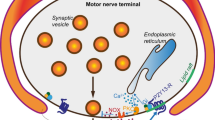
Abstract
β2-Adrenoceptors (β2-ARs) are the most abundant subtype of adrenergic receptors in skeletal muscles. Their activation via a stabilization of postsynaptic architecture has beneficial effects in certain models of neuromuscular disorders. However, the ability of β2-ARs to regulate neuromuscular transmission at the presynaptic level is poorly understood. Using electrophysiological recordings and fluorescent FM dyes, we found that β2-AR activation with fenoterol enhanced an involvement of synaptic vesicles in exocytosis and neurotransmitter release during intense activity at the neuromuscular junctions of mouse diaphragm. This was accompanied by an improvement of contractile responses to phrenic nerve stimulation (but not direct stimulation of the muscle fibers) at moderate-to-high frequencies. β2-ARs mainly reside in lipid microdomains enriched with cholesterol and sphingomyelin. The latter is hydrolyzed by sphingomyelinases, whose upregulation occurs in many conditions characterized by muscle atrophy and sympathetic nerve hyperactivity. Sphingomyelinase treatment reversed the effects of β2-AR agonist on the neurotransmitter release and synaptic vesicle recruitment to the exocytosis during intense activity. Inhibition of Gi protein with pertussis toxin completely prevented the sphingomyelinase-mediated inversion in the β2-AR agonist action. Note that lipid raft disrupting enzyme cholesterol oxidase had the same effect on β2-AR agonist-mediated changes in neurotransmission as sphingomyelinase. Thus, β2-AR agonist fenoterol augmented recruitment and release of synaptic vesicles during intense activity in the diaphragm neuromuscular junctions. Sphingomyelin hydrolysis inversed the effects of β2-AR agonist on neurotransmission probably via switching to Gi protein-dependent signaling. This phenomenon may reflect a dependence of the β2-AR signaling on lipid raft integrity in the neuromuscular junctions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β2-Adrenoceptors (β2-ARs) are the most abundant type of adrenergic receptors in skeletal muscles [1]. Their activation by catecholamines during stressful conditions can contribute to acute enhancement of the muscle performance due to action on Ca2+ cycling, contractile properties, and metabolism in the muscle fibers [2, 3]. Long-term activation of β2-ARs is required for elevation of anabolic processes, skeletal muscle hypertrophy, and a slow-to-fast fiber-type shift, thereby adapting the muscle for higher force development [4,5,6] and counteracting muscle wasting conditions [1, 4, 7]. In addition, sympathetic activity via β2-adrenergic signaling can maintain total and self-renewing number of satellite cells in skeletal muscles [8], enhancing regeneration and functional repair of the muscle [9].
In skeletal muscles, sympathetic nerves, utilizing norepinephrine as the main neurotransmitter, tightly innervate neuromuscular junctions (NMJs) [10,11,12], where motor commands are translated to the muscle fibers. Depression of neuromuscular transmission is one of the peripheral mechanisms of neuromuscular fatigue [13, 14]. Also, a decline in neuromuscular transmission is a possible contributor to sarcopenia [13, 15], Duchenne muscular dystrophy [16], muscle disuse-induced atrophy [17], and amyotrophic lateral sclerosis [18].
Hypothetically, NMJs can serve as an important hub for action of β2-AR agonists. Indeed, pharmacological β2-AR activation alleviated NMJ dysfunction in amyotrophic lateral sclerosis [19] and myasthenic syndromes [20,21,22,23]. It is assumed that one of the key mechanisms for therapeutic action of β2-AR agonists is a stabilization of postsynaptic architecture and increased synaptogenesis via cAMP-dependent pathway [22]. Particularly, β2-AR agonists exerted preserving effects on nicotinic acetylcholine receptor (nAChR) clusters, endplate areas, and formation of postsynaptic junctional folds [24, 25] as well as increased activity of α2-Na,K-ATPase in the junctional membrane [26]. α2-Na,K-ATPase interacts with nAChRs, contributing to enhancement of neuromuscular transmission [27] and stabilization of junctional lipid rafts [28].
Far less is known about a presynaptic action of β2-AR agonists in various skeletal muscles. However, it may be an essential component for the enhancement of muscle performance especially at intense/prolonged activity or in neuromuscular diseases, when safety factor of the neurotransmission decreases [29]. Several recent studies suggest the presence of β2-adrenergic regulation of neurotransmitter release [30,31,32,33]. Particularly, prolonged and chronic treatment with β2-AR agonists increased both spontaneous exocytosis and evoked release in plantar lumbricalis muscle of young mice [30]. At lowered external Ca2+ conditions, β2-AR agonist slightly increased the probability of evoked release at single stimuli but desynchronized the unitary exocytotic events in mouse diaphragm [32, 33].
Here, we investigate the action of fenoterol, a selective β2-AR agonist widely used for asthma treatment, on neurotransmitter release and recruitment of synaptic vesicles into exocytosis during intense activity in the mouse diaphragm NMJ. In addition, the effects of the β2-AR agonist were tested after treatment with sphingomyelinase (SMase). This enzyme hydrolyzes plasmalemmal sphingomyelin, causing a disruption of cholesterol- and sphingomyelin-rich membrane microdomains [34], where the β2-ARs mainly reside [35, 36]. Upregulation of SMase occurs in many conditions associated with skeletal muscle atrophy and damage as well as sympathetic nervous system hyperactivity [37,38,39,40,41,42,43]. Therefore, we hypothesize that the changes in β2-AR agonist effects after SMase treatment have pertinence to pathological remodeling of β2-adrenergic regulation of the neuromuscular transmission.
Methods
Animals
Mice (line BALB/c) were used in experiments. Animals were maintained in ventilated cages (four per cage) at a 12-h light/12-h dark cycle; water and food were available ad libitum. Temperature and humidity were kept at 20–24 °C and 30–60%, respectively. Three- to five-month-old mice (both sexes) were anesthetized and quickly decapitated with a guillotine. Then, the diaphragm with phrenic nerve stubs was excised and embedded in a chamber with physiological solution for dissection into two preparations of the hemidiaphragm–phrenic nerve. The experimental protocol met the requirements of the EU Directive 2010/63/EU and was approved by the Local Ethical Committee of Kazan Federal Scientific Centre (#23/7; May 12, 2023) and Kazan Medical University (Protocol #1/January 25, 2022). The current study was conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. The mice were not intentionally randomized, and investigators were not blinded to the nature of chemicals used.
Solutions, Reagents, and Treatments
Isolated mouse phrenic nerve stub-hemidiaphragm preparation was pinned to Sylgard-coated bottom of the experimental chamber (volume of 5 ml), and the nerve stub was loosely drawn into a suction electrode connected to Model 2100 stimulator (A-M Systems, USA). The chamber was continuously perfused (5 ml/min) with a physiological solution (pH 7.4; 25.0 ± 0.3 °C) containing 129 mM NaCl (Cat. # S9888), 5 mM KCl (Cat. # P9541), 2 mM CaCl2 (Cat. # C5670), 1 mM MgSO4 (Cat. # M3409), 1 mM NaH2PO4 (Cat. # S8282), 20 mM NaHCO3 (Cat. # S8875), 11 mM glucose (Cat. # G8270), and 3 mM HEPES (Cat. # 54,457) and saturated with carbogen. These reagents were purchased from Sigma. The muscle contractions were blocked by pretreatment for 15–20 min with skeletal muscle voltage-gated sodium channel (Nav1.4) inhibitor GIIIB µ-conotoxin (0.5 µM; Peptide Institute Inc., Japan) followed by a rinsing with toxin-free physiological saline. Treatment with neutral SMase (0.01 U/ml; Cat. # S9396; Sigma), which hydrolyzes membrane sphingomyelin, lasted 15 min [34]. Cholesterol oxidase (0.2 U/ml Cat. # 228,250; Sigma) was applied for 15 min [44]. Injection of pertussis toxin (PTX; Sigma, Cat. # 70,323–44-3) was used to inhibit Gi/o proteins. Mice received the intraperitoneal injection of PTX (150 μg/kg in sodium phosphate buffer) 72 h before the experiments [45]. Fenoterol (1, 10 µM; Cat. # F1016; Sigma) and ICI-118.55 (0.1 µM; Cat. # I127; Sigma) were used to selective activation and inhibition of β2-ARs [46, 47]. Application of fenoterol lasted 15 min, and ICI-118.55 was applied for 30 min.
Electrophysiological Recording
Microelectrode recording of postsynaptic responses, namely, end plate potentials (EPPs) and miniature end plate potentials (MEPPs), was carried out as described previously [48]. Briefly, muscle fibers in the junctional region were impaled by Pyrex glass microelectrodes (filled with 2.5 M KCl) with a tip resistance of 5–10 MΩ under visual control (Olympus BX51WI microscope with DIC-optics, × 40 objective). The signals were recorded using an amplifier TEV-200A (Dagan Corporation, USA) with a bandwidth of 0.03 to 10 kHz and analog-to-digital converter (Digidata 1322A, Axon Instruments, USA). Postsynaptic signals were recorded in muscle fibers with a stable resting membrane potential. Threshold for the MEPP detection was set at a level of 0.2 mV, and signal-to-noise ratio was > 5:1. To evoke EPPs, the phrenic nerve stump was stimulated by rectangular stimuli (duration of 0.1 ms) of supramaximal amplitude at low (0.5 Hz) or higher (20 Hz) frequencies. The postsynaptic signals were digitized at 50 kHz, filtered, and stored for analysis using Clampfit 8.2 software (Axon Instruments, Inc., USA). Amplitudes, 20–80% rise time, and e-fold decay time of MEPPs and EPPs were calculated. Recorded signal amplitudes were normalized to a standard resting potential of − 75 mV. For analysis of quantal content, normalized EPP amplitudes were corrected for nonlinear summation [49]. About 150–200 MEPPs were recorded to estimate the MEPP frequency. Quantal content (m) of EPPs was estimated as a ratio between corrected mean EEP amplitude and normalized mean MEPP amplitude [50].
