Abstract
PbO nanoparticles have been employed as an efficient catalyst for the solvent-free synthesis of tetrahydrobenzo pyrans (yields 81–91%) and benzylidene malonitriles (yields 90–96%) at room temperature using green chemistry approach. PbO nanoparticles were found to be highly efficient, eco-friendly and recyclable heterogeneous catalyst. PbO nanoparticles were prepared by hydrothermal method and characterized by IR, XRD, BET Surface area, SEM, EDAX and TEM with SAED techniques.

One-pot synthesis of tetrahydro benzo pyrans and benzylidene malonitriles using PbO nanoparticle under solvent-free condition at room temperature has been presented. PbO nanoparticles were found to be highly efficient, ecofriendly and recyclable catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Benzopyrans and their derivatives are known to show several pharmacological properties such as spasmolytic, diuretic, antianaphylactin, antisterility and are used as anticancer agents.[1] The polyfunctionalized benzopyrans are also used as cosmetics, pigments and biodegradable agrochemicals.[2] The benzylidene malonitrile derivatives have been found to posses inhibitory activity to HER2,[3] EGFR,[4] IGF1R[5] and have been used for treatment of cancer.[6] Many synthetic procedures have been reported for tetrahydro benzo pyrans and benzylidene malonitriles.
The tetrahydro benzo pyrans have been previously synthesized by two or three-component condensations including the use of catalysts like potassium phosphate,[7] ZnO-beta zeolite,[8] Ce(SO4)2.4H2O,[9] s-proline,[10] caro’s acid-silica gel,[11] hexadecyl trimethyl ammonium bromide,[12] sulphonic acid functionalized silica,[13] tetra butyl ammonium bromide,[14] rare earth perfluorooctanoate,[15] basic quaternary ammonium salt,[16] phenyl boronic acid,[17] LiBr,[18] TEAA,[19] PEG- 400,[20] basic ionic liquid,[21] amines[22] and (NH4)2HPO4.[23] The benzylidene malonitriles were synthesized using catalysts such as calcium oxide,[24] TEBA,[25] PEG,[26] base,[27] NH2SO3NH4,[28] MgBr2.OEt2,[29] organo-base mediation,[30] quaternary ammonium salts,[31] Na2S/ Al2O3,[32] mpg-C3N4,[33] mesoporous base,[34] zirconia,[35] amine supported on silica gel[36] and MgC2O4/SiO2.[37]
Recently, metal oxide nanoparticles in the form of nanocatalyst have emerged as viable alternatives to conventional materials in various fields of chemistry and attracted marvelous interest of chemists. Metal oxide nanoparticles are known to be promising heterogeneous catalysts in a variety of organic transformations.[38] Nanoparticles have the potential for improving the efficiency, selectivity and yield of catalytic processes. In particular, the PbO nanoparticles provide higher surface to volume ratio in the reaction. Higher selectivity of PbO nanoparticles towards reaction proceeds through less waste and fewer impurities, which could lead to safer technique and reduced environmental impact. PbO nanoparticles have been investigated as catalysts in the organic reactions including the Paal–Knorr reaction and oxidative coupling of methane.[39, 40] Thus, the significant catalytic property with operational simplicity, high reactivity, environmental friendliness, reduced reaction times and reusability of PbO nanoparticles have prompted us to employ as catalyst for multicomponent reaction.
In view of the importance of PbO nanoparticles as a catalyst in organic synthesis, we report here a simple solvent-free synthesis of tetrahydrobenzo pyrans and benzylidene malonitriles. In this study, PbO nanoparticles were synthesized by hydrothermal method and characterized by IR, XRD, BET Surface area, SEM, EDAX and TEM with SAED techniques. In view of emerging importance of heterogeneous catalyst, we wish to explore the applications of PbO nanoparticles as a catalyst in organic synthesis.
2 Experimental
The synthesized PbO nanoparticles were characterized by FTIR using Shimadzu 8400 s instrument. XRD pattern was recorded using Phillips-1710 diffractometer with Cu–kα radiation (λ = 1.54 Å). The surface area was recorded with the help of Quantachrome Autosorb Automated Gas Sorption System. SEM and EDAX were recorded using JEOL-JEM-6360 microscope. TEM was recorded with SAED using CM-200 Philips Microscope. Melting points were determined using open capillary tubes on Veego melting point apparatus and are uncorrected. FTIR spectra were recorded on Shimadzu 8400 s spectrometer using KBr pellets. 1H NMR spectra were recorded on a Bruker Advance II 400 MHz spectrometer in DMSO-d 6 with TMS as an internal standard.
