Abstract
In this study the preparation and characterization of a zinc MOF which gives blue luminescence and photocatalytic activity have been described The reaction of 2cyanopyridine, Zn(NO3)2⋅4H2O and NaN3 yielded a new 3D Zn(II) coordination polymer {(2PTZ)2Zn}n (1), where 2PTZ =5(2pyridyl)tetrazolate via in situ [2 + 3] cycloaddition reaction of the nitrile and the azide. Compound 1 was characterized by elemental analyses, IR spectroscopy, X-ray powder diffraction (XRPD), scanning electron microscopy (SEM) and single-crystal X-ray crystallography. Crystal structure of the compound belongs to monoclinic space group C2/c with Z = 2 In 1 the crosslinkage of the PTZ ligands and Zn(II) atoms forms an infinite 1D chain network. Further the one–dimensional chains are assembled into threedimensional network by hydrogen bonding. Thermal photoluminescence and photocatalytic properties of 1 were also been investigated.

The reaction of 2-cyanopyridine, Zn(NO3)2·4H2O and NaN3 yielded a new 3D Zn(II) coordination polymer, {(2-PTZ)2Zn}n {2-PTZ = 5-(2-pyridyl)tetrazolate} via in situ [2+3] cycloaddition reaction of the nitrile and the azide. Thermal, photocatalytic properties and photoluminescence of the complex were also investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In last few years tetrazole ligands have been widely used to synthesize metal–organic and H-bonded frameworks of various topologies.[1–5]The versatility of the tetrazole building blocks provides the required template to create a variety of new materials with interesting properties and diverse applications such as gas storage,[6,7]sensors,[8] separation process,[9] drug delivery,[10] catalysis,[11] chemical sensing,[12]and photocatalysis.[13] Cycloaddition reactions of azides and cyanides for synthesis of tetrazoles are known for almost 110 years. First report was in 1901 wherein 5-aminotetrazole was made by the reaction of hydrazoic acid (HN3) with cyanamide.[14] Sharpless et al., in the year 2002, introduced the term “click chemistry” as a chemical philosophy that describes chemistry to be tailored for generating compounds by joining small units together in a practical, quick, and reliable way.[15–17]Tetrazoles exhibit strong networking ability and in most of the reported MOFs, they usually act as mono- or bidentate ligands.[18,19]In addition, the five- membered heterocyclic ring could also serve as a hydrogen-bond acceptor, thereby permitting the expansion of the polymeric framework through hydrogen bonding interactions.[20]
The factors such as solvent system, pH, coordination behaviour of the metal ion, structural features of the polydentate organic ligand, metal/ligand ratio and the possible second organic ligand deeply affect the self- organization of MOFs.[21–23]Although in literature a number of MOF materials and metal complexes are reported, research related to luminescent MOFs and complexes is limited especially for ligand-based emissions.[24–26]Most of the luminescent MOFs attribute the property to the presence of lanthanide elements into their frameworks[27] either as metal centers or as dopant ions. Ligand-based emissions in MOFs are advantageous because both nature and structure of the linkers can be tuned more effectively in the framework. Such possibilities may prove to be important for the practical application of these materials. Mahata et al.[28]first reported the use of MOFs as photocatalysts to degrade the organic pollutants. Since then, many MOFs have been excited under UV–visible light {owing to the ligand-to-metal-charge-transfer (LMCT) states} and used as photocatalysts.[29] However, most of these MOFs are unstable in water which limits their applications. Interestingly, MOFs based on tetrazole ligands have shown to be highly stable in solvents, particularly in water. It has also been established that MOFs with conjugated organic ligands are promising candidates for photocatalytic applications[29] due to their luminescent properties.
In the present work, we report on the reaction of zinc nitrate with 2-cyanopyridine and NaN3 in presence of triethylamine under solvothermal condition at 160°C which generated a luminescent 3D coordination polymer, [Zn(2-PTZ)2]n, which was structurally characterized by single-crystal X-ray diffraction. In addition, the thermal stability, photoluminescence and photocatalytic properties of 1 have also been investigated.
