Abstract
Incorporating sulfanilic acid as a hydrophobic Brønsted acid inside the nanospaces of SBA-15 led to a water-tolerant solid acid catalyst, SBA-15-PhSO3H, which showed excellent catalytic performance in synthesis of uracil-fused spirooxindoles in aqueous ethanol. The synthesized compounds were evaluated for their antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging assay.

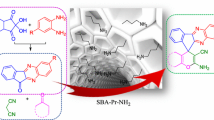
Green synthesis of uracil-fused spirooxindoles using sulfanilic acidfunctionalized SBA-15 as a reusable heterogeneous acid catalyst and the antioxidant activity of the synthesized compounds are described.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Multi-component reactions (MCRs) are an attractive strategy to access complex structures in a single synthetic operation from simple building blocks and they offer high efficiency and atom economy.[1] This reduces time and saves both energy and raw materials. On the other hand, the use of environmentally benign reagents and solvents in combination with heterogeneous and reusable catalysts in MCRs represents one of the more powerful green chemical technology procedures.[2] Solid catalysts are generally preferable in catalysis because of their easy separation, recyclability, high thermal stability and low pollution effects.[3] SBA-15 as a mesoporous silica material is a promising candidate for the immobilization of organocatalyst due to its high surface area, porosity, uniform pore size distribution and hydrothermal stability.[4] To achieve strong acidic sites, SO3H groups have been functionalized on the surface of the mesoporous walls, giving excellent catalytic properties in a series of acid-catalyzed reactions.[5–14]
Spirooxindoles, as interesting N-heterocycles, have attracted much attention because of their biological properties including antimicrobial,[15] antitumoral,[16] antibiotic agents,[17] and inhibitors of human NK-1 receptor.[18] Moreover, spirooxindole system is the core structure of many pharmacological agents and natural alkaloids (figure 1). For instance, spirotryprostatin B, a natural alkaloid isolated from the fermentation broth of Aspergillus fumigatus, has been identified as a novel inhibitor of microtubule assembly,[19] also pteropodine and isopteropodine have been shown to modulate the function of muscarinic serotonin receptors.[20]
Owing to their pharmaceutical importance, various synthetic methods for producing spirocyclic oxindoles have been reported.[21–31]Despite the efficiency of the reported protocols, some of them suffer from drawbacks such as long reaction time, harsh reaction conditions, corrosiveness, toxicity, cost, as well as unreusability of catalyst.
Thus, in continuation of our studies in developing environmentally benign methodologies for the synthesis of heterocyclic compounds,[32–34] we herein report the efficient synthesis of uracil fused spirooxindoles via three component reaction of isatin derivatives with cyclic 1,3-diketones and 1,3-dimethyl-6-amino uracil in the presence of SBA-15-PhSO3H as a reusable heterogeneous acid catalyst in aqueous ethanol (scheme 1). These spirooxindoles have also been screened for their antioxidant potency by DPPH radical scavenging.
2 Experimental
2.1 Materials and apparatus
The chemical materials used in this work were purchased from Merck or Aldrich and used without purification. Mesoporous silica SBA-15 and sulfanilic acid functionalized SBA-15 were synthesized according to reported procedures in the literature.[32,35] IR spectra were recorded on a Bruker Tensor 27 spectrometer. Elemental analyses were carried out on a Perkin-Elmer 2400II CHNS/O instrument. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 AVANCE spectrometer at 400.13 and 100.61 MHz, respectively. Chemical shifts are given in parts per million (δ) relative to internal tetramethylsilane standard, and coupling constants (J) are reported in hertz. Thermogravimetric analysis (TGA) was recorded on a Stanton Redcraft STA-780 (London, UK). Transmission electron microscopy (TEM) images were obtained using a Philips CM10 microscope operated at 200 kV. X-ray powder diffraction (XRD) patterns were recorded by an X-ray diffractometer (XRD, GBC MMA Instrument) with Be-filtered Cu K α radiation. Melting points were determined on a Thermo Scientific IA9200.