2.1 Synthesis of PbO nanoparticles
A mixture of citric acid (2.5 mmol) and sodium hydroxide (10 ml, 0.1 N) in distilled water was added to a magnetically stirred methanolic solution of lead nitrate (2 mmol). The reaction mixture was stirred for 2 h at room temperature. The white polycrystalline product was filtered, washed with distilled water and dried at 110°C for 2 h. The solid product was calcinized at 500°C for 2 h. Over the course of this process, the white PbO nanoparticles turned pale yellow colour.
2.2 Synthesis of tetrahydro benzo pyran derivatives
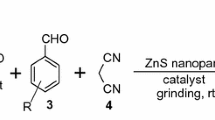
A mixture of aromatic aldehydes (1 mmol), malononitrile (1 mmol), dimedone (1 mmol) and PbO nanoparticles (50 mg) were ground at a room temperature with a mortar and pestle. The reaction was monitored by thin-layer chromatography (TLC). After completion of reaction, the product was washed with distilled water. The crude product was dried and recrystallized from ethanol to afford pure compounds with high yield (table 1; scheme 1).
2.3 Spectroscopic data
2.3a Compound (table 1, 4a):
IR (KBr, cm − 1): 3392, 3319, 3182, 2954, 2192, 1681, 1656, 1360 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 0.94 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.08 (d, J = 16.0 Hz, 1H), 2.23 (d, J = 16.0 Hz, 1H), 2.50 (m, 2H, CH2), 4.11 (s, 1H), 7.06 (s, br, 2H, NH2), 7.19 (m, 3H, ArH), 7.33 (m, 2H). MS (m/z) = 294 (M + 1).
2.3b Compound (table 1, 4b):
IR (KBr, cm − 1): 3303, 3041, 2994, 2246, 1653, 1613, 1490, 850 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 1.07 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.26 (s, 2H, CH2), 2.46–2.48 (m, 2H, CH2), 4.43 (s, 1H, CH), 6.58 (s, 2H, NH2), 7.18–7.28 (m, 4H, ArH). MS (m/z) = 328 (M + 1).
2.3c Compound (table 1, 4c):
IR (KBr, cm − 1): 3400, 3300, 3040, 2990, 2240, 1680, 1510, 840 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 1.03 (s, 3H, CH3), 1.11 (s, 3H, CH3), 2.21 (d, J = 16.0 Hz, 1H), 2.22 (d, J = 16.0 Hz, 1H), 2.43 (s, 2H), 3.77 (s, 3H), 4.36 (s, 3H), 4.55 (s, 2H), 6.84 (d, J = 8.7 Hz, 2H), 7.15 (d, J = 8.7 Hz, 2H). MS (m/z) = 324 (M + 1).
2.3d Compound (table 1, 4e):
IR (KBr, cm − 1): 3394, 3323, 3213, 2970, 2193, 1683, 1523, 1365 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 0.99 (s, 3H, CH3), 1.06 (s, 3H, CH3), 2.14 (d, J = 16.0 Hz, 1H), 2.30 (d, J = 16.0 Hz, 1H), 2.53–2.57 (m, 2H, CH2), 4.39 (s, 1H), 7.24 (s, 2H, NH2), 7.48 (d, 2H, J = 8.4 Hz, ArH), 8.21 (s, J = 8.4 Hz, 2H, ArH). MS (m/z) = 339 (M + 1).
2.4 Synthesis of benzylidene malonitrile derivatives (scheme 2)
A mixture of aromatic aldehydes (1 mmol), malononitrile (1 mmol) and PbO nanoparticles (40 mg) was ground in the presence of sunlight at room temperature. The reaction was monitored by TLC. After completion of reaction, the crude product was washed with distilled water, dried and recrystallized from alcohol to afford pure product (table 2).
2.5 Spectroscopic data
2.5a Compound (table 2, 3c):
IR (KBr, cm − 1): 3107, 2225, 1945, 1610, 1595, 1529, 1479 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 7.86 (s, 1H), 8.31 (s, 1H, = CH), 8.33–8.45 (m, 3H). MS (m/z) = 200 (M + 1).