2 Experimental Section
2.1 Materials and Methods
Zn(NO3)2.6H2O was procured from Merck India. 2-Cyanopyridine and sodium azide were procured from Sigma-Aldrich. All the starting reagents were used without further purification. Thermogravimetric analysis data were collected using a Hi-Res TGA 2950 Thermogravimetric analyzer from TA Instruments. C, H and N analyses were performed on a Perkin–Elmer 2400 CHN Elemental Analyzer. Fourier transform infrared (FTIR) spectroscopy was done on a Jasco FT-IR-5300 spectrophotometer (as KBr pellet) within 4000-400 cm −1 region. The photoluminescence spectrum was recorded on an ocean optics system within 385–750 nm range.
2.2 Synthesis of 1
A mixture of Zn(NO3)2.6H2O (298 mg, 1mmol), 2-cyanopyridine (208 mg, 2 mmol), sodium azide (195 mg, 3 mmol), triethylamine (202 mg, 2 mmol) and 15 mL of DMF was placed in a 20 mL Teflon-lined stainless-steel reactor and heated at 160°C for 24 h, under autogenous pressure and then brought to room temperature at a rate of 5°C/h. Colourless crystals suitable for X-ray analysis were obtained in a good yield (71% based on metal salt). Microanalysis - C12H8N10Zn (357.65): calcd. (%) C 40.37, H 2.04, N 39.21, Found (%) C 40.45, H 2.43, N 40.01. Selected peaks in IR spectrum (KBr, cm −1): 1638 (w), 1612 (s), 1571 (m), 1469 (m), 1444 (s), 1400 (m), 1138 (m), 757 (m), 732 (m), 513 (w).
2.3 Structure Determination and Refinement
A single crystal suitable for X-ray crystallography was mounted on a nylon loop. Single-crystal X-ray data of 1 were collected at 173(2) K by using a Bruker TXS diffractometer with Mo K α radiation (k = 0.71073 Å) The structure was solved by direct methods with SIR97[30] and refined through full-matrix least squares techniques on F 2 by the SHELX-97 program.[31] All non-hydrogen atoms were refined anisotropically, while the hydrogen atoms were located geometrically and refined isotropically. Pertinent crystal data and structural refinement for 1 are summarized in table 1.
3 Results and Discussion
The IR spectrum of 1 exhibits several characteristic strong and medium bands There are no absorptions around ∼2300 cm −1 and ∼2100 cm −1 indicating the absence of cyano and azide groups while the peaks in the range 1571–1447 cm −1 indicate that the [2 + 3] cycloaddition reaction between cyano group and azide anion has taken place.
3.1 Crystal Structure Description of 1
The single-crystal X-ray structural analysis reveals that 1 is a three-dimensional framework. The asymmetric unit contains one Zn(II) cation on a special position and one tetrazole ligand. The Zn(II) cation is located on a twofold axis of the space group C2/c leading to a C2-symmetric octahedral coordination sphere consisting of four tetrazole nitrogen atoms (two N1 and two N2) and two pyridyl nitrogen atoms (two N5) provided by four PTZ ligands. The Zn–N1 [2.1119(17) Å] and Zn–N2 [2.1249(19) Å] distances all fall into the normal bond length ranges i.e., 2.113 to 2.154 Å, corresponding to the typical values in Zn(II)-nitrogen compounds,[23,32]but the Zn–N5 distance [2.2259(19) Å] is slightly longer. The bond angles N–Zn1–N are in the range from 76.32(7)° to 97.11(11)° for cis-standing nitrogen atoms, while they are 172.03(6)° and 170.87(10)° for trans-standing nitrogen atoms. Selected bond lengths and angles are given in table 2.
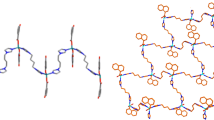
The rings of the PTZ ligand are not coplanar and form a dihedral angle of 12.15(12)° (figure 1). PTZ acts as a tridentate ligand and provides two nitrogen donors to one zinc centre, both from the pyridyl as well as the tetrazole group, while the one nitrogen of the tetrazole group coordinates to an adjacent zinc atom. Thus, each PTZ coordinates to two different zinc centres forming a M2 L 4 type coordination polymer along [001][33] (figures 2a and 2b).