2.2 Synthesis and functionalization of SBA-15
Mesoporous SBA-15 and (3-chloropropyl)trimethoxysilane modified SBA-15 (SBA-15-Cl) materials were synthesized according to our previous report.[32] Grafting sulfanilic acid on SBA-15-Cl surface was as follow:[35] 1.0 g of SBA-15-Cl was dispersed in 30 mL dry toluene. To this suspension, 1.5 g (8.6 mmol) of sulfanilic acid was added and followed by 1.2 mL (8.6 mmol) of triethylamine acting as a base. The mixture was heated for 48 h at 110 ∘C to produce a brown solid. The product was filtered off and washed with toluene, dichloromethane and finally with acidified ethanol. The sample was dried at 110 ∘C for 24 h and ground to powder.
2.3 General procedure for the synthesis of uracil fused spirooxindoles
A mixture of isatins (1.0 mmol), 1,3-dimethyl-6-amino uracil (1.0 mmol), cyclic 1,3-diketones (1.0 mmol) and SBA-15-PhSO3H (0.15 g, 10 mol%) in 4:1 water:ethanol (5.0mL)wasstirredat80 ∘C.Aftercompletionofthereaction, as monitored with TLC, hot methanol (5.0 mL) was added to the reaction mixture and filtered to separate the catalyst which will be recycled for other reactions. Pure products were afforded by evaporation of the solvent, followed by recrystallization from ethanol. The products 4a-j were characterized on the basis of their elemental analysis, 1H and 13C NMR, IR and mass spectra.
2.4 Selected spectral data
2.4.1 1-Ethyl-1\(^{\prime }\),3\(^{\prime }\),8\(^{\prime }\),8\(^{\prime }\)-tetramethyl-8\(^{\prime }\),9\(^{\prime }\)-dihydro-1\(^{\prime }\)H- spiro[indoline-3,5\(^{\prime }\)-pyrimido[4,5-b] quinoline]-2,2\(^{\prime }\),4\(^{\prime }\), 6\(^{\prime }\)(3\(^{\prime }\)H,7\(^{\prime }\)H,10\(^{\prime }\)H)-tetraone (4g)
White powder; M.p. >300 ∘C; IR (KBr cm −1)\(\nu _{\max }\): 3226, 3060, 2956, 1663, 1609, 1509, 1375, 1241, 1131 cm −1; 1H NMR (400.13 MHz,DMSO-d6) δ:0.90and1.01(2s,6H,2CH3),1.21(t, J = 7.6 Hz, 3H, CH3), 1.91 and 2.10 (ABq, J = 16.0 Hz, 2H, CH2), 2.58 (s, 2H, CH2), 2.96 and 3.46 (2s, 6H, 2NCH3),3.60-3.70(m, 2H, CH2), 6.79-6.81 (m, 2Harom), 6.94 (d, J = 6.8 Hz, 1Harom), 7.09 (t, J = 7.6 Hz, 1Harom), 9.13 (br s, 1H, NH) ppm; 13C NMR (100.6 MHz, DMSO-d6) δ: 12.2,26.9,27.5,28.0,28.9,29.7,30.8, 32.3, 34.5, 48.9 (Cspiro), 50.9, 90.2, 107.0, 111.0, 121.2, 123.1, 127.8, 135.7, 144.2, 150.6, 159.7, 178.2, 193.7 ppm; MS (EI,70eV) m/z:434.2(M +);Anal.CalcdforC24 H 26 N 4 O 4 (434.49): C, 66.34; H, 6.03; N, 12.89%. Found: C, 66.41; H, 6.09; N, 12.82%.
