Abstract
Oestrogen deprivation as a consequence of menopause alters the brain neuronal circuit and results in the development of neurobehavioural symptoms later. Hormone replacement therapy to some extent helps to overcome these abnormalities but is associated with various adverse events. Lithium therapy is being used to manage multiple neuropsychiatric disorders and is reported to maintain structural synaptic plasticity, suppress neuroinflammation, and promote adult neurogenesis. The present study examined the effect of lithium treatment on the neurobehavioural impairments in ovariectomized rat model mimicking clinical postmenopausal condition. A protective effect of lithium treatment was observed on the reconsolidation of spatial and recognition memory along with depression-like behaviour in ovariectomized rats. The Golgi-Cox staining revealed increased dendritic length and spine density in the pyramidal neurons of the CA1 region of the hippocampus, layer V of the somatosensory cortex, and layer II/III of the prefrontal cortex in the treated group. A significant reduction in pro-inflammatory markers, Il2, II6, and Il1b, was observed in the hippocampus, somatosensory cortex, and prefrontal cortex following lithium treatment. mRNA expression studies of Gfap and Pparg, along with histopathological analysis, suggested reactive astrogliosis to be a major contributor of neuroinflammation in ovariectomized rats that was normalized following lithium treatment. Further, the treatment inhibited Gsk-3β activity and maintained the normal level of β-catenin, CREB, and BDNF. The results revealed a defensive role of lithium against ovariectomy-induced neurobehavioural impairments, thus suggesting it to be a potential therapeutic agent for managing postmenopausal neurological symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Middle-aged women are always at a higher risk of developing neurobehavioural conditions than coeval men due to gradual oestrogen depletion as a consequence of menopause onset [1]. Oestrogen regulates reproductive functions and engages in memory formation, behavioural functions, neuroprotection, and synaptic plasticity [2, 3]. The transition from the reproductive to non-reproductive stage in women is proposed to be a prodromal phase of neurodegenerative disease [1]. Dendritic spines are small protrusions present in the neuronal dendrites of the hippocampus, prefrontal cortex, and neocortex regions that are highly sensitive to oestrogen fluctuations [2, 4]. Several preclinical studies have supported the reduction in spine density in the apical dendrite of pyramidal neuron of layer V in the female rodents following ovariectomy. These alterations were in the different neocortical regions, apical dendrite of the CA1 region of the hippocampus, and apical and basal dendrite of pyramidal neurons in the prefrontal cortex [5,6,7].
Neuroinflammatory events are the major contributors to postmenopause-associated neurological conditions [1]. Some preclinical studies showed increased expression of interleukins (IL-1β, IL-6, and TNF-α) in the brain of middle-aged ovariectomized (ovx) rats that ultimately led to disruption of neuronal circuits and interfered with normal cognitive and behaviour processes [3, 8]. These pro-inflammatory cytokines are also known to play a crucial role in the pathogenesis of several neuropsychiatric conditions [9]. Astrocytes are a type of glial cells that, despite being devoid of electrical impulse, participate in several physiological processes of the brain [10]. The available information suggested that reactive gliosis and its inflammatory properties significantly promote and sustain neuronal death in neurodegenerative diseases [11]. Oestrogen deprivation affects the neuronal circuit and enhances the blood–brain barrier (BBB) permeability in the middle-aged ovx rats [12]. In rodents, chronic BBB damage is associated with the accumulation of blood-derived neurotoxic proteins such as thrombin, fibrin, hemosiderin, haemoglobin, plasmin, and iron, which leads to neuronal damage, either directly via neurotoxins or by neuroinflammation [13].
The beneficial effect of exogenous oestrogen is reported to combat postmenopause-linked neurobehavioural symptoms by enhancing structural synaptic plasticity and reducing neuroinflammation [8]. However, studies have shown the risk of dementia associated with exogenous oestrogen treatment due to the atrophic changes in the brain, resulting in cortex and hippocampus volume reduction [14, 15]. Chronic treatment of oestrogen following a critical window period in middle-aged ovx rats enhanced the BBB permeability [16], thus suggesting a potential risk of the therapy towards neurodegenerative diseases. Therefore, it is important to identify molecules or drugs that replace the need for exogenous oestrogen and minimize postmenopausal conditions.
Lithium, a gold standard drug in psychiatric medicine, is mainly used to treat bipolar disorder and major depression [17]. Some preclinical studies suggested the neuroprotective effect of lithium via inhibiting autophagy, neuroinflammation, and enhancing structural synaptic plasticity [18, 19]. Furthermore, lithium has been reported to regulate various signalling cascades, including glycogen synthase kinase 3 (Gsk-3), cAMP response element-binding protein (CREB), and brain-derived neurotrophic factor (BDNF) [17]. Our previous study showed that differential activation of Gsk-3β is involved in the development of neurobehaviour impairments in middle-aged ovx rats [3]. Activated Gsk-3β is also known to engage in the prolonged stimulation of neuroinflammatory process, which eventually leads to neurodegenerative diseases [20]. Moreover, activated Gsk-3β also interferes with the mammalian target of rapamycin (mTOR) signalling, resulting in impairment of the cognition process [3], which was improved following lithium treatment [21]. Therefore, in the present study, we hypothesized that treatment of lithium might suppress postmenopause-associated neurobehaviour symptoms via inhibiting neuroinflammation and maintaining the structural synaptic plasticity in the discrete brain regions. We used middle-aged ovx rat model that mimics postmenopausal conditions to study the effect of lithium.
Materials and Methods
Experimental animals and care
The available literature shows that around 10-month-old female rats start experiencing irregularity in their oestrous cycle, indicating initiation of reproductive cessation [22]. Therefore, the study was conducted on 10-month-old Sprague Dawley female rats. The rats were housed in standard polycarbonate cages (three animals/cage) at temperature of 25 ± 2℃, relative humidity of 50–60%, and 12 h day/night cycle in the animal house of CSIR-IHBT. Food and water were provided ab libitum to the animals throughout the experiment. All the experimental procedure on animals were performed during the day cycle, and the protocol was duly approved by Institutional Animal Ethics Committee (IAEC) established by CPCSEA, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India.
Ovariectomy and experimental protocol
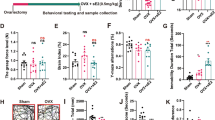
The surgical procedure for ovariectomy in SD female rats was performed following anaesthesia with ketamine and xylazine (detailed in Supplementary Information, Supplement 1). A separate group of rats (n = 8) indicated as sham-N served as sham and received similar surgical procedure, except removal of ovaries. All the animals were kept for 12 weeks’ post-surgery to develop the postmenopausal symptoms [3]. Successfully recovered ovx rats were randomly divided into two groups (n = 8/group). An ovx rats group (ovx-V) received normal standard chow diet (Golden feeds, New Delhi, India) for 10 weeks, whereas the other group (ovx-LI) was provided with a chow diet containing lithium carbonate (2.4 g/Kg of chows) during the same period [23]. The therapeutic concentration of lithium in the serum was maintained by 2.4 g of lithium carbonate per kg of chow to achieve 0.97 ± 0.20 mM, equivalent to 0.6–1.2 mM in humans [24]. It has been observed that long-term lithium therapy is required to maintain its therapeutic concentration in the brain to cure psychiatric conditions [25, 26]. A previous preclinical study has shown that treatment of lithium for 10 weeks increases the learning process in rats [27]. Therefore, 10 weeks’ administration of lithium was selected in the current study. Following the treatment, the animals were subjected to neurobehavioural tests. All the animals were sacrificed after 24 h of the behavioural analysis, and the brain was isolated. The hippocampus, somatosensory cortex, and prefrontal cortex regions were selected as these areas are engaged in the cognitive process and are primarily affected during behaviour disorders. The brain of four animals selected randomly from each group was used for histopathological analysis and immunohistochemistry, while the remaining brains were dissected to isolate all the three regions for gene expression and western blotting studies. The uterus was also isolated from all the animals to calculate adjusted weight. A schematic representation of the experimental protocol is shown in Fig. 1.
