Abstract
The regulation of oxyradicals and PSII activity by UV-B (280–315 nm) and UV-A (315–400 nm) components were investigated in the leaves of maize [Zea mays L. var: HQPM.1]. The impact of ambient UV radiation on the production of superoxide (O2˙−) and hydroxyl (˙OH) radicals were analysed in the leaves of 20-day-old plants. The amount of O2˙− and ˙OH radicals and the radical scavenging activity were significantly higher in the leaves exposed to ambient UV radiation as compared to the leaves of the plants grown under UV exclusion filters. Smaller amount of oxyradicals in the leaves of UV excluded plants was accompanied by a substantial increase in quantum yield of electron transport (φEo), rate of electron transport (ψo) and performance index (PIABS), as indicated by chlorophyll a fluorescence transient. Although higher amounts of oxyradicals invoked higher activity of antioxidant enzymes like superoxide dismutase and peroxidase under ambient UV, they also imposed limitation on the photosynthetic efficiency of PSII. Exclusion of UV components (UV-B 280–315 nm; UV-A 315–400 nm) translated to enhanced photosynthesis, growth and biomass. Thus, solar UV components, especially in the tropical region, could be a major limiting factor in the photosynthetic efficiency of the crop plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants in the tropical regions are exposed to high ambient levels of UV-B compared to those in the temperate zones. Exclusion of the UV components (UV-B 280–315 nm; UV-A 315–400 nm) from the solar spectrum can be an important tool to assess the impact of ambient UV radiation on the performance of the crop plants. Maize is the third important worldwide crop after wheat and rice. Most of the UV radiation effects on plants have been done by using supplementary UV-B radiation. Supplementary UV-B radiation often creates conditions unlikely to occur in nature, because of the unrealistically high UV radiation levels, inadequate level of UV-A, low photosynthetic photon flux density (PPFD) or other technical difficulties (Edwards 1992; Adamse et al. 1997). A few studies have, however, been made on the impact of ambient UV radiation on growth and photosynthesis in tropical plants. Exclusion of solar UV-B and UV-A/B caused enhanced growth and yield in soybean (var. JS-7105) (Varalakshmi et al. 2003). Removal of UV-B from natural UV components caused a large increase in the growth of Cyamopsis species, a marginal increase in Vigna radiata but did not alter growth in Vigna mungo (Amudha et al. 2005). Marginal difference in growth has also been reported in maize (cv. SM-600) after the exclusion of UV-B (Pal et al. 1997). An intraspecific difference in sensitivity to UV radiation is identified among different wheat varieties (Kataria and Guruprasad 2012)
With respect to photosynthetic components, the amount of chlorophyll was shown to increase in the leaves of Cyamposis by excluding UV and this was accompanied by an increase in the rate of oxygen evolution (Amudha et al. 2005). There were also structural changes in the chloroplast and three low-molecular-weight polypeptides (17, 23 and 33 kDA) of Cyamposis were present in higher amount in PSII after UV-B exclusion (Amudha et al. 2005).
Most of the damages to photosynthetic apparatus by artificial UV-B light sources have been related to higher production of oxyradicals after UV-B irradiation. Enhanced production of active oxygen species that include superoxide radical (O2˙−), hydroxyl radical (˙OH), singlet oxygen (1O2) and hydrogen peroxide (H2O2), which can cause oxidative damage to membrane lipid, nucleic acids and proteins, has been observed after irradiation with UV-B (Foyer and Mullineaux 1994). It is widely accepted that UV-B damages the donor side of PSII by inactivating the Mn cluster of water oxidation (Messinger 2004). Hydroxyl radicals are the dominating reactive oxygen induced by UV-B radiation in the thylakoids (Hideg and Vass 1996). Production of highly damaging ˙OH radicals in the heart of the Mn cluster by UV-B has been suggested as one of the possible mechanism of UV-B-induced damage (Szilard et al. 2002). Plants respond to UV-B-induced production of active oxygen species by enhancing the activity of antioxidant enzymes like superoxide dismutase, guaiacol peroxidase, ascorbic acid peroxidase and glutathione reductase (Takeuchi et al. 1995; Rao et al. 1996; Jain et al. 2004; Guruprasad et al. 2007). UV-B irradiance also enhances the endogenous levels of ascorbic acid and glutathione in plants (Rao and Ormrod 1995; Takeuchi et al. 1996; Jain et al. 2004).