2.5b Compound (table 2, 3d):
IR (KBr, cm − 1): 3350, 3026, 2223, 1569, 1511 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 6.91 (d, J = 6.4 Hz, 2H, ArH), 7.89 (s, 2H, ArH), 7.97 (s, 1H, = CH). MS (m/z) = 171 (M + 1).
2.5c Compound (table 2, 3e):
IR (KBr, cm − 1): 2231, 1607 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 8.12 (d, 2H, J = 8.8 Hz, ArH), 8.31 (d, J = 8.8 Hz, 2H, ArH), 8.50 (s, 1H, = CH). MS (m/z) = 200 (M + 1).
2.5d Compound (table 2, 3g):
IR (KBr, cm − 1): 2229, 1584 cm. − 1 1H NMR (400 MHz, DMSO-d 6): 7.51 (d, 2H, J = 8.5 Hz, ArH), 7.70 (s, 1H, = CH), 7.85 (d, 2H, J = 8.5 Hz, ArH). MS (m/z) = 189 (M + 1).
3 Results and discussion
The FT-IR spectra of PbO nanoparticles show bands at 574, 642 and 844 cm − 1 due to Pb–O vibrations (figure 1). The absorption band around 3400 cm − 1 is due to the presence of water molecules. The XRD pattern (figure 2) suggests that the PbO nanoparticles contain 111, 002, 200, 210, 022, 222, 311 and 131 crystal planes. XRD pattern of the synthesized PbO confirmed the formation of a single orthorhombic structure (JCPDS Card No. 76–1796) with space group Pca 21 (29). Sharp diffraction peaks indicated good crystallinity. The broadening of peaks indicated that the particles are in nano regime and are in good agreement with observed SEM images. The average particle size of PbO nanoparticles was determined using Debye–Scherer formula [41] and was found to be 69 nm.
The SEM image (figure 3) showed the morphology and size of the synthesized PbO nanoparticles which, suggested the surface of the PbO nanoparticles are spongy and discrete in appearance. The elemental analysis (EDAX) confirmed the material contain Pb and O elements (figure 4). The TEM image revealed that the synthesized PbO material is orthorhombic with several hexagonal shaped crystallites (figure 5). The dark spot in the TEM micrograph (figure 5) can be alluded to synthesized PbO nanoparticles as SAED pattern associated with such spots reveals occurrence of PbO in total agreement with the XRD data. The average size of the PbO nanocrystallite by TEM was found to be 69 nm.
The N2 adsorption–desorption isotherms and BJH pore size distribution of PbO nanoparticles (figure 6) revealed that the samples have typical IV N2 adsorption–desorption isotherms with H1 hysteresis which indicated that the sample reserve the cylindrical mesopores. The BJH pore size distribution demonstrated that all the samples have a narrow pore diameter range. Based on the N2 adsorption–desorption isotherms, the specific surface area (SBET) of PbO nanoparticles obtained from BET method was 31.99 m2/g, the average pore volume (VP) and pore diameter (dp) were 0.02256 cc/g and 30.86 Å (figure 6).
3.1 Catalytic results
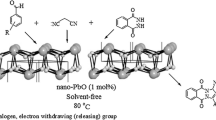
In continuation of our work on the synthesis of heterocyclic molecules using nanoparticles for cyclization and condensation reactions.[42, 43] We report here facile synthesis of tetrahydrobenzo pyrans and benzylidene malonitriles by grinding under solvent-free condition using PbO nanoparticles. To optimize the reaction condition for synthesis of tetrahydrobenzopyrans, benzaldehyde, malononitrile and dimedone were used as a reactant.
In order to verify the role of grinding, the reaction mixture was left over night when it was observed that reaction remained incomplete and in the absence of catalyst does not proceed even after grinding. Under the optimized reaction conditions, a range of substituted tetrahydrobenzo pyran derivatives were synthesized by grinding at room temperature (table 1) using PbO as catalyst. In order to study the scope of the reaction, several substituted aromatic aldehydes with electron-donating as well as electron-withdrawing groups were employed. The reaction proceeded smoothly with good yields. The aromatic aldehydes with hydroxyl group required longer reaction time and gave lower yield. The heterocyclic aldehydes also reacted smoothly to give corresponding derivatives.