Schematic representation of experimental timeline. 10-month-old SD female rats underwent the ovariectomization (ovx) procedure and were kept for 90-day post-surgical procedure to develop post- menopause-associated neurobehavioural impairment. After 90 days, the rats were randomly divided into three groups named as sham-N, ovx-V, and ovx-LI, respectively. sham-N and ovx-V were continuing on the normal chow diet, while ovx-LI groups received lithium containing chow diet for further 75 days. Animals were sacrificed following the 24 h of last behaviour parameter, and brain was isolated to perform the mRNA expression, protein expression, and histopathological analysis. ovx: ovariectomy; MWM: Morris water maze; NORT: Novel object recognition test; OFT: Open field test; FST: Forced swim test; sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; and h: Hours
Assessment of cognitive functions
The Morris water maze test (MWMT) test was conducted for subsequent 5 days following 10 weeks’ treatment to animals. The procedure involved learning phase of 4 days to record escape latency (time to trace a submerged platform). On the next day of the last learning phase, the extent of memory retention was recorded in terms of exploration in a target quadrant. After MWMT, recognition memory in rats was accessed using a novel object recognition test (NORT). In the test, ability of an animals to distinguish a familiar object from a novel object was noted as discrimination ratio and preference index. The details of cognitive tests are included in Supplementary Information (Supplement 1).
Behavioural assessment
The anxiety-like behaviour and locomotor function of rats were studied using an open field test (OFT). Forced swim test (FST) was carried out 1 h after OFT to assess depression-like behaviour in experimental animals, and details are described in Supplementary Information (Supplement 1).
Quantitative real-time PCR analysis (qRT-PCR)
The isolated brain parts were homogenized in Trizol reagent (Sigma-Aldrich, USA) and processed to pellet down the RNA [28]. The RNA was quantified and reverse transcribed into the cDNA following RNAse treatment. A complete qRT-PCR method with details of primers used in the study is described in Supplementary Information (Supplement 1), Table S1. A relative expression of each gene with respect to sham-N was calculated using 2^ΔΔCT method.
Western blotting
The crude protein was extracted from the isolated brain parts using Trizol method as described earlier [28]. Total protein was denatured in 1X sample loading buffer, separated on SDS–polyacrylamide gel electrophoresis, transferred to a PVDF membrane and incubated with primary antibodies [Gsk-3β, p-Gsk-3β(Ser9), β-catenin, mTOR, p-mTOR (Ser2448), BDNF, CREB, and p-CREB (Ser133)]. The protein expression was quantified using ImageJ software following normalization with β-actin. A detailed methodology is included in Supplementary Information (Supplement 1).
Histopathological analysis
The isolated brain was washed in freshly prepared PBS. The brain was sagittal separated into two equal halves. A half was fixed in the formalin for the histopathological and immunohistochemistry analysis, while the remaining part was used for the Golgi-Cox staining.
Golgi-Cox (GC) staining
GC staining was performed to understand the structural synaptic plasticity. The left hemisphere of brain was dissected to separate the prefrontal cortex and somatosensory region. Both the dissected regions were processed for GC staining [29]. The details of staining procedure, and section analysis for dendritic morphology and spine density [30] are described in Supplementary Information (Supplement 1). For the quantitative analysis of pyramidal morphology, only those neurons which fulfil the following criteria were selected: (1) well-impregnated pyramidal neuron characterized by triangular soma shape; (2) cell body within the middle of specified subregions of the hippocampus (CA1 region), layer V of the somatosensory cortex as well as II/III layer of the prefrontal cortex; (3) neuron relatively isolated from the neighbouring neuron; (4) the presence of at least two primary basal dendritic shafts; and (5) apical dendrite without truncated branches.
Immunohistochemistry
The right hemisphere was kept in formalin and finally embedded in paraffin wax. The sections were processed [described in Supplementary Information (Supplement 1)] to study localized expression of glial fibrillary acidic protein (GFAP) [31] and neuronal nuclear protein (NeuN).
Nissl staining
Nissl staining was performed to understand the morphology and pathology of the neuron. Briefly, the 5 µM section of paraffin-embedded brain tissue was taken on the slide and processed for Nissl staining using a 1% solution of cresyl violet [32], as is further described in Supplementary Information (Supplement 1). The sections on slides were analysed under a bright-field microscope (Olympus BX53F) to count the number of dark-stained dead neurons. In addition, to reduce the ambiguity in results, the average number of the dark neurons/field from the sham-N group was deducted from the rest of the experimental groups [32].
Statistical analysis
The results are represented as a mean ± standard error. Distribution of the data was assessed by Shapiro–Wilk test. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was performed for normally distributed data, whereas the parameters with skewed data were analysed by Kruskal–Wallis test and Mann–Whitney U post hoc. Student’s t test was applied to find the statistic significant difference between the groups in Nissl staining. Repeated measure of ANOVA was done to assess the escape latency in MWM and difference in number of intersections by Sholl method. Mauchly’s test of sphericity was used with the repeated measure of ANOVA, and degree of freedom was corrected by using epsilon Greenhouse–Geisser to more conservative value for the factor in which sphericity assumption was violated. Further effective size was also calculated by applying a formula, r = Z/√N, for Mann–Whitney U test (where N = total samples number, r = correlation coefficient, and Z = standardized value for the U value). Epsilon-squared formula was applied for Kruskal–Wallis test, as \({E}_{R}^{2}\) = \(\frac{H}{(\mathrm{n}2-1)/ (\mathrm{n}+1)}\) [where n = the total number of observations, H = value earned in the Kruskal–Wallis test (the Kruskal–Wallis H test statistic), and \({E}_{R}^{2}\) = coefficient, which assumes the value from 0 (no relationship) to 1 (a perfect relationship). Cohen’s d was used to calculate the effective size during pair-wise comparison, while Eta square (η2) is done for one-way ANOVA. A value of P < 0.05 for the studied parameters was considered as significant.
Results
The detailed results including variation between the sample mean/difference within the sample, exact P value among different groups, and the effective size are given in the Supplementary Information (Supplement 1).
Ovariectomy reduced uterine weight
Ovariectomization significantly (P < 0.05) reduced the uterine/body weight in the ovx-V group (0.162 ± 0.0186) compared to sham-N group. Interestingly, no change (P = 0.93) in uterine weight was observed in ovx-LI group (0.147 ± 0.01) as that of ovx-V; however, it remained significantly (P < 0.05) reduced in comparison with sham-N group (0.442 ± 0.045).
Lithium treatment improved ovariectomy-induced neurobehavioural impairments
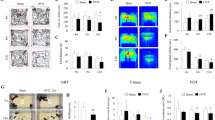
The escape latency, a measure of consolidation process of spatial memory in MWMT, was significantly (P < 0.05) decreased day-wise within the experimental groups. However, insignificant change in the escape latency was observed between the experimental groups (Fig. 2A). The ovx-LI group and sham-N group spent significantly (P < 0.05) more time in the target quadrant during probe trial on day 5 in comparison with the ovx-V group, thus suggesting that lithium rescued the reconsolidation process of spatial memory (Fig. 2B). In NORT, episodic memory was also altered in ovx-V group, indicated by a significant (P < 0.05) decrease in the discrimination ratio (Fig. 2C) and preference index (Fig. 2D) as compared to sham-N group. However, lithium treatment significantly increased (P < 0.05) both the parameters when compared with the ovx-V group.