Enhancement in the production of oxyradicals and activation of antioxidant defence enzymes has always been documented under higher irradiation levels of artificial UV-B radiation in previous studies. There has been no data on the production of oxyradicals and the extent of damage to photosynthetic apparatus under natural UV components of sunlight. We have investigated the sensitivity of maize leaves to UV components of the solar spectrum under tropical sunlight. Production of oxyradicals in the maize leaves was measured using electron paramagnetic resonance spectroscopy in the presence and absence of solar UV components. Simultaneously, we also measured the accompanying changes in antioxidant enzymes and polyphasic chlorophyll a fluorescent transient, growth and biomass. We present the result that indicates the extent of imposition of limitation to photosynthesis by natural solar UV components due to the enhanced production of oxyradicals.
2 Material and methods
2.1 Experimental design
All the field experiments under natural sunlight were conducted in the Botanical Garden of School of Life Sciences, Devi Ahilya University, Indore (latitude 22.4°N), India.
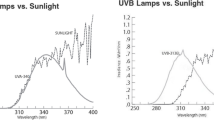
Maize [Zea mays L. Var.HQPM.1] seeds were collected from Indian Agricultural Research Institute, New Delhi. Seeds of uniform size and shape were selected and sown in plastic bags filled with mixture of sand, soil and manure (1:2:1) and placed in metal mesh chambers (4 ft L × 3 ft W × 3 ft H) covered with polyester filters (Garware polyesters Ltd., Mumbai) that excluded either UV-B (<315 nm) or UV-A/B (<400 nm). The control plants were grown under a polyester filter transmissible to ambient solar UV-B and UV-A radiation or in a chamber that had no filter. The transmission characteristics of the filters were measured by Shimadzu (UV-1601) spectrophotometer (figure 1). The transmission characteristics of the filters did not change during the experimental period and these filters did not emit any fluorescence in the visible region. Metal cages received full solar radiation during the day without any shading. Seedlings were exposed to/excluded from solar UV radiation from the time of emergence, and different phenological and physiological measurements were measured 20 days after emergence. There was no significant temperature difference between filter control and UV-excluded chambers as horizontal holes in the chamber allowed passive air ventilation. The experiments were conducted in a randomized block design with three replicates for each treatment.
2.2 Radiation measurement
Absolute solar irradiance and intensity of UV-B or UV-A/B were measured using a radiometer solar light-PMA-2100, USA. The details of radiation measurements are given in table 1.The PAR intensity for normal plant growth was observed to be optimal saturating light.
2.3 Growth analysis
Growth parameters like shoot length, root length, fifth leaf area, fresh weight and dry weight of shoot and fifth leaf were measured at day 20 after emergence. Area of a leaf was taken by pressing the leaf on a millimeter graph paper and tracing the exact outline. The area was measured by weighing the graph cuttings of the leaf. The calibration curve was prepared by weighing 0–1000 mm2 area of graph paper. For dry weight determination, plant parts were dried at 60°C for 72 h. Five plants from each replica was taken for recording these parameters (n = 15).
2.4 UV-absorbing substances
UV-absorbing substances were extracted from 1 cm2 leaf disc in 5 mL methanolic-HCL (99:1, v/v). Absorbance was measured by Shimadzu (UV-1601) spectrophotometer at 305 nm and expressed as Units/mg FW (Tevini et al. 1991).
2.5 Enzyme preparations and assays
2.5.1 Superoxide dismutase [EC 1.15.1.1]
Extracts were prepared from 0.1 g of leaf homogenized at 4°C. Extraction media for superoxide dismutase (SOD) had 3 mL of extraction buffer, containing 50 mM phosphate buffer (pH 7.4), 1 mM ethylenediaminetetraaceticacid (EDTA), 1 g polyvinyl pyrollidine (PVP) and 0.5% (v/v) triton X-100. The homogenate was centrifuged at 10,000 g for 20 min and the supernatant fraction was used for the assay. Total SOD (EC 1.15.1.1) activity was assayed by inhibition of the photochemical reduction of nitro blue tetrazolium chloride (NBT), as described by Beauchamp and Fridovich (1971).The reduction in NBT was followed by reading absorbance at 560 nm. Blanks and controls were run in the same way but without illumination and enzyme respectively. One unit of SOD was defined as the amount of enzyme that produced 50% inhibition of NBT reduction under the assay conditions (Giannopolitis and Ries 1977).