Recording of Muscle Contraction
Recording of isomeric contractions was performed using a force transducer SIH Muscle Tester connected to SI-BAM21-LC amplifier (World Precision Instruments, Inc.) as described previously in details [51]. The central tendon of the hemidiaphragm was tied with linen thread hook attached to a stainless steel rod that was then connected to a force transducer. The contractions were elicited (1) by direct stimulation of the muscle via Pt wire electrodes placed to the muscle surface in the presence of d-tubocurarine (2 µM, Cat # 5.05145; Sigma), a blocker of nicotinic acetylcholine receptors, and (2) by indirect stimulation of phrenic nerve via a suction electrode. Stimuli were rectangle with suprathreshold amplitude and duration of 0.1 ms. Stimulus trains at 20, 50, 70, and 100 Hz (from 50 stimuli) divided by 20-s rest interval were applied twice (through 1 min) before and after 15 min treatment with fenoterol or vehicle alone. Amplitudes of the tetani at corresponding frequencies of the stimulation were calculated. Then, values before the application of fenoterol were taken as 100%. Before the measurements, the resting tension was adjusted to an optimal muscle length and the muscle was equilibrated for 20–25 min.
Imaging of Synaptic Vesicle Exocytosis
Motioning of exocytotic dye release from synaptic vesicles was performed with using Olympus BX51WI microscope equipped by confocal spinning disk unit (Olympus), high-sensitive cameras (Dhyana 400BSI V2, Tucsen and DP72, Olympus), CoolLED pE-4000 light source (CoolLED Ltd.), and water-immersion objectives (UPLANSapo 60xw and LumPlanPF 100xw). The cameras were controlled by Mosaic (Tucsen) and Cell P (Olympus) software. Optosplit II bypass image splitter (Cairn Research) was used for dual channel imaging. The regions of interest (ROIs) were illuminated only at moments (less than 1 s) of the image recording. Image analysis (selection of ROIs, background subtraction, calculation of fluorescence intensity in arbitrary units) was performed using Image-Pro software (Media Cybernetics).
Synaptic vesicles were labeled with FM (styryl) dyes, which reversibly interact with the outer monolayer of surface membranes and are then uptaken into synaptic vesicles by compensatory endocytosis. Subsequent stimulation of the nerve causes exocytotic FM-dye release from the dye-preloaded synaptic vesicles [52]. FM4-64 (red; Cat. # T13320; Thermo Fisher Scientific) and FM1-43 (green; Cat. # T-35356, Thermo Fisher Scientific) dyes, having similar kinetics of binding and unbinding from plasmalemma [53, 54], were used to load different groups of synaptic vesicles, as described previously in details [55]. Briefly, the phrenic nerve was stimulated with two 20-Hz stimulus episodes (30 s and 150 s) separated by a 15-s rest interval, within which the muscle was intensively rinsed with physiological saline containing 30 μM ADVASEP-7 (Cat. # 70,029; Biotium), a water-soluble scavenger for FM dyes. FM4-64 dye (5 µM) and FM1-43 dye (5 µM) were present in the bathing solution during the 1st and 2nd stimulus episodes, respectively. After loading of the FM dyes, the muscle was perfused (5 ml/min) for 40 min with dye-free physiological solution. Next, the phrenic nerve stimulation at 20 Hz led to evoked exocytotic release of the both dyes.
FM-dye fluorescence (excited by a 480/15 nm light) was detected using 715 nm long-pass (for FM4-64) and 535/40 nm (for FM1-43) emission filters, installed in OptoSplit II bypass image splitter allowing to distinguish FM4-64 and FM1-43 fluorescence. A bleed-through of FM1-43 fluorescence into 715 long-pass filter was precluded by reducing the gain settings below a FM1-43 fluorescence threshold.
Nerve terminal FM-dye fluorescence (∆F = FROI − Fb) was calculated in ROI as a mean pixel intensity (in arbitrary units) after subtraction of the background fluorescence (Fb) defined in a 4 × 30 μm2 region outside the NMJ. The nerve terminal fluorescence immediately before onset of unloading stimulation (∆Fmax) was set as 1.0, and a ratio of ∆F/∆Fmax was calculated during 10 min of the dye-unloading stimulation. There were no significant changes in the ∆F/∆Fmax ratio for 10 min at the resting conditions (without the phrenic nerve stimulation). Nerve terminal fluorescence was assessed for the two dyes independently.
Statistics
Data are presented as mean ± standard deviation (SD). Statistical analysis was carried out using OriginPro (OriginLab Corporation) and GraphPad Prism software (GraphPad Software, Inc.). Sample size (n, indicated in each figure legend) is a number of independent experiments on separate muscles from individual mice (n = number of animals). The sample size was determined based on a reasonable value of SD. Normality (by Shapiro–Wilk test) and variance homogeneity (by two sample F-test for variance) were tested. There were no exclusions of outliers. Significance difference was assessed by repeated two-way ANOVA with post hoc Bonferroni multiple comparisons test, a two-tailed, paired t-test (parametric data), or Mann–Whitney U-test (nonparametric data). *P < 0.05, **P < 0.01 and ***P < 0.001 were considered as statistically significant.
Results
Effects of β2-Adrenergic Agonist on Spontaneous and Evoked Neurotransmitter Release
Application of a selective β-AR agonist fenoterol (1 µM) did not modify MEPP amplitude and kinetics (rise and decay time). Fenoterol had no effect on MEPP frequency (Fig. 1A). Accordingly, fenoterol did not change postsynaptic membrane sensitivity to ACh and the rate of spontaneous exocytosis.
Influence of fenoterol (Fen) on parameters of spontaneous and evoked neurotransmitter release. A Changes in MEPP amplitude (Ampl), rise time (Rt), and decay time (Tau) as well as MEPP frequency after treatment with β2-AR agonist Fen. B Effect of Fen on EPP amplitude, rise time, and decay time as well as quantal content (m) of EPPs elicited by single stimuli at 0.5 Hz. A, B, inserts—the typical traces of MEPPs and EPPs, respectively. A, B n = 10 muscles per group; Y-axis, normalized effect of Fen, where the values before Fen addition were taken as 100%. C Changes in quantal content (m) of EPPs during intermittent stimulation at 10 Hz in control and after treatment with Fen. Each stimulus train consisted of 64 stimuli, and interstimulus train interval was 0.5 s. Shown averaged curves. Peaks between the stimulus trains are labeled by (I)–(V). Insert, cumulative quantal content released by 10-Hz intermittent stimulation for 38 s. D Quantification of relative quantal content of the peaks. Right, representative traces. C, D n = 13 (Cntr) and 11 (Fen) muscles; quantal content of EPPs immediately prior to onset of the stimulation was set as 100%. A–D Data are represented as mean ± SD. C, D *P < 0.05, **P < 0.01—by Mann–Whitney U-test between Cntr and Fen
At a low frequency stimulation (0.5 Hz) of phrenic nerve, fenoterol did not affect EPP amplitude, rise, and decay time as well as quantal content (Fig. 1B). This reflects no changes in evoked exocytosis during a low frequency activity.
During 10-Hz intermittent stimulation with 0.5-s resting intervals between stimulus trains (consisting of 64 stimuli), quantal content of EPP quickly decreased by ⁓ 25% during the 1st stimulation episode and then stabilized; the 1st stimulus of each following stimulus train caused a release of more quanta than the last stimulus in the previous stimulation episode. This led to a sawtooth-like pattern of the quantal content changes with the peaks (Fig. 1C, D) arising from a neurotransmitter release facilitation. This facilitation seems to depend on synaptic vesicle delivery to the active zone during the 0.5-s rest interstimulus period. Fenoterol increased the peak amplitudes (Fig. 1C, D), and as a result, 10-Hz intermittent stimulation for ⁓40 s led to release slightly more quanta (P = 0.043) compared to control (Fig. 1C, insert). Thus, fenoterol did not affect evoked exocytosis in response to single stimuli but slightly enhanced neurotransmitter release at 10-Hz intermittent stimulation.
Pharmacological Activation of β2-Adrenergic Receptor Increases Neurotransmitter Release at Moderate-Frequency Activity
During quiet breathing, phrenic motoneurons discharge at a frequency of 20–30 Hz [56]. Intermittent stimulation of phrenic nerve at 20 Hz for 3 min caused three-step changes in quantal content: after a fast drop and plateau phase, quantal content continued to decrease (Fig. 2A). Fenoterol markedly decrease the initial decline of EPP quantal content and prolonged the plateau phase during the 20-Hz stimulation (Fig. 2A). Eventually, 21 ± 13% (P = 0.004) more quanta were released for 3 min of 20-Hz intermittent stimulation in fenoterol-treated NMJs (Fig. 2B).