Effect of lithium treatment on neurobehaviour impairment in ovx rat. (A): Average escape latency from day 1 to day 4 in MWMT; (B): Probe trial on day 5 to assess the time spent in the target quadrant in MWMT; (C) and (D): Recognition memory analysis by calculating the discrimination ration and percentage of preference index by NORT; (E): Locomotion analysis (Mean total virtual zone entries) in OFT; (F): Anxiety-like behaviour analysis (Mean central square entries) in OFT; and (G): Depression-like behaviour by recording immobility time in FST. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; MWMT: Morris water maze test; NORT: Novel object recognition test; OFT: Open field test; FST: Forced swimming test; and s: Seconds
Further, OFT revealed insignificant change in total number of virtual square entities among different groups, indicating no effect on total locomotion (Fig. 2E). There was induction of anxiety-like behaviour in ovx-V group evidenced by a significant (P < 0.05) decrease in the central square entries in comparison with the sham-N group. Interestingly, lithium treatment in ovx-LI group do not overcome the anxiety-like behaviour when compared with ovx-V group, as insignificant change in central square entries was observed among both the groups (Fig. 2F). Furthermore, ovx-V group also showed a significant (P < 0.05) increase in immobility period in FST as compared to the sham-N group (Fig. 2G). Thus, suggested appearance of depression-like behaviour in vehicle control group following ovx. However, lithium treatment ameliorated depression-like behaviour, as a significant decrease (P < 0.05) in the immobility period was observed in ovx-LI group in comparison with ovx-V group. The results of cognitive-behaviour tests suggested that ovx leads to the alteration in the neurobehaviour function, while lithium treatment rescued the rats from these abnormalities.
Lithium improved neuronal population and maintained pyramidal neuron architecture
Nissl staining was performed to evaluate the neuronal morphology in all the selected regions of the brain. The somatosensory cortex and CA1 region of the hippocampus in ovx-V group showed darker neuron population when studied using Nissl staining. A significant (P < 0.05) reduction in dark neuron density was observed in the regions following lithium treatment in the ovx-LI group compared to ovx-V group. However, the hippocampal CA3 region did not show any significant change in the dark neuron population among all the experimental groups. The prefrontal cortex of the ovx-V group also showed a higher density of Nissl-stained neurons, which significantly (P < 0.05) reduced post-lithium treatment in the ovx-LI group (Fig. 3A).
Histopathological analysis to assess the effect of lithium treatment on neuronal population. (A): Analysis of Nissl-stained dark neurons in the ss-cortex, hippocampus (CA1 and CA3 regions) and pf-cortex; (B): Analysis of NeuN protein expression as DAB-stained area (%) in the ss-cortex, hippocampus (CA1 and CA3 regions) and pf-cortex; (C): Representative image of Nissl-stained dark neurons in the ss-cortex, hippocampus (CA1 and CA3 regions) and pf-cortex (Scale bar 50 μm); and (D): Representative image of NeuN protein expression in the ss-cortex, hippocampus (CA1 and CA3 regions) and pf-cortex (Scale bar 100 μm). *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; ND: No dark neuron; ss-Cortex: Somatosensory cortex; CA1: Cornu Ammonis 1; CA3: Cornu Ammonis 3; pf-cortex: Prefrontal cortex; and AoI: Area of interest. The average number of dark neurons/ fields observed in the sham-N group subtracted from rest of other section to avoid the artefacts, hence represented as ND
NeuN is a protein marker used to define mature neuron population. The expression studies of NeuN validated the results of Nissl staining in our study. There was a significant decrease (P < 0.05) in NeuN positive cells reactivity in the somatosensory cortex, CA1 region of the hippocampus, and the prefrontal cortex of ovx-V group compared to sham-N group. The group treated with lithium showed a significant increase (P < 0.05) in NeuN reactivity compared to ovx-V group (Fig. 3B). However, insignificant change in NeuN expression was observed in the CA3 region among all the studied groups.
Morphological analysis of pyramidal neurons was also carried out to understand the effect of lithium on dendritic arborization. The effect was studied on the both apical and basal dendrite of the pyramidal neurons. There was a significant decrease (P < 0.05) in total apical and basal dendritic length in all the three regions of the brain in ovx-V group compared to sham-N group. Lithium treatment significantly increased the dendritic length in ovx-LI group in comparison with ovx-V group; however, no significant change was observed in the apical dendrite of the somatosensory cortex following the lithium treatment in the ovx-LI group (Fig. 4A). Furthermore, a significant (P < 0.05) reduction in average dendritic length of apical dendrite was also observed in ovx-LI group compared to ovx-V group in all the three regions of the brain. However, insignificant change in the average dendritic length of basal dendrite was observed among all the studied groups (Fig. 4B). The number of nodes and branch termination points was also significantly (P < 0.05) increased in both the apical and basal dendrite following lithium treatment in ovx-LI group in comparison with ovx-V group (Fig. 4C and D).
Effect of lithium treatment on pyramidal neuron morphology. (A): Quantification of total dendritic length of apical and basal pyramidal neuron in the ss-cortex, hippocampus, and pf-cortex; (B): Quantification of average dendritic length of apical and basal pyramidal neuron in the ss-cortex, hippocampus, and pf-cortex; (C): Quantification of number of nodes in the apical and basal dendrite of pyramidal neurons with consecutive 20 μm spaced concentric ring in the ss-cortex, hippocampus, and pf-cortex; and (D): Quantification of number of branch termination in the apical and basal dendrite of pyramidal neurons with consecutive 20 μm spaced concentric ring in the ss-cortex, hippocampus, and pf-cortex
In this study, dendritic bifurcation and spine density of pyramidal neuron were also quantified, as decrease in the both the processes interferes with neuronal signalling that results in development of neurobehavioural impairment. There was a significant (P < 0.05) decrease in dendritic bifurcation of apical, as well as basal dendrite in all the studied brain regions in ovx-V group compared to sham-N group. However, lithium treatment showed a significant increased bifurcation at both the sections (P < 0.05) in ovx-LI group as compared to ovx-V group (Fig. 5B-D). Similarly, to the dendritic bifurcation, the ovx-V group also showed a significant (P < 0.05) decrease in the spine density of the apical and basal dendrite in all the three regions of brain compared with the sham-N group. However, lithium treatment showed a significant (P < 0.05) recovery in spine number of apical and basal dendrites in ovx-LI group when compared to ovx-V group. However, insignificant change in spine density was observed between ovx-LI and ovx-N groups (Fig. 6). The results suggested that lithium treatment increased dendritic bifurcation and spine number recovery that might be responsible for improvement in neurobehavioural functions.
Effect of the lithium treatment on the dendritic arborization. (A): Representative image of apical and basal traced neurite over Golgi-Cox-stained neurons micrograph of ss-cortex, hippocampus (CA1 regions), and pf-cortex under 40X magnification; Scale bar 20 μm; (B, C, and D): Quantification of apical and basal dendritic bifurcation of pyramidal neurons in the ss-cortex, hippocampus (CA3 regions), and pf-cortex; (B1, C2, and D1): Number of apical dendritic intersection of pyramidal neurons with consecutive 20 μm spaced concentric ring; (B2, C1, and D2): Number of basal dendritic intersection of pyramidal neurons with consecutive 20 μm spaced concentric ring. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; ss-Cortex: Somatosensory cortex; pf-cortex: Prefrontal cortex; and AoI: Area of interest. X-axis in the Fig. 5(B1, B2, C1, C2, D1, and D2) represent distance from the nucleus (μm), whereas Y-axis represents number of intersections
Effect of lithium treatment on the spine density. (A): Representative image of dendritic spine of apical and basal dendrite of pyramidal neuron in the ss-cortex, hippocampus (CA1 regions), and pf-cortex under 100X magnification (oil immersion); Scale bar 10 μm. Dendritic segments of 10 μm length from second order of branching were included in the study. (B, C, and D): Quantification of total spine/10 μm in the apical and basal dendritic of pyramidal neurons from ss-cortex, hippocampus, and pf-cortex, respectively. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V; sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; ss-Cortex: Somatosensory cortex; and pf-cortex: Prefrontal cortex
Lithium downregulated the expression of pro-inflammatory mRNA
A significant (P < 0.05) upregulation in the mRNA expression of pro-inflammatory markers in the discrete brain regions was observed in ovx-V group in comparison with sham-N group. The results revealed that lithium treatment in ovx-LI group after a chronic period of ovx significantly (P < 0.05) downregulated the mRNA expression of Il2, II6, and Il1b genes in all the three studied regions of the brain in contrast to ovx-V group (Fig. 7A).