2.5.2 Peroxidase [EC 1.11.1.7]
Extracts for determination of peroxidase (POD) activity were prepared by homogenizing 0.1 g of leaf in 80% chilled acetone at 4°C. The extract was centrifuged at 5000 g for 10 min. The supernatant was discarded and the pellet was redissolved in 5 mL of 50 mM sodium phosphate buffer (pH 6.4) and centrifuged for 15 min at 10,000 g. The buffered supernatant was used for the cytosolic peroxidase assay. Peroxidase was assayed by the method of Chance and Maehly (1955). The reaction mixture contained 0.5 mL enzyme extract, 1 mL 20 mM guaiacol and 3 mL of 50 mM phosphate buffer. The reaction was started by the addition of 0.03 mL H2O2 (8.82 mM). The initial and final absorbance was recorded at 475 nm for 2 min. The activity was calculated as changes in optical density (O.D.) (min mg protein)−1.
Protein concentration was estimated by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
2.5.3 Electron paramagnetic resonance (EPR) spectroscopy of superoxide (O2˙−) and (˙OH) radicals
50 mg tissue was homogenized in 1 mL phosphate saline buffer (pH 7.4) containing 100 μM EDTA, 100 μM diethyldithiocarbamate (DETC, a superoxide dismutase inhibitor) and 500 μM phenyl N-tert-butylnitrone (PBN, a spin trapping agent). These chemicals were purchased from Sigma-Aldrich, St. Louis, USA. The homogenate was centrifuged at 10,000 g for 15 min at 4°C. Supernatant was collected and incubated for 60 min in ice, and analysed for EPR signals. EPR spectra were recorded using a JEOL JES-FA 300 X-band EPR spectrometer. All O2˙−-PBN adduct spectra were recorded at room temperature with EPR instrument setting: microwave power 5 mW, modulation width 2 mT, amplitude 8000, field centre 338.092 mT with width +/− 5.0 mT, sweep time 4 min and time constant 1 s. Spectra of Xanthine/Xanthine oxidase (X/XO) + PBN was recorded as a standard spectra for superoxide. Superoxide content was further confirmed by the addition of superoxide dismutase while homogenizing, which reduced the intensity of the spin adduct more than 80% (data not shown).
For the determination of .OH content, 50 mg leaf tissue was homogenized in phosphate saline buffer (pH 7.4), 30 μL of dimethyl sulfoxide (DMSO) and 20 μL of 0.45 M 5,5′-dimethyl-1-pyrroline-N-oxide (DMPO). DMPO-˙OH radical adduct were recorded at room temperature with EPR instrument setting: microwave power 10 mW, modulation width 2 mT, amplitude 8000, field centre 338 mT with width +/− 5.0 mT, sweep time 4 min and time constant 0.3 s. Hydroxyl radical produced from fenton reaction was recorded as standard spectra for DMPO-˙OH adduct.
2.5.4 Superoxide anion radical (O2˙−) scavenging activity (SOD-like activity)
All solutions except DMSO were made in phosphate saline buffer (pH 7.4) just before use. The final reaction mixture consisted of 50 μL of 4 mM Xanthine (X), 30 μL of DMSO, 50 μL of sample solution, (sample was prepared by homogenizing 50 mg tissue and centrifuged at 10,000 g for 15 min at 4°C) 20 μL of 0.45 M 5,5′-dimethyl-1-pyrroline-N-oxide (DMPO) and 50 μL of xanthine oxidase (XO 0.4 U–1 mL) (Noda et al. 1997). A standard was run by adding 50 μL of phosphate saline buffer (pH 7.4) instead of sample solution. The instrument setting was just as described for DMPO-˙OH radicals.
2.5.5 Chlorophyll a fluorescence
Chlorophyll a fluorescence transient exhibited by dark-adapted (30 min) fifth leaf of maize (20-day-old maize plants) was measured by a Handy PEA (Plant Efficiency Analyzer, Hansatech Instruments Ltd. King’s lynn Norfolk). The transients were induced by red light (peak at 650 nm) of 600 Wm2 (3200 μE m−2 s−1) provided by an array of six light-emitting diodes, focused on the leaf surface in the clips on a spot of 4 mm diameter to provide homogenous illumination over the exposed area of the sample. Data were recorded for 1 s with 12 bit resolution; the data acquisition was every 10 μs for the first 2 ms and every 1 ms thereafter (Strasser et al. 1995). All the measurements were recorded at 25 ± 1°C. The normalized Chlorophyll a fluorescent transient, when plotted on a logarithmic scale clearly showed a polyphasic fluorescence rise kinetics (O-J-I-P phase). The fluorescence intensity at 20 μs was considered as the intensity of F0 (O phase) when all reaction centers are opened: the fluorescence intensity at 2 ms was J phase, 30 ms was I phase and the maximum fluorescence (Fm) was the P phase (Fp equals here to Fm since the excitation intensity is high enough to ensure the closure of all reaction centers of PSII).
