Abstract
Organic-inorganic hybrid single crystals of [2,3-(CH3)2C6H3NH3]4BiBr6NO3.2H2O were obtained by slow evaporation at room temperature and characterized by X-ray diffraction, IR and solid state NMR. This compound crystallizes in orthorhombic non-centrosymmetric space group Pca21 with the following lattice parameters: a = 25.8217(1), b = 7.8909(2), c = 21.4328(3) Å, V = 4367.07(13) Å3 and Z = 4. The structure was refined to R = 0.057 for 7069 independent reflections. The crystal is built up of 2,3-dimethylanilinium cations, [BiBr6]3−, disordered [NO3] anions and water molecules. The crystal packing is governed by a three-dimensional network of O-H…O, N-H…O and N-H…Br hydrogen bonds and van der Waals interaction. The infrared spectrum has been interpreted on the basis of literature data. Solid state 13C CP-MAS-NMR spectrum is in agreement with the X-ray structure.

Organic-inorganic hybrid single crystal of [2,3-(CH3)2C6H3NH3]4BiBr6NO3. 2H2O crystallizes in orthorhombic, non-centrosymmetric space group Pca21. The crystal packing is governed by a three-dimensional network of O-H…O, N-H…O and N-H…Br hydrogen bonds and van der Waals interaction. The IR and solid state 13C CP-MAS-NMR spectra are in agreement with the X-ray structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For the past three decades, the fields of fibre electronics, optical communication and lasers have experienced tremendous advancements. Non-linear optical (NLO) materials have received much attention due to their applications in optical data storage, electro-optical shutters, colour displays, optical communication and signal processing.[1]
Inorganic NLO materials exhibit excellent mechanical and thermal properties[2–5]but possess relatively modest optical non-linearities because of the lack of extended π-electron delocalization. Currently, these hybrid materials are gaining attention because they share the properties of both organic and inorganic materials, such as higher second order optical non-linearities and stable physico-chemical performance.
Among the extensively studied halometalate hybrids, the halobismuthate (III) family, and in particular the bromobismuthate, is composed of isolated distorted [BiBr6] 3− octahedra or connected by corners, edges, or faces forming one-dimensional chains, or, two three-dimensional framework.[6,7]In the majority of these hybrid materials the anionic moieties are rigid, whereas the organic cations are placed in large cavities of the anionic substructure through hydrogen bonds and electrostatic interactions. In addition, the occurrence of cation-anion interaction, usually of the type N-Hcation…X anion[8]influences the crystal packing
In this paper, we report a new inorganic–organic hybrid compound, [2,3-(CH3)2 C 6 H 3 NH 3]4BiBr6 NO 3. 2H2O, possessing a zero-dimensional structure. This material crystallizes in a non-centrosymmetric space group and could be a good candidate for non-linear optical applications. In addition, the vibrational and solid state 13C MAS NMR spectrum of the material is reported.
2 Experimental
2.1 Synthesis
For the preparation of the title compound, concentrated hydrobromic acid, 48% purity, was added dropwise to a mixture of 2,3-dimethylaniline and ethanol until complete dissolution of the solid phase. Similarly, concentrated hydrobromic acid was added dropwise to dissolve the solid phase persisting in a mixture of bismuth nitrate pentahydrate and water. The two solutions were mixed and stirred for 30 min. The resulting precipitate was filtered off and dissolved in hydrobromic acid. Pale yellow crystals suitable for X-ray analysis were obtained after several weeks by slow evaporation of the solvent at room temperature.
2.2 Physical measurements
The infrared spectrum was recorded in the range of 4000–400 cm−1 with a Perkin-Elmer 1000 FTIR spectrometer using a sample dispersed in spectroscopically pure KBr pressed into a pellet.
The 13C CP-MAS-NMR spectrum was recorded, by use of cross-polarization from protons (contact time 5 ms), on a Bruker DSX-300 spectrometer operating at 75.49 MHz with a classical 4 mm probe head allowing spinning rates up to 10 kHz. The chemical shifts are given relative to tetramethylsilane. It was checked that there was a sufficient delay between the scans allowing a full relaxation of the nuclei.