In the packing of 1, weak contacts of the type C3-H3 ⋯N4 with a H-N distance of 2.475 Å and an C-H-N angle of 172° exist between pyridyl C-H groups and tetrazolyl nitrogen atoms. By two of these contacts ten-membered rings consisting of two donor and two acceptor atoms are formed which can be described with a \(R_{2}^{2}\)(10) descriptor in terms of graph-set analysis[34,35](figures 3a and 3b).
It is still a challenge for chemists to design and construct MOFs with the expected structures and properties, because the molecular structural architectures of MOFs are influenced by various factors, such as the reaction temperature and pH value. A trinuclear cadmium complex, Cd3(OH)2Cl2(4-PTZ)2, has been reported by Xue et al. which was obtained by the reaction of cadmium chloride with 4-cyanopyridine in the presence of NaN3 and water (pH ∼6.5) under hydrothermal condition at 110°C (via in situgeneration of the 4-PTZ ligand).[36] On the other hand, a mononuclear cadmium complex, Cd(4-PTZ)2(H2O), was carried out by the same group wherein reaction of CdCl2 in H2O was carried out with pre-synthesized 4-PTZ (pH ∼4.0) under the same hydrothermal conditions. The authors concluded that pH is responsible for the difference. The structure of previously reported Zn(II) complex with the same ligand (2-PTZ) i.e. [Zn(2-PTZ)2(H2O)2]n[18]is quite different from the structure of 1. The complex [Zn(2-PTZ)2(H2O)2]n was synthesized by a [2 + 3] cycloaddition reaction under hydrothermal heating at 105°C with 2-cyanopyridine, NaN3 and ZnCl2 (pH ∼6.5). The complex [Zn(2-PTZ)2(H2O)2]n crystallizes as a two-dimensional chain with four equatorial nitrogen atoms from two 2-PTZ ligands and two apical water molecules, which was supported by Sharpless’s proposed structures of the intermediate precipitates.[15–17]The reaction of zinc nitrate, 2-cyanopyridine and NaN3 in presence of triethylamine under solvothermal condition (present work) at 160°C (pH ∼8.5) leads to the formation of [Zn(2-PTZ)2]n. This result has further substantiated the mechanism proposed by Sharpless’s for the formation of tetrazoles. The compositional and structural difference between [Zn(2-PTZ)2(H2O)2]n and 1 is probably due to the different thermal conditions and pH of their respective reactants mixtures.
3.2 XRPD, SEM
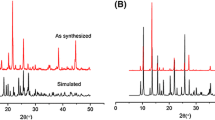
The XRPD pattern of compound 1 was investigated at room temperature. The average crystallite size calculated by Scherrer equation using four strongest reflections (corresponding to (110), (020), (221) and (331)) in 1 is ∼72 nm. As shown in figure 4, the XRPD patterns measured for 1 were in good agreement with the XRPD patterns simulated from the respective single-crystal X-ray data of 1 using the Mercury 1.4 program, demonstrating the phase purity of the product. The dissimilarities in intensities may be due to the preferred orientations in the crystalline powders.
Figure 5 shows SEM image of the as-synthesized crystals of compound 1 which demonstrates that morphologically, the product consists of 3D flower-like microspheres with diameters ranging from 30 to 40 μm. Interestingly, these flower-like microspheres are built from densely packed layers of two dimensional microflakes with a thickness of about 700–800 nm. A close observation reveals thatthese densely packed microflakes bloom out from the central part and appear like a flower.
3.3 UV–Vis diffuse reflectance spectroscopy and Band-gap calculation
The diffuse-reflectance UV/Vis spectrum shows the absorption feature (figure 6) of 1. The main UV absorption band is around 398 nm, which can be assigned to ligand-to-metal charge transfer (LMCT). To obtain the precise value of band gap from the absorption edge, the point of inflection in the first derivative of the absorption spectrum was used. The value of the band gap obtained from the corresponding transition is 3.2 eV. Band gap was calculated by using the Tauc plot showing in figure 6 (inset).