OJIP transient was analysed according to the JIP test and the following parameters were calculated: (1) maximum quantum yield of primary photochemistry (Fv/Fm); (2) the efficiency by which a trapped excitation, having triggered the reduction of QA to Q −A , can move an electron further than Q −A into intersystem electron transport chain (ψo = ET0/TR0); (3) the quantum yield of electron transport (φEo = ET0/ABS) and (4) performance index on absorption basis (PIABS) reflecting the performance of the overall energy flow. These parameters were calculated as described by Strasser et al. (2000; 2004) using the software ‘Biolyzer HP 3’ (the chlorophyll fluorescence analysing program by Bioenergetics Laboratory, University of Geneva, Switzerland).
2.5.6 Statistical analysis
Data are expressed as a mean ± S.E.M. and were analysed by one-way analysis of variance (ANOVA) followed by a post hoc Newman–Keuls Multiple Comparison Test using a trial version of prism 4 software for windows (GraphPad Software, Inc., La Jolla, CA, USA). For the statistical evaluation of the results, significance was defined by a probability level of p < 0.05.
3 Results and discussion
3.1 Superoxide (O2˙−) and hydroxyl (˙OH) radicals
The EPR signals of O2˙−-PBN adducts were recorded in the leaves exposed to and shielded from ambient UV. O2˙−-PBN adduct gives a characteristic EPR spectrum known as triplet of doublet with hyperfine coupling constants of a N = 1.48 mT and a H = 0.28 mT (figure 2A). The standard spectrum of O2˙−-PBN adduct was recorded by generating O2˙− with X/XO system (figure 2A). EPR spectrum of O2˙−-PBN adduct corresponding to the standard from the leaf extracts revealed that the O2˙− radical level was higher under ambient UV (figure 2A). Exclusion of UV-B and UV-A/B reduced the amount of the radical by 40–45% (figure 2A; table 2).
Effect of exclusion of solar UV radiation on reactive oxygen species in maize leaves. (A) EPR spectra of superoxide-PBN (phenyl N-tert-butyl nitrone) spin adduct. [(1) spectra from xanthine/xanthine oxidase, (2) open control, (3) filter control (2 h), (4) -UV-A/B, (5) -UV-B]. (B) EPR spectra of .OH- DMPO (5,5′-dimethyl-1-pyrroline-N-oxide) spin adduct [(1) spectra from Fenton reaction, (2) open control, (3) filter control (2 h), (4) -UV-A/B, (5) -UV-B]. Each spectrum is the representative of 12 individual spectra.
The hydroxyl radicals were recorded as DMPO-˙OH spin adducts stable at room temperature that produced a four line spectrum with 1:2:2:1 intensity ratio (Rosen and Rauckman 1984; Knecht and Mason 1993). The standard spectrum of DMPO-˙OH adduct was produced by Fenton reaction (figure 2B). The corresponding adducts with leaf extracts indicated a reduction in the spectral intensity after the exclusion of the UV-B and UV-A/B (figure 2A; table 2). The reduction was to the extent of 8–11%. Production of ˙OH radical (but not O2˙− radical) was observed in thylakoid membrane irradiated with UV-B (Hideg and Vass 1996). O2˙− radical production has been observed under photosynthetically active radiation (PAR) (Hideg et al. 1994) and UV-B radiation can increase the risk of PAR-induced photoinhibition due to the inhibition of violaxanthine deepoxidation (Pfündel et al. 1992). Alteration of xanthophylls cycle by ambient solar UV radiation has also been reported (Martz et al. 2007). The results presented here indicate the excess production of both O2˙− and ˙OH radical by ambient UV in maize leaves.
3.2 Superoxide anion radical (O2˙−) scavenging activity (SOD-like activity)
Free radical scavenging activity was recorded by observing the changes in the intensity of DMPO-O2˙− spin adduct generated by X/XO after adding the leaf extracts from the plants grown under ambient UV or in the absence of UV (figure 3). Leaf extracts from plants grown under ambient UV showed higher scavenging activity and reduced the intensity of the standard spectrum by 55–60% (figure 3; table 3). Reduction of intensity of the standard spectrum was comparatively lower (31% after exclusion of UV-B and 23% after exclusion of UV-A/B) in the leaves of UV-excluded plants. Thus, under ambient UV there is a higher production of free radical and a higher scavenging activity in the maize leaves.