2.4.2 Ethyl2-(1\(^{\prime }\),3\(^{\prime }\),8\(^{\prime }\),8\(^{\prime }\)-tetramethyl-2,2\(^{\prime }\),4\(^{\prime }\),6\(^{\prime }\)-tetraoxo- 2\(^{\prime }\),3\(^{\prime }\),4\(^{\prime }\),6\(^{\prime }\),7\(^{\prime }\),8\(^{\prime }\),9\(^{\prime }\),10\(^{\prime }\)-octahydro-1\(^{\prime }\)H-spiro[indoline-3, 5’-pyrimido[4,5-b]quinolin]-1-yl)acetate (4h)
White powder; M.p. >300 ∘C; IR (KBr cm −1)\(\nu _{\max }\): 3231, 3062, 2956, 1662, 1614, 1499, 1373, 1202, 1107 cm −1; 1H NMR (400.13 MHz, DMSO-d6) δ: 0.91 and 1.02 (2s, 6H, 2CH3), 1.22 (t, J = 7.2 Hz, 3H, CH3), 1.92 and 2.10 (ABq, J = 16.0 Hz, 2H, CH2), 2.60 (s, 2H, CH2), 2.96 and 3.47 (2s, 6H, 2NCH3), 4.12-4.17 (m, 2H, CH2), 4.36 and 4.38 (ABq, J = 16.8 Hz, 2H, CH2), 6.80 (d, J = 7.6 Hz, 1Harom), 6.84 (t, J = 7.6 Hz, 1Harom), 6.96 (d, J = 6.4 Hz, 1Harom), 7.10 (t, J = 7.8 Hz, 1Harom), 9.06 (br s, 1H, NH) ppm; 13C NMR (100.6 MHz, DMSO-d6) δ: 14.5, 26.9, 28.0, 28. 9, 30.9, 32.3, 43.1, 48.23 (Cspiro), 50.8, 61.0, 90.1, 108.1, 110.9, 121.9, 123.1, 127.8, 135.2, 144.2, 150.6, 159.6, 159.6, 168.4, 179.0, 193.5 ppm; MS (EI, 70 eV) m/z: 492.3 (M +); Anal. Calcd for C26 H 28 N 4 O 6 (492.52): C, 63.40; H, 5.73; N, 11.38%. Found: C, 63.35; H, 5.81; N, 11.42%.
2.4.3 1-Benzyl-1\(^{\prime }\),3\(^{\prime }\),8\(^{\prime }\),8\(^{\prime }\)-tetramethyl-8\(^{\prime }\),9\(^{\prime }\)-dihydro-1\(^{\prime }\) H-spiro[indoline-3,5\(^{\prime }\)-pyrimido [4,5-b]quinoline]-2,2\(^{\prime }\), 4\(^{\prime }\),6\(^{\prime }\)(3\(^{\prime }\)H,7\(^{\prime }\)H,10\(^{\prime }\)H)-tetraone(4i)
White powder; M.p. >300 ∘C; IR (KBr cm\(^{-1}) \nu _{\max }\): 3185, 3065, 2958, 1705, 1662, 1610, 1513, 1376, 1181, 1105 cm −1; 1H NMR (400.13MHz,DMSO-d6) δ:0.93and1.03(2s,6H,2CH3), 1.97and 2.16 (ABq, J = 15.6 Hz, 2H, CH2), 2.62 (s, 2H, CH2),3.00and3.48(2s,6H,2NCH3),4.75and4.92(ABq, J = 16.0 Hz, 2H, CH2), 6.40-7.65 (9Harom), 9.13 (br s, 1H, NH) ppm; 13C NMR (100.6 MHz, DMSO-d6) δ: 26.9, 28.0, 28.9, 30.9, 32.4, 44.7, 47.8 (Cspiro), 50.9, 90.3, 107.9, 110.7, 121.7, 123.1, 127.2, 127.7, 128.6, 135.2, 137.7, 144.6, 150.6, 155.1, 159.8, 169.0, 171.1, 179.2, 193.8 ppm; MS (EI, 70 eV) m/z: 496.2 (M +); Anal. Calcd for C29 H 28 N 4 O 4 (496.56): C, 70.15; H, 5.68; N, 11.28%. Found: C, 70.23; H, 5.74; N, 11.20%.
2.5 Antioxidant activity
Radical scavenging activity of the spirooxindoles 4a-j was determined against stable DPPH radical spectrophotometrically.[36] A stock solution (1.0 mg mL −1) of compounds was prepared in dimethyl sulfoxide (DMSO). Then, 1.0 mL of each compound solution was added to 1.0 mL of a 0.004% methanol solution of the DPPH radical and shaken vigorously. After 30 min of incubation in the dark at room temperature, the absorbance was observed against a blank at 517 nm. The assay was carried out in triplicate and the percentage of inhibition was calculated using the following formula:
Where Ac is the absorbance value of the control sample and As is the absorbance value of the tested sample.