Similarly, for the synthesis of benzylidene malonitriles, benzaldehyde and malononitrile were used as model reaction to optimize the reaction condition. As mentioned above, the role of grinding was important for completeness of reaction. Under the optimized reaction conditions a range of substituted benzylidene malonitrile derivatives were synthesized by grinding at room temperature (table 2). The reactions proceeded smoothly for electron-withdrawing and electron-donating aromatic aldehydes with high yields. The aromatic aldehydes with hydroxyl groups and chloro groups at ortho position required longer reaction time and gave lower yields. The strong electron-donating aromatic aldehydes reacted quickly with high yields.
The role of catalyst was also studied for the model reaction with different amount of catalysts such as 10, 20, 30, 40, 50, 60 and 70 mg. It was observed that 50 mg of catalyst was sufficient to promote the reaction and greater amount of the catalyst did not improve the yields (table 3). In the synthesis of benzylidene malononitriles, to study the amount of catalyst required, the model reaction of benzaldehyde and malononitrile (scheme 2) was used with different amount of catalysts such as 10, 20, 30, 40, 50, 60 and 70 mg. It was found that the use of 40 mg of the catalyst was sufficient to promote the reaction (table 3).
To study the reusability of catalyst, it was separated after the completion of the reaction, washed with acetone and dried at 100°C and reused in the model reaction for four consecutive runs (table 4). Further, recycled catalyst was characterized by different analytical techniques. We observed that the particle size and crystal morphology of reused PbO nanoparticles was nearly the same. After every use, a little loss of catalytic activity was observed which may be attributed to microscopic change in the structure of the catalyst.
4 Conclusions
We have developed a convenient, efficient protocol for one-pot synthesis of tetrahydrobenzo pyrans and benzylidene malonitriles in the presence of nanocrystalline PbO catalyst by grinding at room temperature. The attractive features of this procedure are simple work-up, mild reaction condition, short reaction time, excellent yield, solvent-free reaction and utilization of nanoparticles as a reusable catalyst.
References
a) Montandon J B, Zijlstra F J and Wilson J H P 1989 Int. J. Tissue React. 11 107; b) Brooks G T 1998 J. Pestic. Sci. 22 41
a) Hafez E A A, Elnagdi M H, Elagamey A G A and EL-Taweel F M A 1987 Heterocycles 26 903; b) Abdel Galil F M, Riad B Y, Sherif S M and Elnagdi M H 1982 Chem. Let. 11(8) 1123
Osherov N, Gazit A, Gilon C and Levitzki A 1993 J. Bio. Chem. 268 11134
Kamath S and Buolamwini J K 2003 J. Med. Chem. 46(22) 4657
Li R, Pourpak A and Morris S W 2010 J. Med. Chem. 52(16) 4981
Lee C T, Adachi Y and Carbone D P 2005 Gene. Ther. Mol. Biol. 9 77
Pore D M, Undale K A and Dongare B B 2009 Catal. Lett. 132(1–2) 104
Katkar S S, Lande M K, Arbad B R and Gaikwad S T 2011 Chin. J. Chem. 29(1) 199
Islami D M and Mosaddegh E 2009 Phosphorus Sulfur Silicon Related Elem. 184(12) 3134
Balalaie S, Bararjanian M, Amani A M and Movassagh B 2006 Synlett 2 263
Oskooie H A, Heravi M M, Karimi N and Zadeh M E 2011 Synth. Commun. 41(3) 436
Jin T S, Wang A Q, Wang X, Zhang J S and Li T S 2004 Synlett 5 871
a) Ziarani G M, Abbasi A, Badiei A and Aslani Z 2011 E-J. Chem. 8(1) 293; b) Bahareh S, Alireza H and Somayeh B 2011 J. Chem. Res. 35(11) 666