Superoxide radical scavenging activity in maize leaves after the exclusion of solar UV radiation.(1) X/XO + DMPO, (2) X/XO + DMPO + open control (OC), (3) X/XO + DMPO + filter control (FC), (4) X/XO + DMPO + UV-A/B, (5) X/XO + DMPO + UV-B. Each spectrum is the representative of 12 individual spectra (X/XO, xanthine/xanthine oxidase, DMPO, 5,5′-dimethyl-1-pyrroline-N-oxide).
3.3 Superoxide dismutase and peroxidase activity
Spectrophotometric estimation of SOD and POD activity in the leaf extracts of ambient grown plants indicated a significantly higher activity compared to the control plants grown under UV-exclusion filter (table 4). The reduction in the activity of SOD was up to 60% after the exclusion of UV radiation (table 4). Activity of POD was reduced to a lesser extent; 40% by exclusion of UV-B and 20% by exclusion of UV-A/B (table 4). The spectrophotometric assay of SOD and POD further supported the EPR data of reduction in the antioxidant activity. There was a prominent difference in the O2˙− content, but only a marginal difference in the ˙OH radical, between the ambient grown and UV-excluded plants. One of the possible reason for this difference would be the higher activity of POD in maize leaves, which prevents further reduction of H2O2 to ˙OH but may accelerate the H2O2 breakdown.
Although there was a significant difference in the activity of antioxidant enzymes like SOD and POD, UV-absorbing substances in the maize leaves remained unaltered even after the exclusion of ambient UV (table 4). Correia et al. (1999) also have made similar observation in maize.
3.4 Chlorophyll a fluorescence
Polyphasic chlorophyll a fluorescence transient was measured to evaluate the effect of ambient solar UV radiation on the photochemical efficiency of PSII. The time course of fluorescence yield in dark-adapted intact leaves from plants grown in ambient UV and after UV exclusion plotted on logarithmic time scale showed the separation of OJIP phase (figure 4).The OJIP transient represents the successive but overlapping filling up of PSII electron acceptor pools such as QA, QB and PQ, whose oxido-reduction states are closely controlled by PSII functions (Govindjee 1995). The O phase corresponds to minimal fluorescence yield, originated mostly from chlorophyll a of PSII light-harvesting complex (Butler 1978). The J phase represents the maximum of Q −A (Hsu 1993) and the following fluorescence raise JI transient, characterize the closure of the remaining PSII open centres, resulting the accumulation of Q −A Q −B (Strasser et al.1995). The maximum yield of chlorophyll a fluorescence is attained when the PQ pool become reduced, therefore the IP fluorescence step is a consequence of Q −A Q 2−B (Strasser et al.1995). The intensity of fluorescence yield in the JIP phase was enhanced by the exclusion of solar UV-B and UV-A/B. A very significant reduction in maximum chlorophyll fluorescence (Fm = Fp) yield in control plants indicates a reduction in the amount PSII centres that are able to reduce QA. Either decreased absorption cross-sectional area of PSII-associated light-harvesting antenna or retarded electron flow from the donor side of PSII to the PQ pool that need to be reduced before Q −A can accumulate results in lower fluorescence yield at Fm.
Fv/Fm does not show significant difference between UV-excluded and open field grown plants (table 5), which shows that exclusion of UV radiation did not influence the number of quanta absorbed per unit time (Mehta et al. 2010). The most sensitive parameters calculated by the equations of the JIP test is performance index (PI), which is an indicator of sample vitality, was significantly enhanced by the exclusion of solar UV components (table 5). The PI is a function of three independent functional steps of photosynthesis, i.e. RC/ABS, φPo and ψo (Srivastava et al. 1999). PI was enhanced by 81% and 43% after the exclusion of UV-B and UV-A/B, respectively (table 5). This result indicated the requirement of UV-A for the better harvesting of the sunlight. The enhancement in PI is attributable to high efficiency of ψo, since ψo was up to 22% and 9% higher after UV-B and UV-A/B, respectively. The yield of electron transport (φEo = ET0/ABS), which is a product of the φPo (= Fv/Fm) and ψo, can also be assessed from the fluorescence transients. An enhancement of 28% and 14% was recorded in ET0/ABS after the exclusion of UV-B and UV-A/B, respectively (table 5). The relationship between log PIABS (the driving force for photosynthesis) and ET0/ABS can be considered as a typical property of a plant’s ability to transform light energy into chemical energy (NADH), which is subsequently directed in to metabolic reactions in the biochemical process of photosynthesis (Hermans et al. 2003). A plot of log (PIABS)rel vs (ET0/ABS)rel indicated that the highest efficiency for transforming light energy to chemical energy was shown by the plants grown after exclusion of UV-B (figure 5). This plot also emphasized the need for the presence of UV-A part of the solar spectrum to achieve the highest efficiency although the absence of both UV-B and UV-A components enhances the efficiency above the ambient plants (figure 5). Thus, UV exclusion enhances the ability of maize plants to convert light energy to chemical energy that can be used to reduce CO2 to carbohydrate, which can lead to larger accumulation of biomass.
