (KBr, cm−1): 1728.10 and 1685.67 (C=O), 1107.06 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 7.03 (d, 1H, CH), 7.15–8.02 (m, 9H, Ar-H), 8.66 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 120.9, 122.9, 124.6, 125.9, 127.6, 128.9, 130.2, 130.9 131.5, 132.7, 133, 135.7, 138.9, 144.9, 148.2, 158.3, 160.5, 178.6; MS, [M+], m/z 309 (100%), [M+ +2], m/z 311 (30%), [M+ +4], m/z 313 (5%); Anal. Calcd for C18H11ClO3 (310.73): C, 69.58; H, 3.57. Found: C, 69.62; H, 3.52.
Synthesis of 3-(4-chlorophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4c)
It was obtained from reaction of compound (3) with 4-chlorobenzaldehyde. IR (KBr, cm−1): 1728.10 and 1685.67 (C=O), 1107.06 (C–O–C). 1HNMR (CDCl3-d 6, δ, ppm): 6.36 (d, 1H, CH), 6.90 (d, 1H, CH), 7.02–8.48 (m, 9H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 120.5, 123.4, 124.6, 127.5, 128.4, 128.6, 128.9, 130.5, 130.9, 131.5, 131.7, 132.6, 132.9, 144.4, 145.6, 157.2, 158.6, 182.9; MS, [M+], m/z 309 (100%), [M+ +2], m/z 311 (33%), [M+ +4], m/z 313 (3%); Anal. Calcd for C18H11ClO3 (310.73): C, 69.58; H, 3.57. Found: C, 69.55; H, 3.51.
Synthesis of 3-(2-bromophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4d)
It was obtained from reaction of compound (3) with 2-bromobenzaldehyde. IR (KBr, cm−1): 1724.24 and 1683.74 (C=O), 1184.21 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 6.86 (d, 1H, CH), 7.02–7.93 (m, 9H, Ar-H), 8.00 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 120.1, 120.9, 121.5, 121.9, 124.6, 125.6, 127.6, 127.9, 128.6, 128.9, 129.4, 129.9, 130.9, 145.6, 149.3, 159.6, 161.9, 178.5; MS, [M+], m/z 354 (100%), [M+ +2], m/z 356 (25%), [M+ +4], m/z 358 (2%); Anal. Calcd for C18H11BrO3 (355.18): C, 60.87; H, 3.12. Found: C, 60.81; H, 3.10.
Synthesis of 3-(3-bromophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4e)
It was obtained from reaction of compound (3) with 3-bromobenzaldehyde. IR (KBr, cm−1): 1728.10 and 1685.67 (C=O), 1107.06 (C–O–C). 1HNMR (CDCl3-d 6, δ, ppm): 7.08 (d, 1H, CH), 7.11–7.99 (m, 9H, Ar-H), 8.05 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 1209, 123.5, 124.6, 125.9, 126.9, 127.8, 128.7, 129, 129.4, 130, 131.5, 131.6, 134.6, 140, 147.3, 150.6, 158.3, 179.2; MS, [M+], m/z 354 (100%), [M+ +2], m/z 356 (20%), [M+ +4], m/z 358 (1.6%); Anal. Calcd for C18H11BrO3 (355.18): C, 60.87; H, 3.12. Found: C, 60.81; H, 3.09.
Synthesis of 3-(4-bromophenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4f)
It was obtained from reaction of compound (3) with 4-bromobenzaldehyde. IR (KBr, cm−1): 1739.67 and 1677.95 (C=O), 1107.06 (C–O–C). 1HNMR (CDCl3-d 6, δ, ppm): 7.03 (d, 1H, CH), 7.11–7.94 (m, 9H, Ar-H), 8.23 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 121.9, 122.3, 123.6, 124.6, 125.3, 125.9, 128.6, 128.9, 129.5, 129.9, 130.5, 132.3, 135, 145.6, 150, 160.3, 164.2, 165.1, 180; MS, [M+], m/z 354 (100%), [M+ +2], m/z 356 (18%), [M+ +4], m/z 358 (2.5%); Anal. Calcd for C18H11BrO3 (355.18): C, 60.87; H, 3.12. Found: C, 60.90; H, 3.14.