Effect of lithium treatment on the messenger ribonucleic acid expression of targeted genes. (A): Expression of pro-inflammatory related genes (ll2, ll6, and ll1b) in the ss-cortex, hippocampus, and pf-cortex; (B): Expression of reactive astrogliosis-related genes (Gfap and Pparg) in the ss-cortex, hippocampus, and pf-cortex; and (C): Expression of junction protein-related genes (Ocln and Tjp1) in the ss-cortex, hippocampus, and pf-cortex. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; ll2: Interleukin 2, ll6: Interleukin 6; ll1b: Interleukin 1 beta; Gfap: Glial fibrillary acidic protein; Pparg: Peroxisome proliferator-activated receptor gamma; Ocln: Occludin; Tjp1: Tight junction protein 1; ss-cortex: Somatosensory cortex; and pf-cortex: Prefrontal cortex
Lithium improved neuroinflammation via reactive astrogliosis suppression
Gfap is a marker of reactive astrogliosis that is upregulated in several neurodegenerative conditions and triggers a variety of neuroinflammatory events. The results showed a significant (P < 0.05) upregulation of Gfap expression in all the three regions of the brain in ovx-V group, when compared with sham-N group (Fig. 7B). However, lithium treatment downregulated its expression in the ovx-LI group when compared with ovx-V group. On the contrary, lithium treatment in ovx-LI group showed a significant (P < 0.05) upregulation of Pparg expression (P < 0.05) in all the three regions of the brain in comparison with ovx-V group. The expression of Pparg was significantly (P < 0.05) reduced in ovx-V group as compared to sham-N group in all the studied regions (Fig. 7B).
Further to confirm reactive astrogliosis, morphological analysis of the GFAP positive cells was performed. There was a significant increase (P < 0.05) in total astrocyte process length, average astrocyte process length, and number of the nodes in ovx-V group in all the three studied regions compared to sham-N group (Fig. 8(B1-B3, C1-C3, and D1-D3). Interestingly, lithium treatment in ovx-LI group significantly (P < 0.05) attenuated the observed changes as compared to ovx-V group. Increased astrocyte process bifurcation is considered as a characteristic feature of reactive astrogliosis. In this study, reactive astrogliosis was studied by using concentric ring method of Sholl [24]. A significant (P < 0.05) increase in the astrocyte process bifurcation was observed in all the three brain regions of ovx-V group compared to sham-N group (Fig. 8(B 4, C 4, and D 4). The astrocyte process bifurcation was normalized following the lithium treatment in ovx-LI group as a significant (P < 0.05) decrease is observed in comparison with ovx-V group. The results suggested lithium to be a potential agent to overcome reactive astrogliosis and to maintain homeostasis in the brain following ovx.
Effect of lithium treatment on the reactive astrogliosis. (A): Representative image of GFAP- DAB-stained micrograph of ss-cortex, hippocampus (CA1 regions), and pf-cortex under 100X magnification, Scale bar 10 μm; (B-D): Quantification of reactive astrogliosis in the ss-cortex, CA1 region of the hippocampus, and pf-cortex; (B1, C1, and D1): Average astrocytic process length in (μm); (B2, C2, and D2): Total astrocytic process length in (μm); (B3, C3, and D3): Numbers of nodes by concentric ring method; and (B4, C4, and D4): Number of intersections of astrocytic process with consecutive 4 μm spaced concentric ring. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; GFAP: Glial fibrillary acidic protein; ss-Cortex: Somatosensory cortex; and pf-cortex: Prefrontal cortex
Effect of lithium treatment on the protein expression. (A, B, and C): Graphic representation of relative fold change of protein expression relative to sham-N in the ss-cortex, hippocampus, and pf-cortex. The phosphorylated forms of p-CREB, p-mTOR, and p-Gsk-3β were normalized with their native, i.e. CREB, mTOR, and Gsk-3β, respectively, whereas all the native forms of the protein BDNF, CREB, mTOR, β-catenin, and Gsk-3 β were normalized with β-actin. (A1, B1, and C1): representative Western blotting image of BDNF, p-CREB, CREB, p-mTOR, m-TOR, β-catenin, p-Gsk-3β, Gsk-3β, and β-actin in the ss-cortex, hippocampus, and pf-cortex. *P < 0.05 as compared to sham-N; **P < 0.05 as compared to ovx-V. sham-N: Group subject to sham surgery with normal chow diet; ovx-V: Group subject to ovariectomy with normal chow diet; ovx-LI: Group subject to ovariectomy with lithium chow diet; BDNF: Brain-derived neurotrophic factor; p-CREB: Phosphorylated cAMP-response element binding protein; CREB: cAMP-response element binding protein; p-mTOR: Phosphorylated mammalian target of rapamycin; mTOR: mammalian target of rapamycin; β-catenin: Beta catenin; p-Gsk-3β: Phosphorated glycogen synthase kinase-3 beta, Gsk-3β: Glycogen synthase kinase-3 beta; β-actin: Beta actin; ss-Cortex: Somatosensory cortex; and pf-cortex: Prefrontal cortex
Lithium maintained the BBB integrity by upregulation of Ocln and Tjp1 expression
Increase in the BBB permeability is an indicator of neurodegenerative events in various neurological conditions. The BBB strength depends on the expression of junction proteins, which has been reported to decline following oestrogen deprivation [33]. In the present study, a significant (P < 0.05) downregulation in the mRNA expression of Ocln and Tjp1 was observed in all the studied regions in ovx-V group compared with sham-N group (Fig. 7C). However, lithium treatment significantly upregulated the expression of both the genes in the hippocampus, somatosensory cortex, and prefrontal cortex of ovx-LI group compared to ovx-V and sham-N groups.
Lithium treatment attenuated ovariectomy-induced alteration in proteins expression
Gsk-3β has been well-reported to be involved in numerous of neurobehavioural conditions [20]. The results revealed an insignificant change in total Gsk-3β level in all the three studied brain regions among different groups. However, a significant (P < 0.05) reduction in p-Gsk-3β(Ser9) was observed in the somatosensory cortical region of ovx-V group compared with sham-N group, thus suggested activation of Gsk-3β following ovx. Lithium treatment in ovx-LI group significantly (P < 0.05) upregulated the somatosensory cortical expression of p-Gsk-3β(Ser9) in comparison with ovx-V group (Fig. 9A1). On the contrary, the hippocampus and the prefrontal cortex showed a significant upregulation of p-Gsk-3β(Ser9) in ovx-V group (P < 0.05) compared to sham-N group. Interestingly, there was no significant change in p-Gsk-3β(Ser9) expression observed in both the regions following the lithium treatment in ovx-LI group compared to ovx-V group (Fig. 9B1 and C1).
β-catenin is a major component of Wnt/β-catenin signalling that triggers proteasomal degradation following activation of Gsk-3β. In the present study, the somatosensory cortical region of ovx-V group showed a significant (P < 0.05) downregulation of β-catenin expression compared to sham-N group. However, lithium treatment significantly (P < 0.05) upregulated β-catenin expression in ovx-LI group in comparison with ovx-V group (Fig. 9A1). Surprisingly, the hippocampus and the prefrontal cortex did not show variation in the expression of β-catenin in any of the experimental group (Fig. 9B1 and C1).
mTOR is also an indirect downstream target of Gsk-3β. The selected brain regions did not show any significant change in total mTOR protein expression among different experimental groups. Phosphorylation of mTOR on serine2448 residue is an essential step for the further signalling cascade. The somatosensory cortical region of ovx-V group showed a significant (P < 0.05) downregulation of p-mTOR (Ser2448) compared to sham-N group. Lithium treatment in ovx-LI group significantly (P < 0.05) upregulated p-mTOR (Ser2448) expression when compared ovx-V group (Fig. 9A1). Interestingly, the hippocampus and the prefrontal cortex showed a significant upregulation (P < 0.05) of p-mTOR (Ser2448) in ovx-V group as compared to the sham-N group. However, lithium treatment did not show any significant change in p-mTOR (Ser2448) protein expression in ovx-LI group when compared with ovx-V group. There was significant increase for the expression of same observed in ovx-LI group as compared to sham-N group (Fig. 9B1 and C1).