Effect of β2-adrenergic receptor modulators on neurotransmitter release at intense activity. A Changes in quantal content (m, in %) during 20-Hz intermittent stimulation for 3 min in control (Cntr) and in the presence of β2-adrenergic agonist, fenoterol (Fen). Each stimulus train consisted of 64 stimuli, and interval between the stimulus trains was 0.5 s. Insert, representative traces of EPPs at 0, 60, and 180 s of the stimulation; these time points are denoted by yellow circles on the corresponding curves. Shown averaged curves. B Cumulative quantal content curves, illustrating a number of quanta released during the 20-Hz stimulation (from A). C Quantification of peak amplitudes as the percentage increases in quantal content as compared to the linear segment of the previous stimulation episode (∆m; shown on A). Right, representative traces. A–C n = 16 (Cntr) and 14 (Fen) muscles. D Changes in quantal content (in %) during intermittent stimulation at 20 Hz in muscle treated with β2-adrenergic antagonist (ICI-118.55) or ICI-118.55 in combination with Fen. Shown averaged curves. E Cumulative quantal content released for 3 min of the intermittent stimulation at 20 Hz. D, E n = 12 muscles for ICI and ICI + Fen groups. B, C, E Data are represented as mean ± SD; B, E *P < 0.05 and **P < 0.01– by Mann–Whitney U-test between groups. C **P < 0.01 by a repeated two-way ANOVA followed by Bonferroni post hoc comparisons
Estimation of the peak amplitudes also showed fenoterol-induced increase in the neurotransmitter release facilitation after 0.5-s resting period between stimulus trains (Fig. 2C). Interestingly, the peak amplitudes did not decline during prolonged 20-Hz intermittent stimulation either in control or fenoterol-treated NMJs. Even an increase in the peak amplitudes was observed during the initial 30–40 s of the stimulation in NMJs exposed to fenoterol. It should be noted that ICI-118.55, a selective β2-AR antagonist, completely prevented the effect of fenoterol on neurotransmitter release during 20-Hz stimulation (Fig. 2D, E).
Thus, β2-AR agonist markedly increased the facilitation of neurotransmitter release and total neurotransmitter release upon 20-Hz intermittent stimulation. In addition, the fenoterol-mediated enhancement of neurotransmitter release was persistent after fenoterol washout (Suppl Fig. 1A). This is consistent with a strong binding of fenoterol to β2-ARs [57].
β2-Adrenergic Receptor Activation Increases Recruitment of Synaptic Vesicles into Exocytosis at Intense Activity and Improves Contractile Responses to the Nerve Stimulation
It is assumed that two functional synaptic vesicle pools, recycling and reserve, maintain neurotransmission during a prolonged activity at the vertebrate NMJs. The recycling pool operates mainly during the first 20–30 s of moderate-frequency (20–30 Hz) stimulation and after its depletion synaptic vesicles from the reserve pool mediate neurotransmitter release in a subsequent period [53, 58, 59]. To test the effect of β2-AR activation on the rate of exocytosis of synaptic vesicles formed during the initial (30 s) and following period of moderate-frequency stimulation, red (FM4-64) and green (FM1-43) fluorescent dyes with similar kinetics of dissociation from membranes [53, 54] were used. Particularly, two sequential 20-Hz stimulus trains (with duration of 30 s and 150 s) separated by a 15-s resting washout period were applied to load synaptic vesicles formed during the initial 30-s activity and afterwards with FM4-64 and FM1-43, respectively. Repeated 20-Hz stimulation of the phrenic nerve led to unloading of both FM dyes (Fig. 3A, top).
Effect of β2-adrenergic agonist on exocytotic dye release from synaptic vesicles and contractile responses to intense stimulation. A, B Kinetics of exocytotic FM-dye release from motor nerve terminals dual-labeled with FM4-64 and FM1-43 dyes in control (Cntr) and fenoterol (Fen)-treated muscles. Top, A Protocol of the experiment: nerve terminals were sequentially loaded with FM4-64 (red) and FM1-43 (green) by two 20-Hz stimulus trains separated by 15-s washout period; after loading and 40-min resting period, a repeated 20-Hz stimulation led to dye unloading. Inserts, typical fluorescent images in red and green channels after background subtraction before onset (0) and at 600 s of the stimulation. Scale bars, 15 µm. C The portion of dye fluorescence loss during 300 s and 600 s of 10-min stimulation at 20 Hz in Cntr and Fen-treated nerve terminals. A–C n = 10 (Cntr) and 8 (Fen) muscles. D, E Contractile responses to moderate-to-high frequency stimulation of muscle fibers (direct stimulation, D) and phrenic nerve (indirect stimulation, E). Stimulus trains were applied before and after 15-min exposure to physiological solution without (Time Cntr) or with Fen. Top, typical superimposed traces of tetani in control and after Fen. Bottom, quantification of tetanus amplitude. The averaged amplitude of tetanus in response to the first stimulus train at corresponding frequency was set as 100%. n = 7 per group. A–E Data are represented as mean ± SD. A, B *P < 0.05 and ***P < 0.001 by a repeated two-way ANOVA. C–E *P < 0.05 and ***P < 0.001 by Mann–Whitney U-test
Fenoterol (1 µM) treatment increased the rate of FM4-64 and FM1-43 unloading (Fig. 3A, B). This potentiating effect of fenoterol was more clearly expressed in the case of FM4-64 destaining. Indeed, 28 ± 13% (P < 0.001) more FM4-64 dye was released by 10th min of 20-Hz unloading stimulation in fenoterol-treated vs control NMJs. At the same time, a loss of FM1-43 dye was only 10 ± 8% (P = 0.011) greater than in the control (Fig. 3C). Hence, fenoterol mainly increased recruitment of synaptic vesicles formed during the initial 30 s of previous stimulus train (presumably, recycling pool). Increase fenoterol concentration up to 10 µM did not enhance additionally (as compared to 1 µM fenoterol) the unloading of both FM4-64 and FM1-43 dyes (Suppl Fig. 2A, B).
The increase in synaptic vesicle involvement in exocytosis and neurotransmitter release during intense activity can decrease diaphragm muscle fatigue. Indeed, fenoterol (1 µM) attenuated a depression of contractile responses to repeated moderate- and high-frequency stimulation of the phrenic nerve (indirect stimulation). At the same time, fenoterol did not affect the diaphragm contractions in response to a direct stimulation of the muscle fibers (Fig. 3D, E). Thus, β2-adrenergic modulation can increase a safety factor of the neuromuscular transmission.
Dependence of β2-Adrenergic Regulation of Neurotransmitter Release on Sphingomyelin Integrity in Plasmalemma
β2-ARs mainly reside in planar and caveolar lipid rafts, enriched with cholesterol and sphingomyelin [35, 36]. Sphingomyelin is hydrolyzed to phosphorylcholine and ceramide by secretory and intracellular sphingomyelinases (SMases), which levels increase under the conditions associated with muscle atrophy, e.g., muscle disuse, denervation, amyotrophic lateral sclerosis, heart failure, and inflammation [37,38,39,40,41]. We hypothesize that a partial sphingomyelin breakdown by SMases can affect β2-adrenergic modulation of the neuromuscular transmission, contributing to a dysregulation of muscle activity (Fig. 4).
Effect of β2-adrenergic agonist on neurotransmitter release after treatment with sphingomyelinase (SMase). A Changes in MEPP amplitude (Ampl), rise time (Rt), and decay time (Tau) as well as MEPP frequency in response to fenoterol (Fen) in SMase-pretreated muscles. B Effect of Fen on EPP amplitude, rise, and decay time as well as quantal content (m) of EPPs elicited by single stimuli at 0.5 Hz in SMase-treated muscles. A, B n = 9 muscles per group; Y-axis, normalized effect of Fen, where the values before Fen addition were taken as 100%. C Changes in quantal content of EPPs during intermittent stimulation at 20 Hz in SMase and SMase + Fen-treated muscles. Insert, typical traces of EPPs at 0, 60, and 180 s of the stimulation; these time points are labeled by yellow circles on the corresponding curves. Shown averaged curves. D Cumulative quantal content curves, illustrating a number of quanta released during the 20-Hz stimulation (from C). C, D n = 12 (SMase) and 9 (SMase + Fen) muscles. A, B, D Data are represented as mean ± SD. A, B *P < 0.05 and **P < 0.01 by a two-tailed, paired t-test as compared to pre-Fen baseline. D **P < 0.01 and ***P < 0.001 by Mann–Whitney U-test between SMase and SMase + Fen
Recently, we found that exogenous neutral SMase at concentration of 0.01 U/ml hydrolyzes a portion of plasmalemmal sphingomyelin, leading to a partial disruption of lipid rafts and enhanced synaptic vesicle mobilization at the mice NMJs [34]. Here, effects of β2-AR activation after SMase treatment were evaluated. Fenoterol (1 µM) decreased both MEPP amplitude and rise time, without changing in the MEPP decay time and MEPP frequency in SMase-pretreated NMJs (Fig. 4A). In addition, EPP amplitude, rise, and decay times decreased upon fenoterol application after preexposure to SMase. At the same time, under these conditions, fenoterol did not change quantal content of EPPs at low frequency (0.5 Hz) stimulation (Fig. 4B). Hence, after SMase pretreatment, fenoterol did not change spontaneous exocytosis and evoked neurotransmitter release upon single stimuli but gained the ability to affect postsynaptic membrane properties decreasing the currents via nAChRs.
After SMase pretreatment, fenoterol lost its ability to enhance neurotransmitter release during intermittent 20-Hz stimulation (Fig. 2A; Suppl Fig. 1A), and on the contrary, under these conditions, fenoterol suppressed the neurotransmitter release (Fig. 4C). As a result, 19 ± 5% (P < 0.001) less quanta were released upon fenoterol application in SMase-treated muscles (Fig. 4D). Thus, hydrolysis of plasmalemmal sphingomyelin inversed the effect of β2-adrenergic agonist on neurotransmitter release during 20-Hz activity, causing an appearance of the depressant action of β2-AR activation. This depressant effect of fenoterol was disappeared after β2-agonist washout in SMase-treated muscles (Suppl Fig. 1C, D).
Role of Gi Protein in β2-Adrenergic Regulation of Neurotransmitter Release
β2-ARs can switch a coupling from Gs proteins to PTX-sensitive Gi proteins, which may depend on the lipid microenvironment and lipid raft stability [35, 36, 60, 61]. We hypothesize that after plasmalemmal sphingomyelin hydrolysis, causing a decrease in lipid raft integrity [34], the reversion of β2-AR agonist effect on neurotransmitter release can be mediated by β2-AR switching to Gi protein. To test this possibility, Gi protein was inhibited by pertussis toxin (PTX) injection (Fig. 5).