Synthesis of 3-(2-methoxyphenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4g)
It was obtained from reaction of compound (3) with 2-methoxybenzaldehyde. IR (KBr, cm−1): 1728.10 (C=O), 1164.92 (C–O–C). 1HNMR (CDCl3-d 6, δ, ppm): 3.56 (s, 3H, CH3), 6.86 (d, 1H, CH), 7.02–7.96 (m, 9H, Ar-H), 8.09 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 62.7, 113.5, 118.6, 120.3, 121.6, 123.6, 125.9, 127.6, 128, 128.9, 129, 129.9, 143.9, 150, 155.6, 160.3, 163.5, 163.9, 179; MS, [M+], m/z 305 (100%), [M+ +2], m/z 307 (25%), [M+ +4], m/z 309 (2%); Anal. Calcd for C19H14O4 (306.31): C, 74.50; H, 4.61. Found: C, 74.54; H, 4.57.
Synthesis of 3-(3-methoxyphenyl)-1-(coumarin-3-yl) prop-2-ene-1-one (4h)
It was obtained from reaction of compound (3) with 3-methoxybenzaldehyde. IR (KBr, cm−1): 1735.81 (C=O), 1137.92 (C–O–C). 1HNMR (CDCl3-d 6, δ, ppm): 3.90 (s, 3H, CH3), 6.98 (d, 1H, Ar-H), 7.00–7.85 (m, 9H, Ar-H), 8.10 (d, 1H, CH); 13C NMR (CDCl3-d 6, δ, ppm): 63.2, 112.5, 118.2, 120.9, 122.9, 122.5, 126.9, 127.9, 128, 128.6, 129.3, 129.9, 142.6, 150.3, 154.6, 160.8, 163.6, 165.9, 182.3; MS, [M+], m/z 305 (100%), [M+ +2], m/z 307 (20%), [M+ +4], m/z 309 (1.5%); Anal. Calcd for C19H14O4 (306.31): C, 74.50; H, 4.61. Found: C, 74.45; H, 4.56.
Synthesis of 2-amino-4-(7-substituted coumarin-3-yl)-6-substituted phenyl pyrimidines-(5a–h): general procedure
A mixture of 3-substituted phenyl-1-(coumarin-3-yl) prop-2-ene-1-ones (0.01 mol) and guanidine HCl (0.02 mol) was refluxed in ethanol for 8–10 h. The content was evaporated to dryness and the product so obtained was washed with water repeatedly and recrystallized from ethanol.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(2-chlorophenyl) pyrimidines (5a)
It was obtained from reaction of guanidine HCl with compound (4a). IR (KBr, cm−1): 3151.47 (br, NH), 1758.96 (C=O), 1666.38 (C=N), 1114.78 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.256 (s, 2H, NH2), 6.85–7.72 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm):110.1, 124.2, 125.3, 128.6, 129.1, 129.9, 130, 131.9, 132.5, 135.5, 138.9, 142.6, 143.9, 145.2, 147.6, 157.8, 165.6, 168.5, 170.5; MS, [M+], m/z 348 (100%), [M+ +2], m/z 350 (40%), [M+ +4], m/z 352 (10%); Anal. Calcd for C19H12ClN3O2 (349.77): C, 65.24; H, 3.46; N, 12.01. Found: C, 65.30; H, 3.48; N, 12.06.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(3-chlorophenyl) pyrimidines (5b)
It was obtained from reaction of guanidine HCl with compound (4b). IR (KBr, cm−1): 3352.05 (br, NH), 1758.96 (C=O), 1635.52 (C=N), 1242.07 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.25 (s, 2H, NH2), 6.92–7.36 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 109.2, 122.9, 124.6, 125.9, 127.6, 128.9, 130.2, 131.5, 132.7, 133, 135.7, 138.9, 144.9, 148.2, 158.3, 160.5, 161.4, 163.4; MS, [M+], m/z 348 (100%), [M+ +2], m/z 350 (45%), [M+ +4], m/z 352 (15%); Anal. Calcd for C19H12ClN3O2 (349.77): C, 65.24; H, 3.46; N, 12.01. Found: C, 65.20; H, 3.40; N, 12.0.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(4-chlorophenyl) pyrimidines (5c)