There was insignificant change observed in the level of total CREB protein in the studied brain regions among different experimental groups. Further, we studied p-CREB (Ser133) protein expression to understand the downstream signalling cascade of CREB signalling pathway. The prefrontal cortex of ovx-V group showed a significant (P < 0.05) downregulation of p-CREB (Ser133) expression compared to sham-N group. The level was restored to normal following lithium treatment in ovx-LI group and significantly increased in comparison with ovx-V group (Fig. 9C1). However, no change in the expression of p-CREB (Ser133) was observed in the somatosensory cortex and the hippocampus regions of brain among different experimental groups (Fig. 9A1 and B1). Further, the expression of total BDNF was also analysed, as its promoter consists of CREB response element. There was a significant downregulation (P < 0.05) of total BDNF protein expression in the hippocampus and the prefrontal cortex of ovx-V group, when compared with sham-N group. However, lithium treatment in ovx-LI group significant (P < 0.05) increased BDNF level compared to ovx-V group (Fig. 9B1 and C1). However, insignificant change in the level of BDNF was observed in the somatosensory cortex of all the experimental groups (Fig. 9A1).
Discussion
Alteration in the neuronal circuit following menopause is associated with a variety of cognitive and behaviour impairments. The present study explored the defensive effect of lithium treatment to overcome neurobehavioural abnormalities in ovx rats, a preclinical model that mimics clinical post-menopausal conditions. The results showed that chronic lithium treatment following ovx sustained the structural synaptic plasticity via maintaining the normal polarization state of glial cells, reducing neuroinflammation, and preventing BBB permeability.
Due to increased average life expectancy, one-third age of the majority of women is impacted with menopause. The two ways of seizing the menstrual cycle are either by surgical removal of ovaries in women to treat the reproductive organ-associated complications or through the natural biological process. Deficiency of ovarian hormone following menopause is reported to interfere with normal cognition and behavioural processes [34]. Depression and anxiety are the major psychiatric conditions observed in women following menopause that negatively impact the inflicted person and their quality of life. Deficiency in the sexual hormone creates instability in the monoaminergic neurotransmitter, exposing the female to psychiatric conditions [35]. FST and OFT were performed in the present study to study the ovx-associated depression-like and anxiety-like behaviour, respectively. In line with the previous findings, ovx increased immobility time in FST and decreased central square entries in OFT, indicating induction of behavioural alterations [3, 36] in the ovx-V group. Chronic activation or hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis is reported in depressed patients [37], which disrupts the synaptic plasticity via elevating the cortisol level [38]. Sexual hormones regulate the HPA axis via negative feedback, and during menopause, alterations in these hormones cause an increase in cortisol levels [39]. The hippocampus and the somatosensory cortical regions play a crucial role in the pathogenesis of depression. The dendritic atrophy with decreased spine count in the pyramidal neuron of the hippocampus and the prefrontal cortex is usually observed in depressive disorders [7, 40].
Spines are the major functional unit of neurons involved in the formation and maintenance of memory [40], and alteration in their number is associated with cognitive impairment. Thus, in the present study, the spatial memory functions of the animals were evaluated using MWMT to record consolidation and reconsolidation processes. Following the earlier findings, we did not observe any change in the consolidation process of spatial memory. Still, impairment in the reconsolidation process was recorded following ovx [3, 41] in ovx-V group. The pyramidal neurons in the CA3 region of the hippocampus participate in the consolidation of the spatial memory, whereas neurons of CA1 areas are involved in the reconsolidation process [42]. Likewise, in the present study, we did not find any degenerative changes in the histopathological analysis of the CA3 region of the hippocampus. The observation could be a reason for the normal consolidation process of memory in the ovx-V group. However, degenerative neurons, alteration in the dendritic arborization, and spine number were observed in the CA1 region, which might be responsible for the impaired reconsolidation process in the ovx-V group. The degeneration and alteration of pyramidal neurons were also observed in the neocortical areas linked with non-spatial memory (recognition memory) impairments [43, 44]. The degenerative neurons, alteration in the dendritic arborization, and spine number in all the three studied regions in the ovx-V group further supported behavioural impairments. The results of the present study and literature findings supported the crucial role of the sexual hormone in maintaining the neuronal circuit, which seems to be disrupted following menopause.
Previous studies have proposed the elevated risk of neurobehaviour impairment, breast cancer, endometrium cancer, cerebral stroke, and cardiovascular disease in women after exogenous oestrogen therapy [14, 15, 45]. Hence, as an alternative therapeutic intervention over hormone replacement therapy and managing menopause-associated neurological conditions, lithium carbonate was selected in the present study, as it directly or indirectly regulates signalling cascades that are under the regulation of oestrogen [46]. Lithium salt is not only used to treat bipolar disorder, but it also showed promising effects in major depressive disorder, including treatment-resistant depression [44]. A preclinical study in a mouse model of chronic unpredicted stress supported an antidepressant effect of lithium salt and suggested its ability to instigate the firing rate of neurons in the brain’s frontal cortex [44]. Lithium is often considered a multitargeted molecule that maintains neurotransmitters levels, elevates neurotrophic factors, controls apoptosis, and the second messenger system to overcome clinical depression [44, 47]. In line with the previous reports, we also observed the antidepressant activity of lithium in the ovx rats. However, no protection against anxiety-like behaviour in ovx rats was observed following treatment with lithium.
The pharmacological effect of lithium is not only limited to mood disorders, but it is also known to rescue the hippocampal-dependent memory formation via improving synaptic plasticity and adult neurogenesis [23]. Valdes et al. [46] showed that lithium treatment in ovx mice improved episodic memory through maintaining the normal mRNA expression of the genes related to synaptic plasticity such as BDNF, BCL-2, and NR1 [46]. Similarly, lithium treatment in our study increased dendritic length, maintained dendritic arborization, and increased spine number, resulting in improved cognitive processes in the ovx-LI group. These shreds of evidence suggest that lithium therapy helps to keep the normal structural synaptic plasticity to overcome neurobehaviour impairments following ovx.
Neuroinflammation plays a central role in the pathogenesis of various neurological conditions, which eventually disrupts the neuronal circuit and triggers neurodegeneration cascades in the later stages of life [48]. Ding et al. [49] found that there is a metabolic transition in the brain from glucose to fatty acid following ovx to sustain the normal pool of ATP [49]. This transition is a type of adaption, but it also generates free radicals, leading to neuroinflammation [50]. Glucose deprivation alters the metabolic function of astrocytes, increases cell volume, and initiates transcription of genes that triggers the transformation of normal astrocytes to reactive astrocytes [51]. Activated astrocytes lose their normal functions and release neurotoxic substances that induce neuronal and oligodendrocytes death [52] via triggering the pro-inflammatory cytokine IL-6, IL-1β, and TNF-α expression in the cortex and the hippocampus following ovx [53]. The elevated pro-inflammatory cytokines stimulate the HPA axis [54] and result in the elevation of glucocorticoid level, which finally triggers neurodegenerative events. GFAP, an astrocyte intermediate filament, is reported to be upregulated during reactive astrogliosis in patients affected with bipolar disorder and is reverted to a normal level following lithium treatment [55]. Alteration in the physiological functioning of astrocytes also affects the normal synaptic signalling in bipolar disorder patients that is normalized after lithium therapy [56].