Effects of Gi protein inhibition with pertussis toxin (PTX) on β2-adrenergic agonist-mediated changes in neurotransmitter release. A Influence of fenoterol (Fen) on MEPP and EPP amplitude (Ampl), rise time (Rt), and decay time (Tau) as well as MEPP frequency and quantal content (m) of EPPs elicited by single stimuli at 0.5 Hz in PTX-pretreated mice. n = 7 muscles per group; Y-axis, normalized effect of Fen, where the values prior to Fen addition were taken as 100%. B Fen-mediated changes in quantal content during intermittent 20-Hz stimulation in PTX-treated mice. Shown averaged curves. C Cumulative quantal content illustrating a total number of quanta released for 3-min stimulation at 20 Hz (from B). n = 14 (PTX) and 10 (PTX + Fen) muscles. D Influence of Fen on the parameters of MEPPs and EPPs in PTX + SMase-treated muscles. n = 10 muscles. E Changes in quantal content during intermittent stimulation at 20 Hz in PTX + SMase-treated muscles in control (PTX + SMase) and after application of Fen (PTX + SMase + Fen). F Cumulative quantal content for 3-min stimulation at 20 Hz (from E). E, F n = 12 (PTX + SMase) and 12 (PTX + SMase + Fen). A, C, D, F Data are represented as mean ± SD. C, F **P < 0.01 by Mann–Whitney U-test between groups. D **P < 0.01 by a two-tailed, paired t-test as compared to pre-Fen baseline
Like in control, fenoterol did not affect the amplitude, kinetics, and frequency of MEPPs as well as the amplitude-temporal parameters and quantal content of EPPs at low frequency stimulation (0.5 Hz) in PTX-treated mice (Fig. 5A). Inhibition of Gi protein did not interfere with the ability of fenoterol to enhance the neurotransmitter release during moderate-frequency stimulation (Fig. 5B, C). Fenoterol increased a number of quanta released for 3 min of 20-Hz stimulation by 20 ± 11% (P = 0.005) in PTX-injected mice (Fig. 5C). Note that inhibition of Gi protein itself had no marked effect on the neurotransmitter release during 20-Hz stimulation (Fig. 5C: Cntr vs PTX). The latter is consistent with our previous data [45].
Inhibition of Gi protein prevented the SMase-mediated inversion of β2-AR agonist effect on evoked neurotransmitter release during moderate-frequency activity (Fig. 5D–F). Fenoterol had no effect on amplitude-temporal parameters of MEPPs and EPPs as well as quantal content of EPPs at low frequency stimulation in PTX + SMase-treated NMJs (Fig. 5D). However, the ability of fenoterol to increase neurotransmitter release during 20-Hz stimulation was recovered by Gi protein inhibition in the NMJs exposed to SMase (Fig. 5E, F). Like in control (Fig. 2B), under these conditions, fenoterol increased a total number of quanta released for 3 min of 20-Hz stimulation by 19 ± 12% (P = 0.002) (Fig. 5F). Also, the analysis of the peak amplitudes during intermittent 20-Hz stimulation showed that fenoterol increased the neurotransmitter release facilitation in response to the first stimulus of each subsequent stimulation episode in PTX + SMase-treated NMJs (Suppl Fig. 3). Thus, the transition from stimulatory effect of the β2-AR agonist to inhibitory one after SMase treatment is precluded by inhibition of Gi protein. This points to a switching of β2-AR coupling to Gi protein in response to plasmalemmal sphingomyelin hydrolysis.
It should be noted that fenoterol decreased frequency of spontaneous exocytotic events in PTX + SMase-treated NMJs (Fig. 5D). Also, under these conditions, the fenoterol-induced enhancement of neurotransmitter release during 20-Hz stimulation disappeared after fenoterol washout (Supp. Figure 1 E, F). These effects of fenoterol were different from those observed in the control (Fig. 1A and Suppl Fig. 1 A, B).
Sphingomyelinase Treatment Reverses the Effect of β2-Adrenergic Agonist on Involvement Synaptic Vesicles in Exocytosis: The Role of Gi Protein
Monitoring of FM-dye exocytotic release (unloading) from two groups of synaptic vesicles, which previously captured the FM4-64 and FM1-43 dyes during two sequential 20-Hz stimulus trains, shorter (30 s) and longer (150 s), revealed the inversion of β2-AR agonist effect after pretreatment with SMase (Fig. 6A–C) as compared to control (Fig. 3A–C). Indeed, fenoterol decreased the rate of both FM4-64 and FM1-43 unloading during 20-Hz stimulation of the motor nerve in SMase-treated NMJs (Fig. 6A, B). As a result, fenoterol reduced FM4-64 and FM1-43 dye loss by 11 ± 10% (P = 0.043) and 11 ± 8% (P = 0.005), respectively, after 10-min stimulation as compared to the control action of SMase (Fig. 6C). Thus, β2-AR agonist suppressed an involvement of synaptic vesicles into exocytosis induced by 20-Hz stimulation after treatment with SMase (Fig. 6A–C). Note that in control, fenoterol enhanced the synaptic vesicle recruitment in exocytosis (Fig. 3A–C).
Influence of sphingomyelinase (SMase) on β2-adrenergic agonist-mediated changes in exocytotic FM-dye release during 20-Hz stimulation. A, B Unloading kinetics of FM4-64 and FM1-43 in response to phrenic nerve stimulation at 20 Hz in SMase-treated muscle in control (SMase) and in combination with fenoterol (Fen) application. Top, FM-dye loading protocol was the same as in Fig. 3; after the loading, the muscles were treated with SMase. C Estimation of FM-dye loss for 300 s and 600 s of 20-Hz stimulation (from A and B). A–C n = 8 (SMase) and 7 (SMase + Fen). D, E Kinetics of FM4-64 and FM1-43 unloading during 20-Hz stimulation in PTX + SMase-treated muscles in control (PTX + SMase) and in combination with Fen (PTX + SMase + Fen). F Portions of FM-dye loss for 300 s and 600 s of the 20-Hz stimulation (from D and E). A, B, D, E Inserts, typical fluorescent images after background subtraction before onset (0) and at 600 s of the stimulation. Scale bars, 15 µm. D–F n = 6 (PTX + SMase) and 7 (PTX + SMase + Fen). A, B, D, E *P < 0.05 and **P < 0.01 by a repeated two-way ANOVA. C, F *P < 0.05 and **P < 0.01 by Mann–Whitney U-test between groups
Inhibition of Gi protein with PTX completely restored the ability of β2-AR agonist to increase the rate of FM4-64 and FM1-43 unloading during 20-Hz activity in SMase-pretreated muscles (Fig. 6D, E). Particularly, fenoterol increased FM4-64 and FM1-43 loss by 27 ± 11% (P = 0.003) and 20 ± 4% (P = 0.003), respectively, after 10-min stimulation of the phrenic nerve in PTX + SMase-treated muscles (Fig. 6F). Accordingly, SMase treatment might promote a signaling via β2-AR-Gi protein axis causing the reversion of β2-AR agonist effect on synaptic vesicle recruitment into the exocytosis during moderate-frequency activity.
Cholesterol Oxidase Inverts the Effect of β2-Adrenergic Agonist on Exocytotic FM-Dye Release During Moderate-Frequency Activity
Hypothetically, SMase-mediated changes in the β2-adrenergic regulation of neuromuscular transmission rely on alterations in properties of lipid microdomains. To test this possibility, membrane cholesterol, an essential component of lipid rafts, was targeted by cholesterol oxidase (ChO). Previously, we found that ChO at concentration of 0.2 U/ml oxidized a portion of plasmalemmal cholesterol and effectively disrupts lipid-ordering (raft) phase in the synaptic membranes of the diaphragm NMJs [44].
In contrast to the control conditions, fenoterol suppressed FM-dye exocytotic release during 20-Hz stimulation of the phrenic nerve in ChO-pretreated NMJs (Fig. 7A, B). As a result, fenoterol decreased FM4-64 and FM1-43 dye loss by 17 ± 8% (P = 0.003) and 24 ± 12% (P = 0.001) after 10 min of the stimulation in the NMJs exposed to ChO (Fig. 7C). Accordingly, ChO treatment, acting in a similar way as SMase, reversed the effect of β2-AR agonist on the synaptic vesicle involvement in exocytosis in response to moderate-frequency activity.
Influence of cholesterol oxidase (ChO) on β2-adrenergic agonist-induced changes in exocytotic FM-dye release during 20-Hz stimulation of the phrenic nerve. A, B Kinetics of FM4-64 and FM1-43 unloading in ChO-treated muscles in control (ChO) and in combination with fenoterol (ChO + Fen). Top, schematic representation of experimental protocol: ChO treatment was applied after dye loading and before exposure to Fen and unloading. Inserts, representative fluorescent images after background subtraction before onset (0) and at 600 s of the stimulation. Scale bars, 15 µm. C Portions of FM-dye loss by 300 s and 600 s of the stimulation at 20 Hz. A–C n = 7 (ChO) and 9 (ChO + Fen). A, B **P < 0.01 and ***P < 0.001 by a repeated two-way ANOVA. C *P < 0.05, **P < 0.01, and ***P < 0.001 by Mann–Whitney U-test between groups
Discussion
The main findings of the present study are as follows: (i) pharmacological activation of β2-ARs enhances neurotransmitter release and recruitment of synaptic vesicles into exocytosis during intense activity; (ii) pretreatment with sphingomyelin-hydrolyzing enzyme (SMase) causes a reversion of the β2-AR agonist effect on neurotransmission from stimulatory on inhibitory one; (iii) after SMase treatment, β2-AR-dependent suppression of evoked neurotransmitter release and synaptic vesicle involvement in exocytosis upon 20-Hz stimulation is mediated by Gi protein activation. Accordingly, β2-adrenergic regulation can contribute to maintenance of neuromuscular transmission acting at the presynaptic level. However, under the conditions accompanied by SMase upregulation (e.g., inflammation, muscle disuse, amyotrophic lateral sclerosis, and heart failure) [37,38,39,40,41,42,43], the activation of β2-ARs could suppress the neurotransmission due to switching on Gi protein-dependent signaling. Interestingly, that functional inhibitors of SMase exerted beneficial effects on skeletal muscle structural and functional parameters in models of skeletal muscle disuse [40, 62], exercise-induced muscle damage [42], palmitate-induced insulin resistance [63], and inflammation/infection-induced skeletal muscle atrophy [64, 65].