Correlation of the driving force (DFABS )rel = Log (PIABS)rel as a function of the relative yield of electron transport (ET0/ABS) rel. [Log (PIABS)rel = 100 × [(PIABS)AVG treatment − (PIABS)all avg]/ (PIABS)all avg. (ET0/ABS) rel = 100 × [(ET0/ABS)AVG treatment − (ET0/ABS)all avg]/ (ET0/ABS)all avg]. FC = Filter control, -UV-A/B = UV-A/B excluded plants, -UV-B = UV-B excluded plants. Data are the average of 20 individual plants from each treatment.
Although several studies have shown the sensitivity of PSII to UV-B radiation (Bornman 1989; Melis et al. 1992), the reduction in CO2 assimilation induced by UV-B can occur prior to or in the absence of depressions in PSII and may more likely involve impairment in Calvin cycle, possibly mediated by Rubisco (Nogues and Baker 1995; Lesser and Neale 1996; Allen et al. 1999). In the present study, quantum yield of primary photochemistry (Fv/Fm) did not show much difference between UV-excluded and ambient grown plants. Similarly, Fv/Fm was unaltered by UV exclusion in the studies of Xiong and Day (2001). Xiong and Day (2001) concluded that UV-B effects on photosynthesis may be associated with enzymatic rather than PSII limitation. Results with OJIP curve presented here, however, demonstrate that the components of PSII are significantly affected by solar UV-B although Fv/Fm remains nearly unaffected.
3.5 Growth of maize after UV exclusion
The enhancement in the efficiency of light absorption after the exclusion of UV components translated into enhanced growth parameters in the maize. Leaf area and leaf dry weight were the most altered parameters after the UV exclusion (figure 6). Leaf area was enhanced by 90% and 79% after the exclusion of UV-B and UV-A/B. Although the microclimate was different in the chamber with filters as compared to plants grown in open filed, there was no chamber effect on the growth as no significant difference was found between open field grown plants and the plants grown under the filter that transmitted solar UV components (figure 6). Besides leaf parameters, significant difference in biomass were also recorded in roots and shoots (figure 6) after UV exclusion. The values of PI and plant dry weight when plotted together showed a positive correlation (r 2 = 0.824) after the exclusion of UV radiation. Positive correlation (r 2 = 0.824) was also recorded between PI and plant height. Several plants show similar enhancement of biomass in the aerial and underground parts after the exclusion of solar UV components (see introduction). However, Pal et al. (1997) could only observe marginal difference in maize (cv.SM-600) after UV-B exclusion. The difference in the response to exclusion reported here in Var. HQPM. 1, a single cross-hybrid of maize, may be due to intraspecific variation in the sensitivity of maize varieties to UV-B.
4 Conclusion
The results indicate that solar UV components, especially UV-B, inhibit photosynthetic efficiency of maize leaves. This inhibition can be related to higher production of active oxygen species, which can cause multi-targeted deleterious effect on PSII components. Although the primary photochemistry of PSII remains unaffected by solar UV, the biochemical characters and the transformation of light energy into chemical energy are significantly affected. Enhanced PIABS and ET0/ABS after the exclusion of UV components was accompanied by higher biomass. Photosynthetic efficiency was maximum in the presence of UV-A components of solar spectrum, which requires further experimentation.