3.4 Photocatalytic property
The photocatalytic activity of 1 was evaluated by the degradation of Methylene Blue (MB) in aqueous solution. Experimental details for photocatalysis are available in Supplementary Information. The photodegradation experiment under UV light irradiation was carried out after the dark adsorption equilibrium was achieved. The characteristic absorption of MB at about 650 nm was selected to monitor the adsorption and photocatalytic degradation process (figure S1 in Supplementary Information). The comparison of decomposition rate of MB under UV light in the presence and absence of H2O2 using compound 1 as photocatalyst is depicted in figure 7. The control experiments show that no MB degradation was observed over the photocatalyst 1 and H2O2 without the light irradiation (figure 7e). In contrast, ∼2% MB was self-decolourised after 20 min of UV irradiation (figure 7a), while ∼25% MB was degraded after 20 min (figure 7c). Thus, it can be concluded that 1 shows the photocatalytic activity for MB degradation, although the photodegradation rate is low.
The photocatalytic activity of 1 for MB degradation can be enhanced by the addition of the hydrogen peroxide (H2O2) electron acceptor.[29] As shown in scheme 1, the photocatalytic reaction starts from the generation of electron–hole pairs. The photoexcited holes either react with organic moieties directly or react with OH − to generate ⋅OH for further oxidation reaction. The electron–hole recombination reduces the efficiency of photocatalytic reaction. Therefore, the introduction of an external electron acceptor is expected to suppress the electron–hole recombination and enhance the photocatalytic efficiency. An enhanced photodegradation efficiency with the addition of H2O2 as an electron acceptor can be explained by eq. H2O2 + e −→ OH − +⋅ OH. 20 min UV irradiation induces 70% MB degradation (figure 7d), whereas less than 50% MB was degraded when only H2O2 was used in the reaction (figure 7b). In addition, stability of compound 1 was monitored using XRPD during course of the photocatalytic reaction (figure 5). After photocatalysis, the XRPD pattern of compound 1 is slightly different to that of the original compound implying its stability towards photocatalytic reactions.
3.5 Thermal Analysis
Compound 1 is air-stable and retains crystalline integrity at ambient temperature. The thermogravimetric analysis curve (figure 8) shows it is stable up to 240°C and thereafter the organic groups starts to decompose. The weight loss between 231°C to 275°C has been attributed to the decomposition of three molecules of N2 (calc. 26.61%, obs.25.57%). In the temperature range 383°C to 457°C two cyano-pyridine groups were liberated (calc. 80.54%, obs. 76.92%). These consecutive decompositions suggest the total destruction of the framework.
3.6 Photoluminescence
Subsequently, fluorescence of compound 1 has been investigated at room temperature in the solid state. As shown in figure 9, 1 exhibits a strong emission band at 454 nm upon excitation at 357 nm. The emission of compound 1 may be attributed to the π tz-5s LMCT transition.[25] Thus, compound 1 may find potential applications as a luminescent material in blue light-emitting devices.
4 Conclusions
In conclusion, a new bis[5-(2-pyridyl)tetrazolato]zinc (II) coordination polymer has been synthesized in situ under hydrothermal conditions and characterized by single crystal X-ray structure determination. Compound 1 features a 3D infinite coordination network of dimeric {(2-PTZ)2Zn} repeat units with octahedral coordination around zinc centers. In addition, it displays modest thermal stability and shows strong solid-state blue fluorescent emission. Lastly, compound 1 is a photocatalyst and decolourizes methylene blue under UV irradiation.