β2-AR is the main subtype of ARs in skeletal muscles. Chronic activation of the β2-ARs can decrease muscle wasting in sarcopenia, cancer cachexia, denervation, and neuromuscular diseases [1, 19,20,21,22,23]. Acute activation of the β2-ARs can potentially contribute to the flight-or-fight response, enhancing neuromuscular transmission during stress. Indeed, fenoterol increased neurotransmitter release (Fig. 2A, B) and exocytotic FM-dye loss (Fig. 3) during prolonged 20-Hz stimulation of the motor nerve. This was accompanied by an improvement in contractile function of the diaphragm muscle in response to moderate-to-high frequency stimulation of the phrenic nerve (Fig. 3D, E). At the same time, the activation of β2-ARs did not affect spontaneous exocytosis and evoked neurotransmitter release at low frequency (0.5 Hz) stimulation as well as only slightly increased the neurotransmitter release at intermittent 10 Hz stimulation (Fig. 1C). During intermittent activity at both 10 and 20 Hz, β2-AR agonist markedly increased neurotransmitter release facilitation in the initial period of each subsequent stimulus trains after 0.5-s rest interval (Figs. 1D and 2C). A selective β2-AR antagonist completely prevented the fenoterol-induced elevation of neurotransmitter release (Fig. 2D, E). Accordingly, β2-AR activation can mainly enhance a recruitment of synaptic vesicles into exocytosis probably due to increased vesicle mobilization to the exocytosis sites. Dual labeling of synaptic vesicles with FM dyes suggested that β2-AR agonist could mainly potentiate the mobilization of the vesicles (presumable, from the recycling pool), which were formed by endocytosis during the initial 30 s of moderate frequency activity (Fig. 3A vs Fig. 3B). Interestingly, optogenetic stimulation of postganglionic sympathetic neurons caused an acute increase in MEPP frequency in the lumbricalis muscle. However, this effect of endogenous norepinephrine relied mainly on β1-ARs, since β1-AR antagonist, atenolol strongly suppressed it [66]. Furthermore, norepinephrine is more selective for the β1-ARs as opposed to the β2-ARs, probably due to the relatively slow association rate of norepinephrine to the β2-ARs [67].
Acute administration of fenoterol did not modify significantly the force of diaphragm contractions elicited by direct stimulation (Fig. 3D). Similarly, β2-AR agonist terbutaline as well as a non-subtype selective β-AR agonist isoproterenol had no effect on the contractility of rat diaphragm muscle strips [68, 69]. Also, acute intravenous administration of fenoterol did not change the contractility in nonfatigued diaphragm of dogs [70]. Although a β2-AR agonist salbutamol increased rat diaphragm contraction induced by direct stimulation [71]. In mouse diaphragm strips, β2-AR agonists, clenbuterol and fenoterol at nanomolar concentrations, induced transient positive inotropic effects, which were strongly attenuated by increasing the agonist concentration to 1 µM [72]. Conceivably, such discrepancies may arise due to (i) the complexity of diaphragm muscle containing slow- and fast-twitch muscle fibers, which contractility can be regulated by β-AR in opposite/different manner [68, 69, 73], and (ii) the diversity of β2-AR signaling in combination with biased agonism of β-AR modulators [72, 74, 75]. At the same time, chronic activation of β-ARs [76] or amelioration of sympathetic denervation during aging [77] contributed to the maintenance of contraction force of, at least, fast-twitch muscles. Conversely, sympathectomy led to a significant reduction in both nerve- and muscle-evoked contraction force in the fast lumbricalis muscle [78].
β2-ARs are mainly located in the lipid rafts enriched with sphingomyelin and cholesterol [35]. Plasmalemmal sphingomyelin can be hydrolyzed to ceramide and phosphorylcholine by SMases, whose upregulation and secretion occur during many conditions associated with muscle weaknesses and atrophy, e.g., heart failure [38, 39], muscle disuse [40], sarcopenia [79], amyotrophic lateral sclerosis [18, 41], and inflammation [65]. Increased SMase activity seems to be one of the key triggers of diaphragm dysfunction in heart failure [38, 80] and infection [65]. Also, SMase can have a critical role in exercise-evoked skeletal muscle damage [42]. Here, we found that pretreatment with SMase inversed the action of β2-AR agonist on neurotransmitter release (Fig. 4C, D) and involvement of synaptic vesicles into exocytosis (Fig. 6A–C) during intense activity. Under these condition, β2-AR activation markedly suppressed neuromuscular transmission. Note that SMase itself increased both neurotransmitter and dye release during intense activity (Fig. 4C, D vs Fig. 2A, B; Fig. 6C vs Fig. 3C). This is consistent with the results of our previous study, which showed that SMase acting on plasmalemma increases synaptic vesicle mobilization in the NMJs [34]. Furthermore, SMase treatment can increase depolarization-induced neurotransmitter release from sympathetic nerve terminals [43].
One of reasons for the change in β2-adrenergic regulation of neurotransmission can be a switching of the receptor coupling to Gi protein. It is consistent with this hypothesis that inhibition of Gi protein with PTX completely restored the ability of β2-AR agonist to increase neurotransmitter release (Fig. 5D–F, Suppl Fig. 3B) and synaptic vesicle recruitment to exocytosis (Fig. 6D, E) during prolonged activity. At the same time, the inhibition of Gi protein did not markedly affect the β2-AR-mediated positive regulation of neurotransmitter release in control (Fig. 5A–C). Accordingly, a switching of β2-ARs to Gi protein-dependent signaling can occur after SMase treatment, leading to the depression of neurotransmission in response to β2-AR activation.
Sphingomyelin is an essential component of lipid microdomains, and its interactions with cholesterol can promote lipid raft formation [81, 82]. SMase treatment causes a partial lipid raft disruption and ceramide accumulation in synaptic regions of the mouse diaphragm [34]. Lipid raft stability can control a switching of β2-AR coupling from Gs proteins to Gi proteins [35, 36, 60, 61]. Particularly, lipid raft-disrupting agents, methyl-β-cyclodextrin or oxidized cholesterol, can facilitate β2-AR-Gi protein signaling in isolated atria [36, 60]. Hypothetically, the SMase-mediated reversion of β2-AR agonist effect on the neuromuscular transmission can be related to a decrease in lipid raft integrity and, hence, an increase in coupling of β2-ARs to Gi protein. It is consistent with this hypothesis that treatment with ChO, acting in a similar manner as SMase, caused an inversion of β2-AR agonist effect on synaptic vesicle involvement in exocytosis (Fig. 7). Note that ChO at used concentration as well as SMase partially disrupted lipid rafts in synaptic regions of the mouse diaphragm [34, 44]. Alternatively, products of sphingomyelin hydrolysis (ceramide, sphingosine, and sphingosine 1-phospate) can interfere with β2-adrenergic signaling in the synapses. Sphingosine 1-phosphate induced a heterologous β2-AR desensitization in bronchial airway smooth muscle cells [83]. In turn, heterologous desensitization of β2-ARs mediated by receptor phosphorylation can serve as a “switch” of this receptor coupling from Gs to Gi protein [84].
Fenoterol binding with β2-ARs is a highly favorable process, whereas its unbinding requires overcoming of large free energy barriers [57]. This may be one of the reasons why fenoterol action on neurotransmitter release was persistent after its washout from perfusion (Suppl Fig. 1A). However, after SMase treatment, the action of fenoterol became reversible and disappeared after the agonist washout (Supp Fig. 1 C, E). One possibility is that the change in lipid composition can affect ligand-binding properties of the β2-ARs [85]. Alternatively, sphingomyelin hydrolysis can increase bilayer undulations [86], facilitating a dissociation of the ligand from the β2-AR.
Further molecular and genetic studies are required to uncover the detail mechanisms of lipid-dependence of β2-adrenergic regulation of neurotransmission and its implication in the progression of neuromuscular pathologies. The possible molecular mechanism of change in synaptic vesicle recruitment into exocytosis upon β2-AR activation may be related to cAMP/protein kinase A signaling. Activation of cAMP-dependent enzymes enhanced the recruitment from reserve pool at Drosophila and frog NMJs [87, 88]. Protein kinase A activity can be required for vesicle untethering and delivery from reserve pool to exocytotic sites [89]. In rat NMJs, protein kinase A constitutively increased neurotransmitter release [90, 91]. One of the major presynaptic targets for protein kinase A might be synapsins. Protein kinase A-mediated phosphorylation of synapsin I promoted dissociation of synapsin I from synaptic vesicles, enhancing the rate of exocytosis on stimulation in cultured neurons [92]. In rat neuromuscular junctions, change in protein kinase A-mediated phosphorylation of synapsin 1 and presynaptic SNARE protein SNAP-25 was discovered as a component of the synaptic retrograde regulation [93]. Accordingly, both pre- and postsynaptic β2-ARs could be involved in the regulation of synaptic vesicle mobilization in the NMJs.