It was obtained from reaction of guanidine HCl with compound (4c). IR (KBr, cm−1): 3367.48 (br, NH), 1662.52 (C=O), 1585.38 (C=N), 1226.64 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.25 (s, 2H, NH2), 7.02–7.50 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 110.5, 123.4, 124.6, 127.5, 128.4, 128.6, 128.9, 130.5, 130.9, 131.5, 131.7, 132.6, 132.9, 144.4, 145.6, 157.2, 158.6, 160.9, 163.7; MS, [M+], m/z 348 (100%), [M+ +2], m/z 350 (47%), [M+ +4], m/z 352 (17%); Anal. Calcd for C19H12ClN3O2 (349.77): C, 65.24; H, 3.46; N, 12.01. Found: C, 65.19; H, 3.50; N, 12.02.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(2-bromophenyl) pyrimidines (5d)
It was obtained from reaction of guanidine HCl with compound (4d). IR (KBr, cm−1): 3402.20 (N–H), 1674.10 (C=O), 1546.80 (C=N), 1110.92 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.96 (s, 2H, NH2), 7.25–7.63 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 107.9, 120.5, 121.5, 121.9, 124.6, 125.6, 127.6, 127.9, 128.6, 128.9, 129.4, 129.9, 130.9, 145.6, 149.3, 159.6, 161.9, 162.8, 164.9; MS, [M+], m/z 393 (100%), [M+ +2], m/z 395 (50%), [M+ +4], m/z 397 (20%); Anal. Calcd for C19H12BrN3O2 (394.22): C, 57.89; H, 3.07; N, 10.66. Found: C, 57.85; H, 3.02; N, 10.60.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(3-bromophenyl) pyrimidines (5e)
It was obtained from reaction of guanidine HCl with compound (4e). IR (KBr, cm−1): 3421.48 (N–H), 1654.81 (C=O), 1488.94 (C=N), 1110.92 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.27 (s, 2H, NH2), 6.93–7.63 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm):109.9, 123.5, 124.6, 125.9, 126.9, 127.8, 128.7, 129, 129.4, 130, 131.5, 131.6, 134.6, 140, 147.3, 150.6, 158.3, 160, 165.8; MS, [M+], m/z 393 (100%), [M+ +2], m/z 395 (45%), [M+ +4], m/z 397 (15%); Anal. Calcd for C19H12BrN3O2 (394.22): C, 57.89; H, 3.07; N, 10.66. Found: C, 57.92; H, 3.05; N, 10.60.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(4-bromophenyl) pyrimidines (5f)
It was obtained from reaction of guanidine HCl with compound (4f). IR (KBr, cm−1): 3348.19 (N–H), 1674.10 (C=O), 1542.95 (C=N), 1107.06 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 4.16 (s, 2H, NH2), 6.90–7.73 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 109.3, 122.3, 123.6, 124.6, 125.3, 125.9, 128.6, 128.9, 129.5, 129.9, 130.5, 132.3, 135, 145.6, 150, 160.3, 164.2, 165.1, 167; MS, [M+], m/z 393 (100%), [M+ +2], m/z 395 (55%), [M+ +4], m/z 397 (15%); Anal. Calcd for C19H12BrN3O2 (394.22): C, 57.89; H, 3.07; N, 10.66. Found: C, 57.85; H, 3.01; N, 10.60.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(2-methoxyphenyl) pyrimidines (5g)
It was obtained from reaction of guanidine HCl with compound (4g). IR (KBr, cm−1): 3421.48 (N–H), 1635.52 (C=O), 1600.81 (C=N), 1164.92 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 3.87 (s, 3H, CH3), 4.25 (s, 2H, NH2), 6.92–8.00 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 63.7, 106.3, 113.5, 118.6, 120.3, 121.6, 123.6, 125.9, 127.6, 128, 128.9, 129, 129.9, 143.9, 150, 155.6, 160.3, 163.5, 163.9, 166.3; MS, [M+], m/z 344 (100%), [M+ +2], m/z 346 (25%), [M+ +4], m/z 348 (5%); Anal. Calcd for C20H15N3O3 (345.35): C, 69.56; H, 4.38; N, 12.17. Found: C, 69.62; H, 4.35; N, 12.11.