Apart from GFAP, peroxisome proliferator-activated receptor gamma (Pparg) is a nuclear receptor subfamily that controls the transformation of reactive astrogliosis and neuroinflammation [57]. Lithium treatment was reported to be associated with increased expression of Pparg and reactive astrogliosis perturbations via suppressing the activity of lysyl oxidase [58]. Further lithium treatment also helped to overcome the neuroinflammation via downregulating the expression of the pro-inflammatory marker in the different models of neurodegenerative disease [59, 60]. Similarly, in the present study, lithium treatment upregulated the Pparg expression. At the same time, it downregulated the expression of Gfap, Il2, Il6, and Il1b, reduced total astrocytic process length, and the number of nodes in the ovx-LI group, thus supporting the anti-inflammatory activity of lithium.
Deprivation of sexual hormones and neuroinflammatory events following menopause also enhances BBB permeability by altering tight junction proteins’ expression [33]. These changes result in diapedeses of blood-derived inflammatory molecules towards the brain parenchyma that further participate in inflammation cascades [61]. Lithium treatment has been reported to improve the BBB permeability by maintaining normal expression of the junction proteins leading to antidepressant effect in a rat model [62]. Similarly, in the present study, we also observed upregulation in mRNA expression of Ocln and Tjp1, improving neurobehaviour impairment in ovx rats following lithium treatment. Results of the current study and literature support the ability of lithium to improve and maintain the integrity of BBB after menopause. Hence, lithium treatment may decrease the risk of neurodegenerative disease in women following menopause.
The role of Gsk-3β has been well-explored in the induction of neurobehavioural impairments in the ovx rat model [3]. Furthermore, Gsk-3β instigation in the brain of Dixdc1 KO mice induced depression-like behaviour and disrupted arborization of pyramidal neuronal in the cortex, which was improved after lithium administration [63]. Further, β-catenin downregulation in the ovx-V group confirmed the stimulation of Gsk-3β protein. Being a dual-nature protein, β-catenin appears to function as a cell adhesion molecule to maintain synaptic strength between neurons [64] and in gene transcription control associated with cell proliferation, maturation, and adult neurogenesis pathways [10]. A key component of the Wnt signalling pathway, β-catenin, is degraded after Gsk-3β activation [64], and decreased expression of the total β-catenin protein is reported in the brain of depressed patients [65]. However, lithium treatment maintained a normal protein level and relieved the depression-like behaviour [66], thus suggesting Gsk-3β inactivation.
Another important transcription regulator, a serine-threonine kinases mTOR, is primarily involved in the protein translation cascade, synaptic plasticity, axon sprouting, and myelination, also get inhibited following Gsk-3β activation [67, 68]. Chronic unpredicted stress-induced depression in mouse impaired mTOR signalling that was reverted to normal level following lithium therapy [69]. Alteration in mTOR activity interferes with the normal translation mechanism of synaptic proteins such as postsynaptic density (PSD95), synapsin 1, and GluR1, resulting in abnormal neuronal arborization that further leads to neurobehaviour impairments [70]. Surprisingly, in our study, Gsk-3β was found to be inactive in the hippocampus and the prefrontal cortex of the ovx-V group and remained at the same level following the lithium treatment. Activation of Gsk-3β is not only linked with neurodegeneration, but also contributes towards the long-term potentiation (LTP) and long-term depression (LTD) during memory processing [71]. An impaired reconsolidation process of spatial memory was observed following the inhibition of Gsk-3β in a mouse model, suggesting its role in memory reactivation [41]. In our study, lithium treatment rescued the reconsolidation process without activating Gsk-3β in the ovx-LI group animals.
Memory generation, maintenance, and storage are complex phenomena requiring synchronized involvement of various brain regions and complex interaction of signalling cascades [72, 73]. BDNF is a neurotrophic factor known for its involvement in learning and memory processes via maintaining the structural synaptic plasticity in the brain [73]. Depression-like behaviour following chronic-unpredicted stress in a mouse model showed downregulation in the BDNF signalling [44], upregulated following lithium treatment. Downregulation of BDNF signalling is also linked with structural synaptic plasticity distortion, which further impedes the hippocampus and the prefrontal cortex-dependent spatial and recognition memory reconsolidation process [74,75,76]. BDNF is a multifunctional protein that involves the memory reconsolidation process and regulates the mTOR-dependent translational cascade [77]. As previously reported, downregulation of BDNF expression in the hippocampus impairs the reconsolidation process, which was recovered after exogenous administration of the same protein [77]. Similarly, we also observed normalization of BDNF protein level and cognitive and behaviour process recovery following the lithium treatment.
Lithium is a drug of choice in psychiatric medicine, despite of some associated side effects that usually appear with improper monitoring. Chronic lithium treatment is linked with the development of nephrogenic diabetes insipidus [78]. Nausea, diarrhoea, tremor, and weight gain are common side effects associated with lithium therapy [79]. It has a narrow therapeutic index, and a slight rise in serum lithium level beyond therapeutic concentration (0.5 to 1.2 mM) leads to adverse effects [80]. Such consequences need to be taken into consideration while scheduling lithium therapy.
Conclusion
The present study revealed that lithium treatment prevented neurobehavioural impairments and improved structural synaptic plasticity following ovx. Neuroinflammatory events were also obscured following lithium treatment due to suppression of reactive gliosis and maintenance of the BBB strength. Lithium also regulated Gsk-3β activity, maintained a normal level of BDNF that ultimately helped to overcome neuroinflammation, and maintained the structural synaptic plasticity. Overall, the results supported the efficacy of lithium therapy to overcome the post-menopause-associated neurobehavioural complications after the critical therapeutic window period. However, in-depth research is still required to transfer the lithium from bench to bed in place of the hormone replacement therapy.
Data availability
The data generated during the course of study is included in the manuscript in the form of figures, images, and Supplementary Information (Supplement 1).