Conclusion
Acute activation of β2-ARs with fenoterol enhanced neuromuscular transmission during intense activity via increasing a recruitment of synaptic vesicles into the evoked exocytosis. Sphingomyelin-hydrolyzing enzyme SMase, which partially destabilizes synaptic lipid rafts [34], inversed the fenoterol-induced positive modulation of neuromuscular transmission probably due to switching of β2-adrenergic signaling to PTX-sensitive Gi protein. Similarly, cholesterol-oxidizing enzyme, which partially disrupts the junctional lipid rafts [43], converted the β2-AR agonist stimulatory action on the synaptic vesicle exocytosis to inhibitory one. These data suggest a significant role of lipid microdomains in the β2-adrenergic regulation of neuromuscular transmission in the mouse diaphragm.
Data Availability
All mentioned data are represented in the main manuscript figures and supplementary figures. Other additional data will be made available on reasonable request.
Abbreviations
- ACh:
-
Acetylcholine
- β2-AR:
-
Beta2-adrenoceptor
- ChO:
-
Cholesterol oxidase
- EPP:
-
Endplate potential
- MEPP:
-
Miniature endplate potential
- NMJ:
-
Neuromuscular junction
- nAChR:
-
Nicotinic acetylcholine receptor
- PTX:
-
Pertussis toxin
- SMase:
-
Sphingomyelinase
References
Lynch GS, Ryall JG (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88(2):729–767. https://doi.org/10.1152/physrev.00028.2007
Hostrup M, Kalsen A, Ortenblad N, Juel C, Morch K, Rzeppa S, Karlsson S, Backer V, Bangsbo J (2014) Beta2-adrenergic stimulation enhances Ca2+ release and contractile properties of skeletal muscles, and counteracts exercise-induced reductions in Na+-K+-ATPase Vmax in trained men. J Physiol 592(24):5445–5459. https://doi.org/10.1113/jphysiol.2014.277095
Meister J, Bone DBJ, Knudsen JR, Barella LF, Liu L, Lee R, Gavrilova O, Chen M, Weinstein LS, Kleinert M, Jensen TE, Wess J (2022) In vivo metabolic effects after acute activation of skeletal muscle G(s) signaling. Mol Metab 55:101415. https://doi.org/10.1016/j.molmet.2021.101415
Goncalves DA, Silveira WA, Manfredi LH, Graca FA, Armani A, Bertaggia E, BT ON, Lautherbach N, Machado J, Nogara L, Pereira MG, Arcidiacono D, Realdon S, Kahn CR, Sandri M, Kettelhut IC, Navegantes LCC (2019) Insulin/IGF1 signalling mediates the effects of beta(2) -adrenergic agonist on muscle proteostasis and growth. J Cachexia Sarcopenia Muscle 10(2):455-475. https://doi.org/10.1002/jcsm.12395
Jessen S, Reitelseder S, Kalsen A, Kreiberg M, Onslev J, Gad A, Ortenblad N, Backer V, Holm L, Bangsbo J, Hostrup M (2021) beta(2)-Adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men. J Appl Physiol (1985) 130(3):617–626. https://doi.org/10.1152/japplphysiol.00553.2020
Ohnuki Y, Umeki D, Mototani Y, Jin H, Cai W, Shiozawa K, Suita K, Saeki Y, Fujita T, Ishikawa Y, Okumura S (2014) Role of cyclic AMP sensor Epac1 in masseter muscle hypertrophy and myosin heavy chain transition induced by beta2-adrenoceptor stimulation. J Physiol 592(24):5461–5475. https://doi.org/10.1113/jphysiol.2014.282996
Dutt V, Gupta S, Dabur R, Injeti E, Mittal A (2015) Skeletal muscle atrophy: potential therapeutic agents and their mechanisms of action. Pharmacol Res 99:86–100. https://doi.org/10.1016/j.phrs.2015.05.010
Yuan S, Zheng S, Zheng K, Gao Y, Chen M, Li Y, Bai X (2021) Sympathetic activity is correlated with satellite cell aging and myogenesis via beta2-adrenoceptor. Stem Cell Res Ther 12(1):505. https://doi.org/10.1186/s13287-021-02571-8
Ryall JG, Schertzer JD, Alabakis TM, Gehrig SM, Plant DR, Lynch GS (2008) Intramuscular beta2-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. J Appl Physiol (1985) 105(1):165–172. https://doi.org/10.1152/japplphysiol.00317.2007
Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molgo J, Lochmuller H, Witzemann V, Kettelhut IC, Navegantes LC, Pozzan T, Rudolf R (2016) Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci U S A 113(3):746–750. https://doi.org/10.1073/pnas.1524272113
Petrov AM, Zakirjanova GF, Kovyazina IV, Tsentsevitsky AN, Bukharaeva EA (2022) Adrenergic receptors control frequency-dependent switching of the exocytosis mode between “full-collapse” and “kiss-and-run” in murine motor nerve terminal. Life Sci 296:120433. https://doi.org/10.1016/j.lfs.2022.120433
Straka T, Vita V, Prokshi K, Horner SJ, Khan MM, Pirazzini M, Williams MPI, Hafner M, Zaglia T, Rudolf R (2018) Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle. Int J Mol Sci 19(7). https://doi.org/10.3390/ijms19071935
Fogarty MJ, Gonzalez Porras MA, Mantilla CB, Sieck GC (2019) Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol 122(1):93–104. https://doi.org/10.1152/jn.00061.2019
Hepple RT, Rice CL (2016) Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594(8):1965–1978. https://doi.org/10.1113/JP270561
Arnold WD, Clark BC (2023) Neuromuscular junction transmission failure in aging and sarcopenia: the nexus of the neurological and muscular systems. Ageing Res Rev 89:101966. https://doi.org/10.1016/j.arr.2023.101966
Chang M, Cai Y, Gao Z, Chen X, Liu B, Zhang C, Yu W, Cao Q, Shen Y, Yao X, Chen X, Sun H (2023) Duchenne muscular dystrophy: pathogenesis and promising therapies. J Neurol 270(8):3733–3749. https://doi.org/10.1007/s00415-023-11796-x
Sirago G, Pellegrino MA, Bottinelli R, Franchi MV, Narici MV (2023) Loss of neuromuscular junction integrity and muscle atrophy in skeletal muscle disuse. Ageing Res Rev 83:101810. https://doi.org/10.1016/j.arr.2022.101810
Zakyrjanova GF, Giniatullin AR, Mukhutdinova KA, Kuznetsova EA, Petrov AM (2021) Early differences in membrane properties at the neuromuscular junctions of ALS model mice: Effects of 25-hydroxycholesterol. Life Sci 273:119300. https://doi.org/10.1016/j.lfs.2021.119300
Bartus RT, Betourne A, Basile A, Peterson BL, Glass J, Boulis NM (2016) Beta2-Adrenoceptor agonists as novel, safe and potentially effective therapies for amyotrophic lateral sclerosis (ALS). Neurobiol Dis 85:11–24. https://doi.org/10.1016/j.nbd.2015.10.006
Webster RG, Cossins J, Lashley D, Maxwell S, Liu WW, Wickens JR, Martinez-Martinez P, de Baets M, Beeson D (2013) A mouse model of the slow channel myasthenic syndrome: neuromuscular physiology and effects of ephedrine treatment. Exp Neurol 248:286–298. https://doi.org/10.1016/j.expneurol.2013.06.012
Ghazanfari N, Morsch M, Tse N, Reddel SW, Phillips WD (2014) Effects of the ss2-adrenoceptor agonist, albuterol, in a mouse model of anti-MuSK myasthenia gravis. PLoS One 9(2):e87840. https://doi.org/10.1371/journal.pone.0087840
McMacken G, Cox D, Roos A, Muller J, Whittaker R, Lochmuller H (2018) The beta-adrenergic agonist salbutamol modulates neuromuscular junction formation in zebrafish models of human myasthenic syndromes. Hum Mol Genet 27(9):1556–1564. https://doi.org/10.1093/hmg/ddy062
Webster RG, Vanhaesebrouck AE, Maxwell SE, Cossins JA, Liu W, Ueta R, Yamanashi Y, Beeson DMW (2020) Effect of salbutamol on neuromuscular junction function and structure in a mouse model of DOK7 congenital myasthenia. Hum Mol Genet 29(14):2325–2336. https://doi.org/10.1093/hmg/ddaa116
Clausen L, Cossins J, Beeson D (2018) Beta-2 adrenergic receptor agonists enhance AChR clustering in C2C12 myotubes: implications for therapy of myasthenic disorders. J Neuromuscul Dis 5(2):231–240. https://doi.org/10.3233/JND-170293
Vanhaesebrouck AE, Webster R, Maxwell S, Rodriguez Cruz PM, Cossins J, Wickens J, Liu WW, Cetin H, Cheung J, Ramjattan H, Palace J, Beeson D (2019) Beta2-adrenergic receptor agonists ameliorate the adverse effect of long-term pyridostigmine on neuromuscular junction structure. Brain 142(12):3713–3727. https://doi.org/10.1093/brain/awz322
Arkhipov A, Khuzakhmetova V, Petrov AM, Bukharaeva EA (2022) Catecholamine-dependent hyperpolarization of the junctional membrane via beta2- adrenoreceptor/G(i)-protein/alpha2-Na-K-ATPase pathway. Brain Res 1795:148072. https://doi.org/10.1016/j.brainres.2022.148072
Heiny JA, Kravtsova VV, Mandel F, Radzyukevich TL, Benziane B, Prokofiev AV, Pedersen SE, Chibalin AV, Krivoi II (2010) The nicotinic acetylcholine receptor and the Na, K-ATPase alpha2 isoform interact to regulate membrane electrogenesis in skeletal muscle. J Biol Chem 285(37):28614–28626. https://doi.org/10.1074/jbc.M110.150961
Petrov AM, Kravtsova VV, Matchkov VV, Vasiliev AN, Zefirov AL, Chibalin AV, Heiny JA, Krivoi II (2017) Membrane lipid rafts are disturbed in the response of rat skeletal muscle to short-term disuse. Am J Physiol Cell Physiol 312(5):C627–C637. https://doi.org/10.1152/ajpcell.00365.2016