Synthesis of 2-amino-4-(coumarin-3yl)-6-(3-methoxyphenyl) pyrimidines (5h)
It was obtained from reaction of guanidine HCl with compound (4h). IR (KBr, cm−1): 3413.77 (N–H), 1639.38 (C=O), 1585.38 (C=N), 1157.21 (C–O–C); 1HNMR (CDCl3-d 6, δ, ppm): 3.81 (s, 3H, CH3), 4.04(s, 2H, NH2), 6.86–7.25 (m, 10H, Ar-H); 13C NMR (CDCl3-d 6, δ, ppm): 63.2, 106.6, 112.5, 118.2, 120.9, 122.9, 122.5, 126.9, 127.9, 128, 128.6, 129.3, 129.9, 142.6, 150.3, 154.6, 160.8, 163.6, 165.9, 167.5; MS, [M+], m/z 344 (100%), [M+ +2], m/z 346 (20%), [M+ +4], m/z 348 (8%); Anal. Calcd for C20H15N3O3 (345.35): C, 69.56; H, 4.38; N, 12.17. Found: C, 69.50; H, 4.34; N, 12.15.
Pharmacological screening
Animals
Albino-Swiss mice weighing (20–25 g) were used for studying in vivo analgesic activity. Animals were maintained under standard laboratory conditions (24 ± 2°C relative humidity 60–70%). Study protocol was approved by the institutional Animal Ethics Committee for the Purpose of Control and Supervision on Experiments on Animals (IAEC, Approval No. 711/02/a/CPCSEA) before experiment. Albino-Swiss mice from Laboratory Animal House Section, Department of Pharmaceutical Technology, Meerut Institute of Engineering & Technology, Meerut were used in the study. The animals were kept in polypropylene cages and maintained on balanced ration with free access to clean drinking water. All experimental procedures were conducted in accordance with the guide for Care and use of laboratory animals and in accordance with the Local animal care and use committee.
Analgesic activity (acetic acid-induced writhing response model)
The compounds were selected for screening of their analgesic activity in acetic acid-induced writhing response in Swiss albino mice following the method of Turner (Turner, 1965). Sixty mice were selected and divided into 10 groups (six in each group), starved for 16 h and pretreated as follows, the first group which served as control positive was orally received distilled water in appropriate volumes. The second to ninth groups were received the aqueous suspension of synthesized compounds orally in a dose of 20 mg/kg. The last group was orally received diclofenac sodium in a dose of 20 mg/kg. After 30 min, each mice was administered 1% of an aqueous solution of acetic acid (10 ml/kg) and the mice were then placed in transparent boxes for observation. The number of writhes was counted for 30 min after acetic acid injection. The number of writhes in each treated group was compared to that of a control group. The number of writhing was recorded and the percentage protection was calculated using the following ratio:
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett’s t test for multiple comparisons of all compounds in various pharmacological assays. Data are expressed as mean ± SEM.