3 Results and Discussion
3.1 Synthesis and characterization of catalyst
SBA-15-sulfanilic acid was synthesized according to the following procedure, by silylation/condensation of SBA-15 with (3-chloro propyl)trimethoxy silane, which was then reacted with sulfanilic acid to form sulfanilic acid-functionalized SBA-15 material (scheme 2).
The catalyst structure was characterized by elemental analysis, Fourier-transform infrared spectroscopy, thermogravimetric analysis, X-ray diffraction and transmission electron microscopy (see ??). The amount of acid in SBA-15-PhSO3H was evaluated by the nitrogen or sulfur content, 0.66 mmol/g, on the basis of elemental analysis (C: 7.28%; H: 1.12%; N: 0.93%; S: 2.13%), which was in good agreement with the result obtained from TG analysis. The concentration of exchangeable proton on the SBA-15-PhSO3H, which was determined by exchanging the ionisable proton with excess Na + (aqueous NaCl) following by titrating the H+ liberated with standard NaOH solution,[35] was 0.45 ±0. mmol/g.
3.2 Catalytic study
To assess the catalytic activity of SBA-15-PhSO3H in the synthesis of spirooxindole derivatives, the three-component condensation of isatin 1a, dimedone 2a and 1,3-dimethyl-6-mino uracil 3a in equimolar ratio as model reaction was investigated. In this regard, we attempted to determine the optimum conditions by examining the influence of catalyst, solvent and temperature variations on the progress of the trial reactions. The results of the optimized conditions are summarized in table 1. In our initial endeavor to synthesize uracil fused spirooxindoles, the model reaction was carried out in water in the absence of any catalyst under reflux. It was observed that, the reaction did not proceed to completion even after 24 h and only a trace amount of the desired product 4a was formed (table 1, entry 1).
To explore the suitable reaction conditions, the above model reaction was performed in the presence of various catalysts such as SBA-15, nano SiO2, nano MgO, nano TiO2, DABCO, L-proline, sulfanilic acid, SBA-15-PrSO3H, and SBA-15-PhSO3H in water under reflux conditions (table 1, entries 2-10). From the results, it is obvious that SBA-15-PhSO3H demonstrates superior catalytic activity in green synthesis of spirooxindole and is the best catalyst among those examined (table 1, entry 10). The higher efficacy of SBA-15-PhSO3H might be attributed to the high surface area of ordered SBA-15 and hydrophobic character of phenyl moiety in close proximity to sulfonic acid group as a catalyst active center. Incorporating sulfanilic acid, hydrophobic Brønsted acid, inside the nanospaces of SBA-15 could provide enough hydrophobicity for a fast mass transfer of starting materials over the catalyst and expel out the water which is formed during the reaction from the catalyst surface.[11]
To investigate the effects of solvent, the condensation reaction of the model substrates was examined in different solvents including CH3CN, MeOH, EtOH and solvent system 4:1H2O-EtOH mixture using 10 mol% SBA-15-PhSO3H under reflux conditions (table 1, entries 11-14). Although H2O is superior to organic solvents, even in H2O-EtOH mixture due to better solubility of reactants, a good yield of desired product was obtained in shorter reaction time (table 1, entry 14). Therefore, 4:1 H2O: EtOH mixture was selected as the reaction solvent in the following investigation.
We also evaluated the amount of catalyst required for this transformation. The amount of SBA-15-PhSO3H, which afforded the best yields, was 10 mol%. Increasing the amount of catalyst did not change the yield dramatically (table 1, entry 15), whereas reduction of it significantly decreased the product yield (table 1, entry 16). To further optimize the reaction conditions, the same reaction was carried out in 4:1 H2O:EtOH at temperatures ranging from 25 to 80 ∘C. We observed that, the reaction failed completely at room temperature even after 24 h (table 1, entry 18), and the yield of product 4a was improved and the reaction time was shortened as the temperature was increased to 80 ∘C.