a) Gurumurthi S, Sundari V and Vallippan R 2009 E-J. Chem. 6(S1) S 466; b) Mobinikhaledi A and Fard M A 2010 Acta. Chim. Slov. 57 931
a) Wang L M, Shao J H, Tian H, Wang Y H and Liu B 2006 J. Fluorine Chem. 127 97; b) Mei H and Chun C 2010 J. Chem. Res. 34(10) 568
Zhao L, Li Y, Chen L and Zhou B 2010 J. Org. Chem. 30(1) 124
Nemouchi S, Boulcina R, Carboni B and Debache A 2012 Comptes Rendus Chime. 15(5) 394
a) Saini A, Kumar S and Sandhu J S 2006 Synlett 12 1928; b) Sun W B, Zhang P, Fan J, Chen S H and Zhang Z H 2010 Synth. Commun. 10(4) 587
Balaskar R, Gavade S, Mane M, Pabrekar P, Shingare M and Mane D 2011 Lett. Org. Chem. 8(4) 282
Feng C, Wang Q, Lu C, Yang G and Chen Z 2012 Combinatorial Chem. High Throughput Screening 15(1) 100
Ranu B C, Banerjee S and Roy S 2008 Indian J. Chem. 47B 1108
Yu L Q, Liu F and You Q D 2009 Org. Prep. Proc. Int. 41 77
a) Balalaie S, Bararjanian M, Ahmadi M S, Hekmat S and Salehi P 2007 Synth. Commun. 37(7) 1097; b) Abdolmohammadi S and Balalaie S 2007 Tetrahedron Lett. 48 3299
Peng Y and Song G 2003 Indian J. Chem. 42B 924
Shi D Q, Chen J, Zhuang Q Y, Wang X S and Hu H W 2003 Chin. Chem. Lett. 14(12) 1242
Ye W, Jiang H and Yang X C 2011 J. Chem. Sci. 123(3) 331
Parida K M, Rath D and Mol J 2009 J. Mol. Catal. A: Chem. 310 93
Mogilaiah K, Babu S H, Vidya K and Kumar K S 2010 Indian J. Chem. 49B 390
Abaee M S, Mojtahedi M M, Zahedi M M and Khanalizadeh G 2006 Arkivoc xv 48
a) Cardillo G, Fabbrani S, Gentilucci L, Gianotti M and Tolomelli A 2003 Synth. Commun. 33 1587; b) Wang Y, Shang Z, Wu T, Fan J and Chen X J 2006 J. Mol. Catal. A: Chem. 253 212
a) Wang S, Ren Z, Cao W and Tong W 2001 Synth. Commun. 31 673; b) Jin T S, Wang X, Liu L B and Li T S 2006 J. Chem. Res. 6 346
Heravi M M, Bakhtiari K, Taheri and Oskooie H A 2007 J. Chin. Chem. Soc. 54 1557
Su F, Antonietti M and Wang X 2012 Catal. Sci. Technol. 2 1005
Mondal J, Modak A and Bhaumik A 2011 J. Mol. Catal. A: Chem. 335(1–2) 236
Reddy B M, Patil M K, Rao K N and Reddy G K 2006 J. Mol. Catal. A: Chem. 258(1–2) 302
Isobe K, Hoshi T, Suzuki T and Hagiwara H 2005 Mol. Divers. 9(4) 317
Yuan S, Li Z and Xu L 2012 Res. Chem. Intermed. 38(2) 393
Kumar B V, Naik H S, Girija D and Kumar B V 2011 J. Chem. Sci. 123(5) 615
Pasha S K, Satyanarayana V S V, Sivakumar A, Chidambaram K and Kennedy L J 2011 Chin. Chem. Lett. 22(8) 891
Bytyn W and Baerns M 1986 Appl. Catal. 28 199
Dash P K and Balto Y 2011 Res. J. Nanosci. Nanotechnol. 1 25
Borhade A V, Tope D R and Patil D R 2012 Res. Chem. Intermed. doi:10.1007/s11164-012-0693-8
Borhade A V, Tope D R and Patil D R 2012 J. Chem. Pharm. Res. 4(5) 2501
Acknowledgements
Authors thank the University Grants Commission (UGC), New Delhi, for financial support, University of Pune, Pune and Sophisticated Analytical Instrument Facility (SAIF) Panjab University, Chandigarh for providing spectral analysis facilities. Authors are also thankful to Prof. A G Gadhave for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
Supplementary material given as figures S1–S5 can be seen online www.ias.ac.in/chemsci website.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BORHADE, A.V., UPHADE, B.K. & TOPE, D.R. PbO as an efficient and reusable catalyst for one-pot synthesis of tetrahydro benzo pyrans and benzylidene malonitriles. J Chem Sci 125, 583–589 (2013). https://doi.org/10.1007/s12039-013-0396-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0396-8