References
Abu-Hashem AA, Gouda MA, Badria FA (2010) Synthesis of some new pyrimido[2′,1′:2,3]thiazolo[4,5-b]quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur J Med Chem 45:1976–1981
Amr AE, Nermien MS, Abdulla MM (2007) Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh Chem 138:699–707
Ballell L, Field RA, Chung GAC, Young RJ (2007) New thiopyrazolo [3,4d] pyrimidine derivatives as antimycobacterial agents. Bioorg Med Chem Lett 17:1736–1740
Black JG (2005) Microbiology principles and exploration, 6th edn. Wiley, US
Chikhale RV, Bhole RP, Khedekar PB, Bhusari KP (2009) Synthesis and pharmacological investigation of 3-(substituted 1-phenylethanone)-4-(substituted phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylates. Eur J Med Chem 44:3645–3653
El-Gazzar ABA, Youssef MM, Youssef AMS, Abu-Hashem AA, Badria FA (2009) Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur J Med Chem 44:609–624
El-Sayed Ali T (2009) Synthesis of some novel pyrazolo[3,4-b]pyridine and pyrazolo[3,4-d]pyrimidine derivatives bearing 5,6-diphenyl-1,2,4-triazine moiety as potential antimicrobial agents. Eur J Med Chem 44:4385–4392
Fujiwara N, Nakajima T, Ueda Y, Fujita H, Kawakami H (2008) Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg Med Chem 16:9804–9816
Ghorab MM, Ragab FA, Alqasoumi SI, Alafeefy AM, Aboulmagd SA (2010) Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur J Med Chem 45:171–178
Gupta SV, Baheti K, Bora R, Dekhane D, Chhabrya M, Shingare M, Pawar S, Shishoo CJ, Thore SN (2009) Synthesis of novel bioactive derivatives of 3-(4-chlorophenyl)-2-hydrazino-5,6,7,8-tetrahydrobenzo(b)thieno[2,3-d]pyrimidine-4(3H)-ones. Eur J Med Chem 44:4721–4725
Hockov D, Holy A, Masojidkova M, Andrei G, Snoeck R, Clercq ED, Balzarini J (2004) Synthesis and antiviral activity of 2,4-diamino-5-cyano-6-[2-(phosphonomethoxy)ethoxy]pyrimidine and related compounds. Bioorg Med Chem 12:3197–3202
Keri RS, Hosamani KM, Shingalapur RV, Hugar MH (2010) Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur J Med Chem 45:2597–2605
Kulkarni MV, Kulkarni GM, Lin CH, Sun CM (2006) Synthesis of novel triheterocyclic thiazoles as anti-inflammatory and analgesic agents. Curr Med Chem 13:2795–2818
Lacy A, O’Kennedy R (2004) Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des 10:3797–3811
O’Kennedy R, Thornes RD (eds) (1997) Coumarins—biology, applications and mode of action. Wiley, Chichester
Radulovic N, Stojanovic G, Vukicevic R, Dekic V, Dekic B, Palic R (2006) New 3,4-annelated coumarin derivatives: synthesis, antimicrobial activity, antioxidant capacity and molecular modeling. Monatsh Chem 137:1477–1486
Shafiee A, Motamedi R, Firuzi O, Meili S, Mehdipour AR, Miri R (2010) Synthesis and cytotoxic activity of novel benzopyrano[3,2-c]chromene-6,8-dione derivatives. Med Chem Res 20:466–474
Sherwood ER, Toliver-Kinsky T (2004) Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 18:385–405
Soine TO (1964) Naturally occurring coumarins and related physiological activities. J Pharm Sci 53:231
Sorbera LA, Lesson PA, Castanar J, Castanar RM (2001) Valdecoxib and parecoxib sodium. Drugs Fut 26:133–140
Turner RA (1965) Analgesic. In: Turner RA (ed) Screening methods in pharmacology. Academic Press, London
Wagner E, Al-Kadasi K, Zimecki M, Sawka-Dobrowolska W (2008) Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur J Med Chem 43:2498–2504
Xie F, Zhao H, Zhao L, Lou L, Hu Y (2009) Synthesis and biological evaluation of novel 2,4,5-substituted pyrimidine derivatives for anticancer activity. Bioorg Med Chem Lett 19:275–278
Acknowledgment
This research work is financially supported by the Meerut Institute of Engineering & Technology, Meerut, India-250005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, J.K., Sharma, P.K., Dudhe, R. et al. Analgesic study of novel pyrimidine derivatives linked with coumarin moiety. Med Chem Res 21, 1625–1632 (2012). https://doi.org/10.1007/s00044-011-9675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9675-4