References
Liu W T, Li J Y, Ni Z P, Bao X, Leng J D, Liu J L and Tong M L 2012 Cryst. Growth Des. 12 1482
Cui P, Chen Z, Gao D, Zhao B, Shi W and Cheng P 2010 Cryst. Growth Des. 10 4370
Nasani R, Saha M, Mobin S M and Mukhopadhyay S 2013 Polyhedron 55 24
Singh S, Gupta D K, Noeth H and Pandey A 2013 J. Chem. Crystallogr. 43 82
Gupta D K, Singh S, Mayer P and Pandey A 2011 Inorg. Chem. Com. 14 1485
Suh M P, Park H J, Prasad T K and Lim D W 2012 Chem. Rev. 112 (2) 782
He Y B, Zhou W, Yildirim T and Chen B L 2013 Energy Environ Sci. 6 2735
Kreno L E, Leong K, Farha K, Allendorf M, Duyne V and Hupp T 2012 Chem. Rev. 112 1105
Lin J B, Xue W, Zhang J P and Chen X M 2011 Chem. Commun. 47 926
McKinlay A C, Morris R E, Horcajada P, Ferey G, Gref R, Couvreur P and Serre C 2010 Angew. Chem. Int. Ed. 49 6260
Wanderley M M, Wang C, Wu C D and Lin W B 2012 J. Am. Chem. Soc. 134 (22) 9050
Wang G Y, Yang L L, Li Y, Song H, Ruan W J, Chang Z and Bu X H 2013 Dalton Trans. 42 12865
Zhang T and Lin W 2014 Chem. Soc. Rev. 43 5982
Hantzsch A and Vagt A 1901 Ueber das sogenannte Diazoguanidin. Liebig’s Ann 314 339
Demko Z P and Sharpless K B 2001 J. Org. Chem. 66 7945
Demko Z P and Sharpless K B 2002 Angew. Chem. 114 2214; 2002 Angew. Chem. Int. Ed. 41 2110
Himo F, Demko Z P, Noodleman L and Sharpless K B 2003 J. Am. Chem. Soc. 125 9983
Wang L Z, Qu Z R, Zhao H, Wang X S, Xiong R G and Xue Z L 2003 Inorg. Chem. 42 3969
Singh S, Mayer P and Pandey A 2010 Indian J. Chem. 49A 1345
Wu J Y, Huang S M, Huang Y C and Lu K L 2012 Cryst. Eng. Comm. 14 1189
Robson R, Abrahams B F, Batten S R, Gable R W, Hoskins B F and Liu J 1992 ACS Symp. Ser. T Bein (Ed.) (ACS: Washington, DC) 499 256
Haasnoot G J 2000 Coord. Chem. Rev. 200 131
Su C Y, Cai Y P, Chen C L, Smith M D, Kaim W and Loye H C 2003 J. Am. Chem. Soc. 125 8595
Bauer A, Timofeeva V, Settersten B, Patterson D, Liu H, Simmons A and Allendorf D 2007 J. Am. Chem. Soc. 129 7136
Mishra A, Nayak P K and Periasamy N 2004 Tetrahedron Lett. 45 6265
Nayak P K, Agarwal N, Ali F, Patankar M P, Narasimhan K L and Periasamy N J. Chem. Sci. 122 847
Fordham S, Wang X, Bosch M and Zhou H 2015 “Lanthanide Metal-Organic Frameworks” In Structure and Bonding P Cheng (Ed.) (Berlin: Heidelberg) 163 p. 1
Mahata P, Madras G and Natarajan S 2006 J. Phys. Chem. B 110 13759
Wang C C, Li J R, Lv X L, Zhang Y Q and Guo G 2014 Energy Environ. Sci. 7 2831
Altomare A, Burla M C, Camalli M, Cascarano G L, Giacovazzo C, Guagliardi A, Moliterni A G G, Polidori G and Spagna R 1999 J. Appl. Cryst. 32 115
Sheldrick G M 1997 SHELXL-97: Program for refining crystal structure refinement (University of Goettingen: Goettingen)
Zhu H B, Yang W N and Hu J 2013 J. Coord. Chem. 16 2775
Ma H, Xu Y, Meng Q, Zhang L, Wang R and Sun D 2014 Z. Anorg. Allg. Chem. 640 1219
Bernstein J, Davis R E, Shimoni L and Chang N -L 1995 Angew. Chem. Int. Ed. Engl. 34 1555
Etter M C, MacDonald J C and Bernstein J 1990 Acta Cryst. B 46 256
Xue X, Wang X S, Wang L Z, Xiong R G, Abrahams B F, You X Z, Xue Z L and Che C M 2002 Inorg. Chem. 41 6544
Acknowledgements
The authors gratefully acknowledge the CIR facility of MNNIT, Allahabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary crystallographic data for 1 have been deposited with the CCDC 985644 and can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/Community/Requestastructure/Pages/DataRequest.aspx. (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK (fax: + 44 (1223) 336-033; e-mail: deposit@ccdc.cam.ac.uk). Other supporting information associated with this article i.e., figure S1 and photocatalytic experimental details are available in the electronic form of supplementary data. Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
CHOUHAN, A., PANDEY, A. & MAYER, P. Synthesis crystal structure, photoluminescence and photocatalytic property of a new three dimensional Zinc(II) tetrazole framework. J Chem Sci 127, 1599–1606 (2015). https://doi.org/10.1007/s12039-015-0925-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0925-8