Wood SJ, Slater CR (2001) Safety factor at the neuromuscular junction. Prog Neurobiol 64(4):393–429. https://doi.org/10.1016/s0301-0082(00)00055-1
Rodrigues AZC, Wang ZM, Messi ML, Delbono O (2019) Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca(2+) channels. Mol Cell Neurosci 95:59–70. https://doi.org/10.1016/j.mcn.2019.01.007
Wang ZM, Rodrigues ACZ, Messi ML, Delbono O (2020) Aging blunts sympathetic neuron regulation of motoneurons synaptic vesicle release mediated by beta1- and alpha2B-adrenergic receptors in geriatric mice. J Gerontol A Biol Sci Med Sci 75(8):1473–1480. https://doi.org/10.1093/gerona/glaa022
Tsentsevitsky A, Nurullin L, Tyapkina O, Bukharaeva E (2020) Sympathomimetics regulate quantal acetylcholine release at neuromuscular junctions through various types of adrenoreceptors. Mol Cell Neurosci 108:103550. https://doi.org/10.1016/j.mcn.2020.103550
Tsentsevitsky AN, Kovyazina IV, Bukharaeva EA (2019) Diverse effects of noradrenaline and adrenaline on the quantal secretion of acetylcholine at the mouse neuromuscular junction. Neuroscience 423:162–171. https://doi.org/10.1016/j.neuroscience.2019.10.049
Tsentsevitsky AN, Gafurova CR, Mukhutdinova KA, Giniatullin AR, Fedorov NS, Malomouzh AI, Petrov AM (2023) Sphingomyelinase modulates synaptic vesicle mobilization at the mice neuromuscular junctions. Life Sci 318:121507. https://doi.org/10.1016/j.lfs.2023.121507
Xiang Y, Rybin VO, Steinberg SF, Kobilka B (2002) Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem 277(37):34280–34286. https://doi.org/10.1074/jbc.M201644200
Odnoshivkina YG, Sytchev VI, Petrov AM (2017) Cholesterol regulates contractility and inotropic response to beta2-adrenoceptor agonist in the mouse atria: involvement of Gi-protein-Akt-NO-pathway. J Mol Cell Cardiol 107:27–40. https://doi.org/10.1016/j.yjmcc.2016.05.001
Bryndina IG, Shalagina MN, Protopopov VA, Sekunov AV, Zefirov AL, Zakirjanova GF, Petrov AM (2021) Early lipid raft-related changes: interplay between unilateral denervation and hindlimb suspension. Int J Mol Sci 22(5). https://doi.org/10.3390/ijms22052239
Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF (2014) Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail 16(5):519–525. https://doi.org/10.1002/ejhf.73
Olsson K, Cheng AJ, Al-Ameri M, Tardif N, Melin M, Rooyackers O, Lanner JT, Westerblad H, Gustafsson T, Bruton JD, Rullman E (2022) Sphingomyelinase activity promotes atrophy and attenuates force in human muscle fibres and is elevated in heart failure patients. J Cachexia Sarcopenia Muscle 13(5):2551–2561. https://doi.org/10.1002/jcsm.13029
Petrov AM, Shalagina MN, Protopopov VA, Sergeev VG, Ovechkin SV, Ovchinina NG, Sekunov AV, Zefirov AL, Zakirjanova GF, Bryndina IG (2019) Changes in membrane ceramide pools in rat soleus muscle in response to short-term disuse. Int J Mol Sci 20(19). https://doi.org/10.3390/ijms20194860
Choi BJ, Park KH, Park MH, Huang EJ, Kim SH, Bae JS, Jin HK (2022) Acid sphingomyelinase inhibition improves motor behavioral deficits and neuronal loss in an amyotrophic lateral sclerosis mouse model. BMB Rep 55(12):621–626. https://doi.org/10.5483/BMBRep.2022.55.12.142
Lee YI, Leem YH (2019) Acid sphingomyelinase inhibition alleviates muscle damage in gastrocnemius after acute strenuous exercise. J Exerc Nutrition Biochem 23(2):1–6. https://doi.org/10.20463/jenb.2019.0009
Odnoshivkina JG, Sibgatullina GV, Petrov AM (2023) Lipid-dependent regulation of neurotransmitter release from sympathetic nerve endings in mice atria. Biochim Biophys Acta Biomembr 1865(7):184197. https://doi.org/10.1016/j.bbamem.2023.184197
Zakirjanova GF, Giniatullin AR, Gafurova CR, Malomouzh AI, Fedorov NS, Khaziev AN, Tsentsevitsky AN, Petrov AM (2023) Effects of cholesterol oxidase on neurotransmission and acetylcholine levels at the mice neuromuscular junctions. Archives of Biochemistry and Biophysics 749. https://doi.org/10.1016/j.abb.2023.109803
Zakyrjanova GF, Tsentsevitsky AN, Kuznetsova EA, Petrov AM (2021) Immune-related oxysterol modulates neuromuscular transmission via non-genomic liver X receptor-dependent mechanism. Free Radic Biol Med 174:121–134. https://doi.org/10.1016/j.freeradbiomed.2021.08.013
Odnoshivkina YG, Petrov AM, Zefirov AL (2011) The effects of beta (2) -adrenoreceptor activation on the contractility, Ca-signals and nitric oxide production in the mouse atria. Acta Naturae 3(2):103–112
Odnoshivkina UG, Sytchev VI, Nurullin LF, Giniatullin AR, Zefirov AL, Petrov AM (2015) β2-adrenoceptor agonist-evoked reactive oxygen species generation in mouse atria: implication in delayed inotropic effect. Eur J Pharmacol 765:140–153. https://doi.org/10.1016/j.ejphar.2015.08.020
Zakyrjanova GF, Gilmutdinov AI, Tsentsevitsky AN, Petrov AM (2020) Olesoxime, a cholesterol-like neuroprotectant restrains synaptic vesicle exocytosis in the mice motor nerve terminals: possible role of VDACs. Biochim Biophys Acta Mol Cell Biol Lipids 1865(9). https://doi.org/10.1016/j.bbalip.2020.158739
McLachlan EM, Martin AR (1981) Non-linear summation of end-plate potentials in the frog and mouse. J Physiol 311:307–324. https://doi.org/10.1113/jphysiol.1981.sp013586
Rozas JL, Gomez-Sanchez L, Tomas-Zapico C, Lucas JJ, Fernandez-Chacon R (2011) Increased neurotransmitter release at the neuromuscular junction in a mouse model of polyglutamine disease. J Neurosci 31(3):1106–1113. https://doi.org/10.1523/JNEUROSCI.2011-10.2011
Lenina O, Petrov K, Kovyazina I, Malomouzh A (2019) Enhancement of mouse diaphragm contractility in the presence of antagonists of GABAA and GABAB receptors. Exp Physiol 104(7):1004–1010. https://doi.org/10.1113/EP087611
Betz WJ, Bewick GS (1992) Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255(5041):200–203. https://doi.org/10.1126/science.1553547
Richards DA, Guatimosim C, Betz WJ (2000) Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron 27(3):551–559. https://doi.org/10.1016/s0896-6273(00)00065-9
Wu Y, Yeh FL, Mao F, Chapman ER (2009) Biophysical characterization of styryl dye-membrane interactions. Biophys J 97(1):101–109. https://doi.org/10.1016/j.bpj.2009.04.028
Gafurova CR, Tsentsevitsky AN, Petrov AM (2022) Frequency-dependent engagement of synaptic vesicle pools in the mice motor nerve terminals. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-022-01202-x
Lee KZ, Fuller DD (2011) Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol 179(1):71–79. https://doi.org/10.1016/j.resp.2011.02.014
Plazinska A, Plazinski W, Jozwiak K (2015) Agonist binding by the beta2-adrenergic receptor: an effect of receptor conformation on ligand association-dissociation characteristics. Eur Biophys J 44(3):149–163. https://doi.org/10.1007/s00249-015-1010-4
Richards DA, Guatimosim C, Rizzoli SO, Betz WJ (2003) Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39(3):529–541. https://doi.org/10.1016/s0896-6273(03)00405-7
Rizzoli SO, Betz WJ (2005) Synaptic vesicle pools. Nat Rev Neurosci 6(1):57–69. https://doi.org/10.1038/nrn1583
Sytchev VI, Odnoshivkina YG, Ursan RV, Petrov AM (2017) Oxysterol, 5alpha-cholestan-3-one, modulates a contractile response to beta2-adrenoceptor stimulation in the mouse atria: involvement of NO signaling. Life Sci 188:131–140. https://doi.org/10.1016/j.lfs.2017.09.006
Strohman MJ, Maeda S, Hilger D, Masureel M, Du Y, Kobilka BK (2019) Local membrane charge regulates beta2 adrenergic receptor coupling to Gi3. Nat Commun 10(1):2234. https://doi.org/10.1038/s41467-019-10108-0
Bryndina IG, Shalagina MN, Sekunov AV, Zefirov AL, Petrov AM (2018) Clomipramine counteracts lipid raft disturbance due to short-term muscle disuse. Neurosci Lett 664:1–6. https://doi.org/10.1016/j.neulet.2017.11.009
Verma MK, Yateesh AN, Neelima K, Pawar N, Sandhya K, Poornima J, Lakshmi MN, Yogeshwari S, Pallavi PM, Oommen AM, Somesh BP, Jagannath MR (2014) Inhibition of neutral sphingomyelinases in skeletal muscle attenuates fatty-acid induced defects in metabolism and stress. Springerplus 3:255. https://doi.org/10.1186/2193-1801-3-255
De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K, Desgeorges M, Piraud M, Cheillan D, Vidal H, Lefai E, Nemoz G (2012) TNF-alpha- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle 2(1):2. https://doi.org/10.1186/2044-5040-2-2
Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA (2015) Neutral sphingomyelinase 2 is required for cytokine-induced skeletal muscle calpain activation. Am J Physiol Lung Cell Mol Physiol 309(6):L614-624. https://doi.org/10.1152/ajplung.00141.2015
Wang ZM, Messi ML, Grinevich V, Budygin E, Delbono O (2020) Postganglionic sympathetic neurons, but not locus coeruleus optostimulation, activates neuromuscular transmission in the adult mouse in vivo. Mol Cell Neurosci 109:103563. https://doi.org/10.1016/j.mcn.2020.103563
Xu X, Kaindl J, Clark MJ, Hubner H, Hirata K, Sunahara RK, Gmeiner P, Kobilka BK, Liu X (2021) Binding pathway determines norepinephrine selectivity for the human beta(1)AR over beta(2)AR. Cell Res 31(5):569–579. https://doi.org/10.1038/s41422-020-00424-2
Uzuki M, Yamakage M, Fujimura N, Namiki A (2007) Direct inotropic effect of the beta-2 receptor agonist terbutaline on impaired diaphragmatic contractility in septic rats. Heart Lung 36(2):140–147. https://doi.org/10.1016/j.hrtlng.2006.06.006
Fujimura N, Sumita S, Narimatsu E, Nakayama Y, Shitinohe Y, Namiki A (2000) Effects of isoproterenol on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 161(2 Pt 1):440–446. https://doi.org/10.1164/ajrccm.161.2.9904044
Suzuki S, Numata H, Sano F, Yoshiike Y, Miyashita A, Okubo T (1988) Effects and mechanism of fenoterol on fatigued canine diaphragm. Am Rev Respir Dis 137(5):1048–1054. https://doi.org/10.1164/ajrccm/137.5.1048
Van der Heijden HF, Van Balkom RH, Folgering HT, Van Herwaarden CL, Dekhuijzen PN (1996) Effects of salbutamol on rat diaphragm contractility. J Appl Physiol (1985) 81(3):1103–1110. https://doi.org/10.1152/jappl.1996.81.3.1103
Duarte T, Menezes-Rodrigues FS, Godinho RO (2012) Contribution of the extracellular cAMP-adenosine pathway to dual coupling of beta2-adrenoceptors to Gs and Gi proteins in mouse skeletal muscle. J Pharmacol Exp Ther 341(3):820–828. https://doi.org/10.1124/jpet.112.192997
Ohnuki Y, Umeki D, Mototani Y, Shiozawa K, Nariyama M, Ito A, Kawamura N, Yagisawa Y, Jin H, Cai W, Suita K, Saeki Y, Fujita T, Ishikawa Y, Okumura S (2016) Role of phosphodiesterase 4 expression in the Epac1 signaling-dependent skeletal muscle hypertrophic action of clenbuterol. Physiol Rep 4(10). https://doi.org/10.14814/phy2.12791
Cairns SP, Borrani F (2015) beta-Adrenergic modulation of skeletal muscle contraction: key role of excitation-contraction coupling. J Physiol 593(21):4713–4727. https://doi.org/10.1113/JP270909
Ippolito M, Benovic JL (2021) Biased agonism at beta-adrenergic receptors. Cell Signal 80:109905. https://doi.org/10.1016/j.cellsig.2020.109905
Kim J, Grotegut CA, Wisler JW, Li T, Mao L, Chen M, Chen W, Rosenberg PB, Rockman HA, Lefkowitz RJ (2018) Beta-arrestin 1 regulates beta2-adrenergic receptor-mediated skeletal muscle hypertrophy and contractility. Skelet Muscle 8(1):39. https://doi.org/10.1186/s13395-018-0184-8
Rodrigues ACZ, Messi ML, Wang ZM, Bonilla HJ, Freeman WM, Delbono O (2021) Long-term, induced expression of Hand2 in peripheral sympathetic neurons ameliorates sarcopenia in geriatric mice. J Cachexia Sarcopenia Muscle 12(6):1908–1924. https://doi.org/10.1002/jcsm.12790
Rodrigues ACZ, Messi ML, Wang ZM, Abba MC, Pereyra A, Birbrair A, Zhang T, O’Meara M, Kwan P, Lopez EIS, Willis MS, Mintz A, Files DC, Furdui C, Oppenheim RW, Delbono O (2019) The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol (Oxf) 225(3):e13195. https://doi.org/10.1111/apha.13195
Russ DW, Wills AM, Boyd IM, Krause J (2014) Weakness, SR function and stress in gastrocnemius muscles of aged male rats. Exp Gerontol 50:40–44. https://doi.org/10.1016/j.exger.2013.11.018
Bost ER, Frye GS, Ahn B, Ferreira LF (2015) Diaphragm dysfunction caused by sphingomyelinase requires the p47(phox) subunit of NADPH oxidase. Respir Physiol Neurobiol 205:47–52. https://doi.org/10.1016/j.resp.2014.10.011
Slotte JP (2016) The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim Biophys Acta 1858(2):304–310. https://doi.org/10.1016/j.bbamem.2015.12.008
Murata M, Matsumori N, Kinoshita M, London E (2022) Molecular substructure of the liquid-ordered phase formed by sphingomyelin and cholesterol: sphingomyelin clusters forming nano-subdomains are a characteristic feature. Biophys Rev 14(3):655–678. https://doi.org/10.1007/s12551-022-00967-1
Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ (2016) Effect of sphingosine 1-phosphate on cyclo-oxygenase-2 expression, prostaglandin E2 secretion, and beta2-adrenergic receptor desensitization. Am J Respir Cell Mol Biol 54(1):128–135. https://doi.org/10.1165/rcmb.2014-0443OC
Daaka Y, Luttrell LM, Lefkowitz RJ (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390(6655):88–91. https://doi.org/10.1038/36362
Manna M, Niemela M, Tynkkynen J, Javanainen M, Kulig W, Muller DJ, Rog T, Vattulainen I (2016) Mechanism of allosteric regulation of beta(2)-adrenergic receptor by cholesterol. Elife 5. https://doi.org/10.7554/eLife.18432
Ingolfsson HI, Carpenter TS, Bhatia H, Bremer PT, Marrink SJ, Lightstone FC (2017) Computational lipidomics of the neuronal plasma membrane. Biophys J 113(10):2271–2280. https://doi.org/10.1016/j.bpj.2017.10.017
Kuromi H, Kidokoro Y (2000) Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron 27(1):133–143. https://doi.org/10.1016/s0896-6273(00)00015-5
Petrov AM, Giniatullin AR, Zefirov AL (2008) Role of the cAMP cascade in the turnover of synaptic vesicles of the frog motor nerve terminal. Neurochem J 2(3):175–182. https://doi.org/10.1134/s1819712408030069
Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47(3):365–378. https://doi.org/10.1016/j.neuron.2005.06.018
Santafe MM, Garcia N, Lanuza MA, Tomas M, Tomas J (2009) Interaction between protein kinase C and protein kinase A can modulate transmitter release at the rat neuromuscular synapse. J Neurosci Res 87(3):683–690. https://doi.org/10.1002/jnr.21885
Losavio A, Muchnik S (2000) Facilitation of spontaneous acetylcholine release induced by activation of cAMP in rat neuromuscular junctions. Life Sci 66(26):2543–2556. https://doi.org/10.1016/s0024-3205(00)00588-9
Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, Benfenati F, Baldelli P, Valtorta F (2006) Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci 26(45):11670–11681. https://doi.org/10.1523/JNEUROSCI.3321-06.2006
Polishchuk A, Cilleros-Mane V, Just-Borras L, Balanya-Segura M, Vandellos Pont G, Silvera Simon C, Tomas M, Garcia N, Tomas J, Lanuza MA (2023) Synaptic retrograde regulation of the PKA-induced SNAP-25 and Synapsin-1 phosphorylation. Cell Mol Biol Lett 28(1):17. https://doi.org/10.1186/s11658-023-00431-2
Acknowledgements
ANT was supported by assignment for Kazan Institute of Biochemistry and Biophysics, FRC Kazan Scientific Center of Russian Academy of Sciences.
Funding
This work was supported by the Russian Science Foundation (grant numbers 21–14-00044, https://rscf.ru/project/21-14-00044/ (#3.3–3.6 Result’s sections); 23–15-00124, https://www.rscf.ru/project/23-15-00124/ (#3.1 and 3.2 Result’s sections); and 23–75-10022, https://www.rscf.ru/project/23-75-10022/ (#3.7 Result’s section)).
Author information
Authors and Affiliations
Contributions
ANT: electrophysiological experiments, visualization, and data analysis, review and editing; CRG: fluorescent experiments, visualization, and data analysis; NSF: contraction recording and data analysis; AIM: visualization, review and editing; ANK: electrophysiological data analysis; AMP: conceptualization; writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experimental protocol met the requirements of the EU Directive 2010/63/EU and was approved by the Local Ethical Committee of Kazan Federal Scientific Centre (#23/7; May 12, 2023) and Kazan Medical University (Protocol #1/January 25, 2022). The current study was conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Research does not involve human patients.
Consent for Publication
All authors consent to publish the article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chulpan R Gafurova and Andrei N. Tsentsevitsky are first coauthors with equal contribution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gafurova, C.R., Tsentsevitsky, A.N., Fedorov, N.S. et al. β2-Adrenergic Regulation of the Neuromuscular Transmission and Its Lipid-Dependent Switch. Mol Neurobiol 61, 6805–6821 (2024). https://doi.org/10.1007/s12035-024-03991-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-03991-2