To explore the extent and limitations of this reaction, we applied SBA-15-PhSO3H (10 mol%) in the reaction of various substituted isatins 1a-g with cyclic 1,3-diketones 2a and 2b, and 1,3-dimethyl-6-amino uracil 3a or 6-amino thiouracil 3b in 4:1 H2O: EtOH at 80 ∘C, furnishing the respective uracil fused spirooxindoles 4a-j in good yields. The yield of products and time taken for maximum conversion of the substrates in each case, are listed in table 2.

As can be seen from table 2, the presence of electron withdrawing substituents on the aromatic ring accelerated the reaction rate. The nature of substituents did not affect the reaction yields. The structure of products 4a-j was characterized on the basis of their elemental analysis, 1H and 13C NMR, IR and mass spectroscopic data.
To evaluate the recyclability and stability of our catalyst, we designed a set of experiments by conducting successive condensation of model substrates using recovered SBA-15-PhSO3H under optimized conditions. After the completion of the first reaction run, hot methanol was added to the reaction mixture and then filtered to separate the catalyst. The catalyst was thoroughly washed with ethanol and finally dried at 80 ∘C for 3 h. It was found that the recycled catalyst could be used directly for at least five reaction cycles without noticeable drop in the product yield and its catalytic activity (figure 2). It is obvious that the amount of recycled catalyst is reduced after each cycle; therefore, the reactants were taken with respect to the amount of the catalyst recovered after each reaction cycle.
The proposed mechanism for the synthesis of uracil fused spirooxindoles 4 can be described as shown in scheme 3.[30,31]SBA-15-PhSO3H acts as a protonic acid which increases the electrophilicity of the carbonyl carbon of isatin so that undergoes reaction with dimedone 2, leading to the formation of the intermediate 5 (established through isolation). Intermediate 5 on further reaction with aminouracil 3 in the presence of the mesoporous solid Brønsted acid catalyst forms the adduct 6, which undergoes cyclization with the elimination of water molecule to give the spirooxindole 4. It is believed that while the hydrophobic nature of the catalyst affords paths for ecient mass transfer of starting materials to the active sites, at the same time, it can also increase the conversion of substrates to the product by removing the water formed during the reaction from the catalyst surface.
3.3 In vitro antioxidant activity
In vitro antioxidant activity for uracil fused spirooxindoles 4a-j was evaluated by use of the DPPH radical-scavenging method[36] and the results are presented in figure 3. Antioxidant compounds scavenge DPPH radicals by the process of either hydrogen or electron donation and the purple color from the DPPH radical assay solution becomes light yellow which can be quantified by the decrease of absorbance at a wavelength 517 nm. Spirooxindoles 4a-j showed good antioxidant activity (67–96%), which can be attributed to their N-H groups.[37,38] As illustrated in figure 3, compounds 4a, 4c, 4f, 4g and 4j exhibited a high percentage inhibition of DPPH radical activity (85.3 ±0.9%, 82.7 ±0.8%, 92.5 ±1.4%, 89.4 ±1.0%, and 96.2 ±1.5%, respectively) and were the most effective DPPH radical scavengers.
4 Conclusion
In conclusion, we have designed a highly powerful and water-tolerant Brønsted solid acid catalyst that shows remarkable catalytic activity for synthesis of uracil fused spirooxindoles as antioxidant agents. Environmental acceptability, high yield of product, simple workup, easy removal and recyclability of the catalyst are the important features of this atom economical protocol.