Abbreviations
- (ψo):
-
capacity of PSII to transfer trapped excitation that can move an electron into the electron transport chain further than Q -A
- DMPO:
-
5, 5′-dimethyl-1-pyrroline-N-oxide
- ET:
-
flux of electrons from Q –A into the intersystem electron transport chain
- F0 :
-
initial fluorescence
- Fm :
-
maximum fluorescence
- Fv :
-
variable fluorescence
- PBN:
-
phenyl N-tert-butyl nitrone
- PIABS :
-
performance index based on absorption
- POD:
-
peroxidase
- PSII:
-
photosystem II
- RC:
-
reaction centre
- SOD:
-
superoxide dismutase
- TR:
-
excitation energy flux trapped by a RC and utilized for the reduction of QA to Q –A
- UAS:
-
UV absorbing substances
- X/XO:
-
xanthine, xanthine oxidase
- φEo :
-
quantum yield of electron transport
- φPo :
-
maximum quantum yield of primary photochemistry (−Fv/Fm)
References
Adamse R, Reed HE, Krizek DT, Britz SJ and Mirecki RM 1997 An inexpensive set up for assessing the impact of ambient of solar UV radiation on seedlings. J. Nat. Res. Life Sci. Edu. 26 139–144
Allen, DJ, Nogues S, Morison JIL, Greenslad PD, McLeod AR and Baker NR 1999 A thirty percent increase in UV-B has no impact on photosynthesis in well-watered and droughted pea plants in the field. Glob. Change Biol. 5 235–24
Amudha P, Jayakumar M and Kulandaivelu G 2005 Impact of ambient solar UV (280–400 nm) radiation on three tropical legumes. J. Plant Biol. 48 284–291
Beauchamp CO and Fridovich I 1971 Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 44 276–278
Bornman JF 1989 Target sites of UV-B radiation in photosynthesis of higher plants. J. Photochem. Photobiol. B Biol. 4 145–156
Butler WL 1978 Energy distribution in the photochemical apparatus of photosynthesis. Annu. Rev. Plant Physiol. 29 345–378
Chance B and Maehly AC 1955 Assay of catalases and peroxidases; in Methods in enzymology volume II (eds) SP Colowick and NO Kaplan (New York: Academic Press) pp 764–775
Correia CM, Areal ELV, Torres-Pereira MS and Torres-Pereira JMG 1999 Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions II. Physiological and biochemical aspects. Field Crops Res. 62 97–105
Edwards NT 1992 State of the art field exposure techniques do not qualitatively simulate expected global increase in UV-B irradiance. Bull. Ecol. Soc. Am. 73 165
Foyer CH and Mullineaux P 1994 Cause of photooxidative stress and amelioration of defense systems in plants (Boca Raton: CRC Press)
Giannopolitis CN and Ries K 1977 Superoxide dismutases. I. Occurrences in higher plants. Plant Physiol. 59 309–314
Govindjee 1995 Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust. J. Plant Physiol. 22 131–160
Guruprasad KN, Bhattacharjee S, Kataria S, Yadav S, Tiwari A, Baroniya S, Rajiv A and Mohanty P 2007 Growth enhancement of soybean (Glycine max) upon exclusion of UV-B and UV-A components of solar radiation: characterization of photosynthetic parameters in leaves. Photosynth. Res. 94 299–306
Hermans C, Smeyers M, Rodriguez RM, Eyletters M, Strasser RJ and Delhaye JP 2003 Quality assessment of urban trees: A comparative study of physiological characterization, airborne imaging and on site fluorescence monitoring by the OJIP-test. J. Plant Physiol. 160 81–90
Hideg E, Spetea C and Vass I 1994 Singlet oxygen and free radical production during acceptor and donor side induced photoinhibition: studies with spin trapping EPR spectroscopy. Biochim. Biophys. Acta 86 143–152
Hideg E and Vass I 1996 UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Sci. 115 251–260
Hsu BD 1993 Evidence for the contribution of S state transitions of oxygen evolution to the initial phase of fluorescence induction. Photosyn. Res. 36 81–88
Jain K, Kataria S and Guruprasad KN 2004 Effect of UV-B radiation on antioxidant enzyme and its modulation by bezaoquinone and alpha-tocopherol in cucumber cotyledons. Curr. Sci. 87 87–90
Kataria S and Guruprasad KN 2012 Solar UV-B and UV-A/B exclusion effects on intraspecific variations in crop growth and yield of wheat varieties. Field Crop Res. 125 8–13
Knecht KT and Mason RP 1993 In vivo spin trapping of xenobiotic free radical metabolites. Arch. Biochem. Biophys. 303 185–194
Lesser M and Neale PJ 1996 Acclimation of Antarctic phytoplankton to ultraviolet radiation: ultraviolet-absorbing compounds and carbon fixation. Mol. Marine Biol. Biotechnol. 5 314–32