References
McCarthy M, Raval AP (2020) The peri-menopause in a woman’s life: a systemic inflammatory phase that enables later neurodegenerative disease. J Neuroinflammation 17:1–4. https://doi.org/10.1186/s12974-020-01998-9
Khan MM, Dhandapani KM, Zhang QG et al (2013) Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids 78:614–23. https://doi.org/10.1016/j.steroids.2012.12.005
Rana AK, Sharma S, Singh D (2020) Differential activation of Gsk-3β in the cortex and the hippocampus induces cognitive and behavioural impairments in middle-aged ovariectomized rat. Comprehensive Psychoneuroendocrinology 4:100019. https://doi.org/10.1016/j.cpnec.2020.100019
Frankfurt M, Luine V (2015) The evolving role of dendritic spines and memory: interaction (s) with estradiol. Horm Behav 74:28–36. https://doi.org/10.1016/j.yhbeh.2015.05.004
Wallace M, Luine V, Arellanos A et al (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126:176–82. https://doi.org/10.1016/j.brainres.2006.07.064
Kim J, Schalk JC, Koss WA et al (2019) Dorsal hippocampal actin polymerization is necessary for activation of G-Protein-Coupled Estrogen Receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J Neurosci 39:9598–9610. https://doi.org/10.1523/jneurosci.2687-18.2019
Ye Z, Cudmore RH, Linden DJ (2019) Estrogen-dependent functional spine dynamics in neocortical pyramidal neurons of the mouse. J Neurosci 39:4874–4888. https://doi.org/10.1523/jneurosci.2772-18.2019
Zhang WY, Guo YJ, Wang KY et al (2020) Neuroprotective effects of vitamin D and 17ß-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: Role of the AMPK/NF-κB pathway. Int Immunopharmacol 86:106734. https://doi.org/10.1016/j.intimp.2020.106734
Jeon SW, Kim YK (2017) Inflammation-induced depression: its pathophysiology and therapeutic implications. J Neuroimmunol 313:92–98. https://doi.org/10.1016/j.jneuroim.2017.10.016
Rana AK, Singh D (2018) Targeting glycogen synthase kinase-3 for oxidative stress and neuroinflammation: opportunities, challenges and future directions for cerebral stroke management. Neuropharmacology 139:124–136. https://doi.org/10.1016/j.neuropharm.2018.07.006
Flores-Cuadrado A, Saiz-Sanchez D, Mohedano-Moriano A et al (2021) Astrogliosis and sexually dimorphic neurodegeneration and microgliosis in the olfactory bulb in Parkinson’s disease. NPJ Parkinsons Dis 7:1–3. https://doi.org/10.1038/s41531-020-00154-7
Wilson AC, Clemente L, Liu T et al (2008) Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim Biophys Acta Mol Basis Dis 1782:401–407. https://doi.org/10.1016/j.bbadis.2008.02.011
Bell RD, Winkler EA, Sagare AP et al (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427. https://doi.org/10.1016/j.neuron.2010.09.043
Resnick SM, Espeland MA, Jaramillo SA et al (2009) Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 72:135–142. https://doi.org/10.1212/01.wnl.0000339037.76336.cf
Daniel JM, Bohacek J (2010) The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta Gen Subj 1800:1068–1076. https://doi.org/10.1016/j.bbagen.2010.01.007
Bake S, Sohrabji F (2004) 17β-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinol 145:5471–5475. https://doi.org/10.1210/en.2004-0984
Alda M (2015) Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry 20:661–670. https://doi.org/10.1038/mp.2015.4
Shimada K, Motoi Y, Ishiguro K et al (2012) Long-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotion. Neurobiol Dis 46:101–108. https://doi.org/10.1016/j.nbd.2011.12.050
Shim SS, Hammonds MD, Mervis RF (2013) Four weeks lithium treatment alters neuronal dendrites in the rat hippocampus. Int J Neuropsychopharmacol 16:1373–1382. https://doi.org/10.1017/s1461145712001423
Jope RS, Cheng Y, Lowell JA et al (2017) Stressed and inflamed, can GSK3 be blamed? Trends Biochem Sci 42:180–192. https://doi.org/10.1016/j.tibs.2016.10.009
Ma T, Hoeffer CA, Capetillo-Zarate E et al (2010) Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS ONE 5:e12845. https://doi.org/10.1371/journal.pone.0012845
Koebele SV, Bimonte-Nelson HA (2016) Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 87:5–17. https://doi.org/10.1016/j.maturitas.2016.01.015
Contestabile A, Greco B, Ghezzi D et al (2012) Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Investig 123:348–361. https://doi.org/10.1172/jci64650
Chen G, Rajkowska G, Du F et al (2000) Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75:1729–1734. https://doi.org/10.1046/j.1471-4159.2000.0751729.x
Bosetti F, Bell JM, Manickam P (2005) Microarray analysis of rat brain gene expression after chronic administration of sodium valproate. Brain Res Bull 65:331–338. https://doi.org/10.1016/j.brainresbull.2005.01.004
Szczepankiewicz D, Celichowski P, Kołodziejski PA et al (2021) Transcriptome changes in three brain regions during chronic lithium administration in the rat models of mania and depression. Int J Mol Sci 22:1148. https://doi.org/10.3390/ijms22031148
Nocjar C, Hammonds MD, Shim SS (2007) Chronic lithium treatment magnifies learning in rats. Neuroscience 150:774–788. https://doi.org/10.1016/j.neuroscience.2007.09.063
Sharma S, Sharma M, Rana AK et al (2021) Deciphering key regulators involved in epilepsy-induced cardiac damage through whole transcriptome and proteome analysis in a rat model. Epilepsia 62:504–516. https://doi.org/10.1111/epi.16794
Zaqout S, Kaindl AM (2016) Golgi-Cox staining step by step. Front Neuroanat 10:38. https://doi.org/10.3389/fnana.2016.00038
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87: 387–406. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1244622/
Tavares G, Martins M, Correia JS et al (2017) Employing an open-source tool to assess astrocyte tridimensional structure. Brain Struct Funct 222:1989–99. https://doi.org/10.1007/s00429-016-1316-8
Mazumder AG, Patial V, Singh D (2019) Mycophenolate mofetil contributes to downregulation of the hippocampal interleukin type 2 and 1β mediated PI3K/AKT/mTOR pathway hyperactivation and attenuates neurobehavioral comorbidities in a rat model of temporal lobe epilepsy. Brain Behav Immun 75:84–93. https://doi.org/10.1016/j.bbi.2018.09.020
Shin JA, Oh S, Ahn JH et al (2015) Estrogen receptor-mediated resveratrol actions on blood-brain barrier of ovariectomized mice. Neurobiol Aging 36:993–1006. https://doi.org/10.1016/j.neurobiolaging.2014.09.024
Yan YD, Chen YQ, Wang CY et al (2021) Chronic modafinil therapy ameliorates depressive-like behavior, spatial memory and hippocampal plasticity impairments, and sleep-wake changes in a surgical mouse model of menopause. Transl Psychiatry 11:1–4. https://doi.org/10.1038/s41398-021-01229-6
Newhouse PA, Dumas J, Hancur-Bucci C et al (2008) Estrogen administration negatively alters mood following monoaminergic depletion and psychosocial stress in postmenopausal women. Neuropsychopharmacology 33:1514–1527. https://doi.org/10.1038/sj.npp.1301530
Rajkumar AP, Qvist P, Donskov JG et al (2020) Reduced Brd1 expression leads to reversible depression-like behaviors and gene-expression changes in female mice. Transl Psychiatry 10:1–4. https://doi.org/10.1038/s41398-020-00914-2
Iob E, Kirschbaum C, Steptoe A (2020) Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry 25:1130–1140. https://doi.org/10.1038/s41380-019-0501-6
Sierra-Fonseca JA, Gosselink KL (2018) Tauopathy and neurodegeneration: a role for stress. Neurobiol Stress 9:105–112. https://doi.org/10.1016/j.ynstr.2018.08.009
Veldhuis JD, Sharma A, Roelfsema F (2013) Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrin Metab Clin 42:201–225. https://doi.org/10.1016/j.ecl.2013.02.002
Ko S, Jang WS, Jeong JH et al (2021) (−)-Gallocatechin gallate from green tea rescues cognitive impairment through restoring hippocampal silent synapses in post-menopausal depression. Sci Rep 11:1–20. https://doi.org/10.1038/s41598-020-79287-x
Kimura T, Yamashita S, Nakao S et al (2008) GSK-3β is required for memory reconsolidation in adult brain. PLoS ONE 3:e3540. https://doi.org/10.1371/journal.pone.0003540
Florian C, Roullet P (2004) Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav Brain Res 154:365–374. https://doi.org/10.1016/j.bbr.2004.03.003
Bekinschtein P, Weisstaub N (2014) Role of PFC during retrieval of recognition memory in rodents. J Physiol Paris 108:252–255. https://doi.org/10.1016/j.jphysparis.2014.03.001