References
(a) Kefayati H, Narchin F and Rad-Moghadam K 2012 Tetrahedron Lett. 53 4573; (b) Kumar M, Sharma K and Arya A K 2012 Tetrahedron Lett. 53 4604; (c) Arya A K, Gupta S K and Kumar M 2012 Tetrahedron Lett. 53 6035; (d) Zhu J and Bienayme H 2005 In Multicomponent Reactions (Weinheim: Wiley-VCH)
Clark J H and Macquarrie D J 1996 Chem. Soc. Rev. 25 303
Adam F, Hello K M and Aisha M R B 2011 J. Taiwan Inst. Chem. E 42 843
(a) Martin-Aranda R M and Cejka J 2010 Top. Catal. 53 141; (b) Mondal J, Nandi M, Modak A and Bhaumik A 2012 J. Molecul. Catal. A 363–364 254
Karimi B and Zareyee D 2008 Org. Lett. 10 3989
Karimi B and Vafaeezadeh M 2012 Chem. Commun. 48 3327
Yuan C, Huang Z and Chen J 2012 Catal. Commun. 24 56
Parvin M N, Jin H, Ansari M B, Oh S M and Park S E 2012 Appl. Catal. A 413-414 205
Mohammadi Ziarania G, Mousavi S, Lashgari N and Badiei A 2013 J. Chem. Sci. 125 1359
Rostamnia S, Xin H, Liuc X and Lamei K 2013 J. Molecul. Catal. A 374–375 85
Zareyee D, Moosavi S M and Alaminezhad A 2013 J. Molecul. Catal. A 378 227
Rostamnia S and Hassankhani A 2014 Synlett 25 2753
Mohammadi Ziarania G, Lashgari N and Badiei A 2015 J. Molecul. Catal. A 397 166
Bahrami K, Khodaei M M and Fattahpour P 2015 J. Chem. Sci. 127 167
Abdel-Rahman A H, Keshk E M, Hanna M A and El-Bady S M 2004 Bioorg. Med. Chem. 12 2483
Kornet M J and Thio A P 1976 J. Med. Chem. 19 892
Okita T and Isobe M 1994 Tetrahedron 50 11143
Rosenmond P, Hosseini-Merescht M and Bub C 1994 Liebigs Ann. Chem. 2 151
Usui T, Kondoh M, Cui C B, Mayumi T and Osada H 1998 Biochem. J. 333 543
Kang T H, Matsumoto K, Murakami Y, Takayama H, Kitajima M, Aimi N and Watanabe H 2002 Eur. J. Pharmacol. 444 39
Litvinov Y M, Mortikov V Y and Shestopalov A M 2008 J. Comb. Chem. 10 741
Ghahremanzadeh R, Sayyafi M, Ahadi S and Bazgir A 2009 J. Comb. Chem. 11 393
Ghahremanzadeh R, Imani Shakibaei G, Ahadi S and Bazgir A 2010 J. Comb. Chem. 12 191
Balamurugan K, Perumal S and Menendez J C 2011 Tetrahedron 67 3201
Imani Shakibaei G, Feiz A, Khavasi H R, Abolhasani Soorki A and Bazgir A 2011 ACS Comb. Sci. 13 96
Jadidi K, Ghahremanzadeh R, Mirzaei P and Bazgir A 2011 J. Heterocycl. Chem. 48 1014
Arya A K and Kumar M 2011 Mol. Divers. 15 781
Kumar M, Sharma K and Arya A K 2012 Tetrahedron Lett. 53 4604
Rahmati A and Khalesi Z 2012 Tetrahedron 68 8472
Paul S and Das A R 2013 Tetrahedron Lett. 54 1149
Khalafi-Nezhad A and Mohammadi S 2013 ACS Comb. Sci. 15 512
Baharfar R and Azimi R 2014 Synth. Commun. 44 89
Baharfar R and Shariati N 2014 Aust. J. Chem. 67 1646
Azimi R and Baharfar R 2014 Can. J. Chem. 92 1163
Adam F, Hello K M and Ali T H 2011 Appl. Catal. A 399 42
Blois M S 1958 Nature 26 1199
Kumar M, Sharma K, Samarth R M and Kumar A 2010 Eur. J. Med. Chem. 45 4467
Kadhum A A H, Al-Amiery A A, Musa A Y and Mohamad A B 2011 Int. J. Mol. Sci. 12 5747
Acknowledgements
We are thankful to the Research Council of Mazandaran University for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
All additional information relating to characterization of the catalyst using FT-IR, TGA, XRD and TEM techniques (figures S1–S4), 1H NMR and 13C NMR spectra for compounds 4a-j (figures S5-S24) are available in the supplementary information, which is available free of charge at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BAHARFAR, R., AZIMI, R. Sulfanilic acid functionalized mesoporous SBA-15: A water-tolerant solid acid catalyst for the synthesis of uracil fused spirooxindoles as antioxidant agents. J Chem Sci 127, 1389–1395 (2015). https://doi.org/10.1007/s12039-015-0910-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0910-2