Lowry HO, Rosebrough NJ, Farr AL and Randall RJ 1951 Protein measurements with Folin reagent. J. Biol. Chem. 193 265–275
Martz F, Sutinen MS, Derome K, Wingsles G, Tiitto RJ and Turnen M 2007 Effect of ultraviolet (UV) exclusion on the seasonal concentration of photosynthetic and UV-screening pigments in Scot pine needles. Glob. Change Biol. 13 252–265
Mehta P, Jajoo A, Mathur S and Bharti S 2010 Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 48 16–20
Melis A, Nemson JA and Harrison MA 1992 Damage to functional components and partial degradation of Photosystem II reaction center proteins upon chloroplast exposure to ultraviolet-B radiation. Biochim. Biophys. Acta 1100 312–320
Messinger J 2004 Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data. Phys. Chem. Chem. Phys. 6 4764–4771
Noda Y, Anzai K, Mori A, Kohno M, Shimmei M and Packer L 1997 Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR 30 EPR spectrometer system. Biochim. Mol. Biol. Int. 42 35–44
Nogues S and Baker NR 1995 Evaluation of the role of damage to photosystem II in the inhibition of CO2 assimilation in pea leaves on exposure to UV-B radiation. Plant Cell Environ. 18 781–787
Pal M, Sharma A, Abrol YP and Sengupta UK 1997 Exclusion of UV-B radiation from normal solar spectrum on the growth of mung bean and maize. Agric. Ecosys. Environ. 61 29–34
Pfündel EE, Pan RS and Dilly RA 1992 Inhibition of violaxanthin deepoxidation by ultraviolet-B radiation in isolated chloroplasts and intact leaves. Plant Physiol. 98 1372–1380
Rao MV and Ormrod DP 1995 Impact of UV-B and O3 on the free radical scavenging in Arabidopsis thalilana genotypes differing in flavonoid biosynthesis. Photochem. Photobiol. 62 719–726
Rao MV, Paliyath G and Ormrod DP 1996 Ultraviolet-B and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thalilana. Plant Physiol. 110 125–136
Rosen GM and Rauckman EJ 1984 Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 105 198–209
Srivastava A, Strasser RJ and Govindjee 1999 Greening of peas: parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission and P700. Photosynthetica 37 365–392
Strasser RJ, Srivastava A and Govindjee 1995 Polyphasic chlorophyll a fluorescence transients in plants and cyanobacteria. Photochem. Photobiol. 61 32–42
Strasser RJ, Micheal T and Srivastava A 2000 The fluorescence transient as a tool to characterize and screen photosynthetic samples; in Probing photosynthesis: Mechanisms, regulation and adaptation (eds) M Yunus, U Pathre and P Mohnanty (London: Taylor and Francis) pp 445–483
Strasser RJ, Micheal T and Srivastava A 2004 Analysis of the chlorophyll a fluorescence transient; in A signature of photosynthesis, Advances in photosynthesis and respiration volume 19 (eds) GC Papageorgiou and Govindjee (The Netherlands: Springer) pp 321–362
Szilard A, Sass L and Vass I 2002 Photoinactivation of photosystem II at low light intensity, Mathematical model. Acta Biol. Szeged. 46 167–169
Takeuchi Y, Fukomuto R, Kasahara H and Sakaki T 1995 Peroxidation of lipid and growth inhibition induced by UV-B irradiation. Plant Cell Rep. 14 566–570
Takeuchi Y, Kubo H, Kasahara H and Sakaki T 1996 Adaptive alteration in the activities of scavengers of active oxygen in cucumber cotyledons irradiated with UV-B. J. Plant Physiol. 147 589–592
Tevini M, Braun J and Fieser G 1991 The protective function of the epidermal layer of rye seedlings against ultraviolet- B radiation. Photochem. Photobiol. 53 329–333
Varalakshmi D, Lakshmi N and Guruprasad KN 2003 Physiological changes in soybean CV. JS 71–05 after the exclusion of UV-A and UV-B from the solar radiation. Indian J. Plant Physiol. Special Issue 602–606
Xiong FS and Day TA 2001 Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of antarctic vascular plants. Plant Physiol. 125 738–751
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Jitendra P Khurana
[Shine MB and Guruprasad KN 2012 Oxyradicals and PSII activity in maize leaves in the absence of UV components of solar spectrum. J. Biosci. 37 1–10] DOI 10.1007/s12038-012-9248-9
Rights and permissions
About this article
Cite this article
Shine, M.B., Guruprasad, K.N. Oxyradicals and PSII activity in maize leaves in the absence of UV components of solar spectrum. J Biosci 37, 703–712 (2012). https://doi.org/10.1007/s12038-012-9248-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-012-9248-9