Liu D, Tang QQ, Wang D et al (2020) Mesocortical BDNF signaling mediates antidepressive-like effects of lithium. Neuropsychopharmacology 45:1557–1566. https://doi.org/10.1038/s41386-020-0713-0
Liang J, Shang Y (2013) Estrogen and cancer. Annu Rev Physiol 75:225–240. https://doi.org/10.1146/annurev-physiol-030212-183708
Valdes JJ, Weeks OI (2009) Lithium: a potential estrogen signaling modulator. J Appl Biomed 7:175–188. https://doi.org/10.32725/jab.2009.020
Won E, Kim YK (2017) An oldie but goodie: lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int J Mol Sci 18:2679. https://doi.org/10.3390/ijms18122679
Xu Y, Sheng H, Bao Q, et, (2016) NLRP3 inflammasome activation mediates estrogen deficiency-induced depression-and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun 56:175–186. https://doi.org/10.1016/j.bbi.2016.02.022
Ding F, Yao J, Zhao L et al (2013) Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer’s. PLoS ONE 8:e59825. https://doi.org/10.1371/journal.pone.0059825
Yin F, Sancheti H, Patil I et al (2016) Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med 100:108–122. https://doi.org/10.1016/j.freeradbiomed.2016.04.200
Kogel V, Trinh S, Gasterich N et al (2021) Long-Term Glucose Starvation Induces Inflammatory Responses and Phenotype Switch in Primary Cortical Rat Astrocytes. J Mol Neurosci 1-15. https://doi.org/10.1007/s12031-021-01800-2
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Wyse AT, Siebert C, Bobermin LD et al (2020) Changes in Inflammatory Response, Redox Status and Na+, K+-ATPase Activity in Primary Astrocyte Cultures from Female Wistar Rats Subject to Ovariectomy. Neurotox Res 37:445–454. https://doi.org/10.1007/s12640-019-00128-5
Bellavance MA, Rivest S (2014) The HPA–immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol 5:136. https://doi.org/10.3389/fimmu.2014.00136
Ferensztajn-Rochowiak E, Tarnowski M, Samochowiec J et al (2016) Increased mRNA expression of peripheral glial cell markers in bipolar disorder: the effect of long-term lithium treatment. Eur Neuropsychopharmacol 26:1516–1521. https://doi.org/10.1016/j.euroneuro.2016.07.009
Giridharan VV, Sayana P, Pinjari OF et al (2020) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25:94–113. https://doi.org/10.1038/s41380-019-0448-7
Iglesias J, Morales L, Barreto GE (2017) Metabolic and inflammatory adaptation of reactive astrocytes: role of PPARs. Mol Neurobiol 54:2518–2538. https://doi.org/10.1007/s12035-016-9833-2
Rivera AD, Butt AM (2019) Astrocytes are direct cellular targets of lithium treatment: novel roles for lysyl oxidase and peroxisome-proliferator activated receptor-γ as astroglial targets of lithium. Transl Psychiatry 9:1–4. https://doi.org/10.1038/s41398-019-0542-2
Wang HM, Zhang T, Li Q et al (2013) Inhibition of glycogen synthase kinase-3β by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem Int 63:345–353. https://doi.org/10.1016/j.neuint.2013.07.003
Budni J, Feijo DP, Batista-Silva H et al (2017) Lithium and memantine improve spatial memory impairment and neuroinflammation induced by β-amyloid 1–42 oligomers in rats. Neurobiol Learn Mem 141:84–92. https://doi.org/10.1016/j.nlm.2017.03.017
Na W, Lee JY, Kim WS (2015) 17β-estradiol ameliorates tight junction disruption via repression of MMP transcription. Mol Endocrinol 29:1347–1361. https://doi.org/10.1210/me.2015-1124
Taler M, Aronovich R, Henry Hornfeld S et al (2021) Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord 23:55–65. https://doi.org/10.1111/bdi.12962
Martin PM, Stanley RE, Ross AP (2018) DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling. Mol Psychiatry 23:467–475. https://doi.org/10.1038/mp.2016.184
Teo CH, Soga T, Parhar IS (2018) Brain beta-catenin signalling during stress and depression. Neurosignals 26:31–42. https://doi.org/10.1159/000487764
Karege F, Perroud N, Burkhardt S et al (2012) Protein levels of β-catenin and activation state of glycogen synthase kinase-3β in major depression. A study with postmortem prefrontal cortex. J Affect Disord 136:185–188. https://doi.org/10.1016/j.jad.2011.09.024
Habib MZ, Ebeid MA, El Faramawy Y (2020) Effects of lithium on cytokine neuro-inflammatory mediators, Wnt/β-catenin signaling and microglial activation in the hippocampus of chronic mild stress-exposed rats. Toxicol Appl Pharmacol 399:115073. https://doi.org/10.1016/j.taap.2020.115073
Inoki K, Ouyang H, Zhu T et al (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968. https://doi.org/10.1016/j.cell.2006.06.055
Ignacio ZM, Reus GZ, Arent CO et al (2016) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 82:1280–1290. https://doi.org/10.1111/bcp.12845
Neis VB, Moretti M, Rosa PB et al (2020) The involvement of PI3K/Akt/mTOR/GSK3β signaling pathways in the antidepressant-like effect of AZD6765. Pharmacol Biochem Behav 198:173020. https://doi.org/10.1016/j.pbb.2020.173020
Li N, Lee B, Liu RJ et al (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. https://doi.org/10.1126/science.1190287
Phane Peineau S, Taghibiglou C, Bradley C et al (2007) LTP Inhibits LTD in the Hippocampus via Regulation of GSK3b. Neuron 53:703–717. https://doi.org/10.1016/j.neuron.2007.01.029
Alemany-Gonzalez M, Gener T, Nebot P et al (2020) Prefrontal–hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc Natl Acad Sci U S A 117:11788–11798. https://doi.org/10.1073/pnas.1921314117
Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1. https://doi.org/10.3389/neuro.02.001.2010
Heldt SA, Stanek L, Chhatwal JP et al (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12:656–670. https://doi.org/10.1038/sj.mp.4001957
Cefis M, Prigent-Tessier A, Quirie A et al (2019) The effect of exercise on memory and BDNF signaling is dependent on intensity. Brain Struct Funct 224:1975–1985. https://doi.org/10.1007/s00429-019-01889-7
Brombacher TM, Berkiks I, Pillay S et al (2020) IL-4R alpha deficiency influences hippocampal-BDNF signaling pathway to impair reference memory. Sci Rep 10:1–8. https://doi.org/10.1038/s41598-020-73574-3
Radiske A, Rossato JI, Gonzalez MC et al (2017) BDNF controls object recognition memory reconsolidation. Neurobiol Learn Mem 142:79–84. https://doi.org/10.1016/j.nlm.2017.02.018
Gong R, Wang P, Dworkin L (2016) What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol AM J PHYSIOL-RENAL 311:F1168–F1171. https://doi.org/10.1152/ajprenal.00145.2016
Gitlin M (2016) Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord 4:1. https://doi.org/10.1186/s40345-016-0068-y
Medi B, Stojanovic M, Stimec BV et al (2020) Lithium-pharmacological and toxicological aspects: The current state of the art. Curr Med Chem 27:337–351. https://doi.org/10.2174/0929867325666180904124733
Acknowledgements
The authors are highly grateful to the Director, CSIR‐Institute of Himalayan Bioresource Technology (CSIR‐IHBT), for providing the required facilities. Authors also thankful to the Shiv Kumar Saini for assisting in the surgical procedure and feed preparation. The institutional communication number of the manuscript is 4862.
Funding
The worked carried out in the present study was financially supported by CSIR, New Delhi, under project MLP-0204. SS is thankful to CSIR, New Delhi, for providing CSIR‐SRF (No. 31/054(0137)‐2K18 EMR 1), AKR is grateful to the DST, India, for providing DST‐INSPIRE fellowship, vide letter no: DST/INSPIRE fellowship/[IF160224].
Author information
Authors and Affiliations
Contributions
AKR performed the animal surgery, behaviour experiments, protein expression, histopathology, and wrote the manuscript. SS carried out the gene expression studies. VP analysed the histopathology data. DS conceptualized the idea, analysed the data, wrote, and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was duly approved by the Institutional Animal Ethics Committee (IAEC) of the CSIR-IHBT established by the CPCSEA, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India.
Consent for publication
The authors have provided the consent for publication in the journal Molecular Neurobiology.
Competing interests
None declared.
Consent to participate
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rana, A.K., Sharma, S., Patial, V. et al. Lithium therapy subdues neuroinflammation to maintain pyramidal cells arborization and rescues neurobehavioural impairments in ovariectomized rats. Mol Neurobiol 59, 1706–1723 (2022). https://doi.org/10.1007/s12035-021-02719-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02719-w