2.3 X-ray crystallographic study
A single crystal was carefully selected under polarizing microscope in order to perform its structural analysis by X-ray diffraction. Diffraction data were collected on a Nonius Kappa diffractometer equipped with a CCD detector with graphite-monochromatized MoK α radiation (λ= 0.71069 Å). Intensities were corrected for Lorentz, polarization and absorption effects.[9] The structure was solved by direct methods with the SIR97 suite of programs[10] and refinement were performed on F 2 by full-matrix least-squares methods with all non-hydrogen atoms anisotropic. Hydrogens were included on calculated positions, riding on their carrier atoms, apart from those linked to OW1 and OW2, located in difference Fourier map, whose coordinates were kept fixed. O2 and O3 atoms of the nitrate groups have been found to be disordered over two equivalent positions. All calculations were performed using SHELXL-97[11] implemented in the WINGX system of programs.[12] The crystal data are reported in table 1. The drawings were made with Diamond[13] and ORTEPIII.[14]
3 Results and Discussion
3.1 Description of the structure
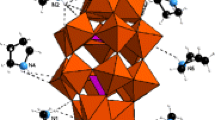
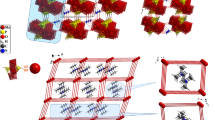
The crystal structure of [2,3-(CH3)2 C 6 H 3 NH 3]4 BiBr6 NO3.2H2O consists of isolated [BiBr6] 3− and [NO3] − anions, four independent 2,3-dimethylanilinium cations and two crystallographically independent water molecules, as shown in figure 1. The Bi atom is hexacoordinated to six Br atoms, in a slightly distorted octahedral geometry (table 2). The Bi-Br bond lengths are in perfect agreement with the mean value of 2.85(1) Å calculated on 16 CSD entries[15] containing isolated BiBr6 complexes. The packing of the different molecular entities is shown in figure 2; the projection along the b axis reveals a layered structure made of alternating inorganic and organic layers, linked trough weak N-H …Br and N-H …O(water) hydrogen bonds (table 3). Almost all N-H and O-H groups are involved in such hydrogen bonding interactions, which are often bifurcated. Inside the anionic layers, hydrogen bonds of O-H …O type are also established between water molecules and nitrate ions, as shown in figure 3. The presence of the [NO3] anions in the anionic sublattice of the organic bismuthate family was already found in other structures.[16]
With regard to the organic cation arrangement, the organization of 2,3-dimethylanlinium groups, not in opposition, is favourable to a non-centrosymmeric arrangement.[17] The aromatic rings are all almost planar, with a maximum mean deviation of 0.0025 Å from the calculated least-squared planes. Adjacent anilinium cations are approximately parallel to each other, but there is no π−π stacking interactions among them since the centroid-to-centroid distances are found to be too large, being in the range of 4.605–4.616 Å.[18] The four 2,3-dimethylanilnium cations are differently connected to the anionic sublattice by weak N-H …Br, N-H …O hydrogen bonds (table 3). It is worthwhile to mention the important role of the water molecules in the cohesion of the atomic arrangement, which act both as proton donors (towards the nitrate anion) and as proton acceptors (from the anilinium cations).
3.2 Infrared spectroscopy
To gain more information on the crystal structure, we have recorded the IR spectrum of the title compound which is shown in figure 4. The assignment of the internal modes of the organic cations is based on the comparison with other compounds associated to the same cation and the literature data.[17–19]The IR spectrum shows, at high wave numbers, the two broad bands at 3550–3450 cm −1 are attributed to ν(OH) of crystallization water molecules and ν(NH\(_{\mathrm {3}}^{\mathrm {+}})\), respectively. The broad bands in the range 3000–2920 cm −1 are attributed to ν(CH) and ν(CH3) stretching modes. The broad band at 1580 cm −1 is assigned to the stretching vibration of C =C and to the bending modes of the NH3 and OH groups. The bands observed at 1480, 1420, 1464 and 1323 cm −1 correspond to the stretching vibrations of C =C and NO3 anions. The weak bands at 1250, 1200 cm −1 and the moderate band centred at 1100 cm −1 are associated with the ν(C-N) and ν(C-C). The vibrations at 1025, 899, 829 cm −1 are caused by the δ(C-H), δ(CC) of the aromatic ring and δ(NO3). The remaining bands in the range 780–485 cm −1 may be assigned to γ(CCC), ρ(NH3) and ρ(NH3).
3.3 NMR results
Carbon signals are often broad and not always possible to distinguish between different carbons chemically very similar. The 13C CP-MAS NMR spectrum of [2,3-(CH3)2 C 6 H 3 NH 3]4 BiBr6 NO 3.2H2O is shown in figure 5. It displays three peaks in the resonance region of the aliphatic carbon atoms, at 14.8, 15.6 and 19.9 ppm. These resonances correspond to the carbon atoms of the eight crystallographically independent methyl groups. The first two peaks are assigned to the carbon atoms of the four crystallographically independent methyls in ortho position of the ammonium groups. The last peak is attributed to the carbon atoms of the four inequivalent methyls in meta position of the ammonium groups having very close chemical environments. The sum of the nine peaks with the chemical shifts spread between 121.1 and 139.7 ppm is assigned to the carbon atoms of the four inequivalent aromatic rings of the title compound. The presence of only nine lines in this resonance region, due to the fact that some of crystallographically independent carbons have similar environments, proves that the asymmetric unit of the title compound contains more than one aromatic ring.
4 Conclusion
The crystallographic and spectroscopic studies have illustrated that [2,3-(CH3)2 C 6 H 3 NH 3]4 BiBr6 NO 3.2H2O crystallizes in the non-centrosymmetric space group Pca21 at room temperature. Its crystal structure can be described by alternation of organic and inorganic layers. The packing of this hybrid compound shows weak and moderate interlocking between the inorganic and organic entities by a multi-directional hydrogen-bonding network. These interactions are known to exist in many systems of biological importance. The water molecules play an important role in stabilizing the structure by a set of intermolecular hydrogen bonds involving both N-H …O and O-H …O types.
References
(a) Maury O, Le Bozec H and Acc H 2005 Chem. Res. 38 691; (b) Green K A, Cifuentes M P, Samoc M and Humphrey M G 2011 Coord. Chem. Rev. 255 2530; (c) Evans O R, Lin W B and Acc H 2002 Chem. Res. 35 511
Chen C and Liu G 1986 Annu. Rev. Mater. Sci. 16 203
Wang X Q, Xu D, Yuan D R, Tian Y P, Yu W T, Sun S Y, Yang Z H, Fang Q, Lu M K, Yan Y X, Meng F Q, Guo S Y, Zhang G H and Jiang M H 1999 Mat. Res. Bull. 34 2003
Marcy H O, Warren L F, Webb M S, Ebbers C A, Velsko S P, Kennedy G C and Catella G C 1992 App. Opt. 31 5051
Qin J G, Liu D Y, Dai C Y, Chen C T, Wu B C, Yang C L and Zhan C M 1999 Coord. Chem. Rev. 188 23
Chaari N, Hamdi B, Chaabouni S and Zouari F 2007 X-ray Struct. Analysis 23 x183
Ciapala P, Jakubas R, Bator G, Zaleski J, Pietraszko A, Drozd M and Baran J 1997 J. Phys. Condens. Matter. 9 627
Neve F, Francescangeli O and Crispini A 2002 Inorg. Chim. Acta. 338 51
Blessing R H 1995 Acta Crystallogr. Sect. A 51 33
Altomare A, Burla M C, Camalli M, Cascarano G, Giacovazzo C, Guagliardi A, Moliterni A G, Polidori G and Spagna R 1999 J. Appl. Crystallogr. 32 115
Sheldrick G M 1997 SHELXL97Program for Crystal Structure Refinement University of Göttingen, Germany
Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Brandenburg K 1998 DIAMOND version 2.0
Burnett M N and Johnson C K 1996 ORTEPIII Report ORNL-6895, Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA
Allen F H, Bellard S, Brice M D, Cartwright B A, Doubleday A, Higgs H, Hummelink T, Hummelink-Peters B G, Kennard O, Motherwell W D S, RodgersJ R and Watson D G 1979 Acta Crystallogr. B 35 2331
Golzar Hossa G M 2013 Acta Crystallogr. E 69 m402
Rayes A, Ben Nasr C and Rzaigui M 2004 Mat. Res. Bull. 39 1113
Janiak J 2000 J. Chem. Soc. Dalton Trans. 3885
Silverstein R M, Bassler G C and Morill T C 1974 In Spectrometric identification of organic compounds 3 rd Edition (New York: Wiley)
Acknowledgements
This work was supported by the Tunisian Ministry of H. E. Sc. R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC 1000448 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK (fax: + 44 (1223) 336-033; e-mail: deposit@ccdc.cam.ac.uk).
Rights and permissions
About this article
Cite this article
ALOUI, Z., FERRETTI, V., ABID, S. et al. Synthesis and Physico-chemical studies of a new non-centrosymmetric organic-inorganic hybrid material: [2,3-(CH3)2C6H3NH3]4 BiBr6NO3.2H2O. J Chem Sci 127, 461–466 (2015). https://doi.org/10.1007/s12039-015-0799-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0799-9