Abstract
Recent decade has seen a surge in identifying modifiable risk and protective factors for cognitive decline associated with natural aging and with common dementing disorders such as Alzheimer’s disease (AD). Ovarian steroid hormone estrogen is extensively studied among these factors with profound effects on many tissues and organs, including the brain. Brain aging in female is also accompanied with decline in estrogen levels. In women, it is characterized by natural depletion of hormone levels and menopause, whereas in rodents, it results in estropause. A decent amount of evidence associates the estrogen (E2, 17βestradiol) with hippocampal activity, an area of brain related to cognition and memory. Presence of estrogen receptors (ER) in the hippocampus gives further evidence to it being one of the target brain regions for the hormone activity. Our findings have revealed that ovariectomy or natural aging leads to decreased synaptic activity, degenerative cytoarchitectural changes and altered protein levels in hippocampal neurons. Further, it was seen that long-term estrogen therapy maintains the synaptic plasticity, regulates apoptotic proteins and affords neuroprotection to the hippocampal neurons through both the nuclear and membrane estrogen receptor mediated pCREB and MAPK activation. Interestingly, normal aging also exhibits immune activation and cell infiltration in the brain. Neuroprotective effects of estrogen may include its anti-inflammatory response via regulating the neuro-immune response and levels of pro-inflammatory cytokines. However, the exact mechanism of anti-inflammatory actions of estrogen in senescent female brain is not yet fully characterized and needs to be undertaken to fully embark on neuro-immune processes in female brain aging. Attention is now largely focused on collective and beneficial approach of estrogen replacement therapy that shall not only support the cognitive function but also prevent neurodegenerative pathologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
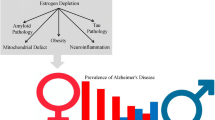
Prevention and treatment of age-related brain disorders require deeper understanding of the basic neurological processes behind the normal and pathological brain aging. Growing evidence suggests that gonadal hormones exert different effects in male and female subjects, perhaps because of underlying dimorphism in some brain processes. These processes are closely related to brain regions involved in memory, cognition and affective state, such as the hippocampus, amygdala, cerebral cortex (Baron-Cohen et al. 2005; McCarthy and Konkle 2005; Cahill 2006; Cosgrove et al. 2007; Wilson and Davies 2007), and regions controlling sensorimotor and reward systems (Dewing et al. 2006; Cantuti-Castelvetri et al. 2007; McArthur et al. 2007a). The loss of gonadal steroid hormones during normal aging increases the risk of disease and dysfunction in hormone-responsive tissues (Baumgartner et al. 1999; Morley 2001). Age -related hormone depletion likely results in diminished neuroprotective actions of hormones in brain and an increased risk to neurodegenerative diseases such as Alzheimer’s disease (AD) (Pike et al. 2006; Rosario and Pike 2008). In case of humans, the decline of estrogen production in female is fast and generally accompanied by slow progression of cognitive disturbances, and in some cases by dementia (Henderson 2008). In AD, postmenopausal women have a greater toll than men (Swaab 2004). Estrogen has been identified as a vital protective factor in women in providing them the benefit against the diseases which are prevalent in men. However, a rapid decline in estrogen after menopause leads to the loss of this advantage. The knowledge of the significance of physiological and pharmacological actions of estrogen in brain is indispensable for realizing the full translational prospective role of this ubiquitous female steroid hormone in maintaining the health and wellbeing. This chapter is focused to deliver current knowledge of neuroprotective effects of estrogen in the context of cognitive decline and neurodegeneration in postmenopausal women. To study the impact of estrogen loss on brain plasticity and cognitive functions , the ovariectomized rat is a suitable experimental model for clinical representation of menopause in animals. We will discuss pleiotropic nature of estrogen, mechanism of action in brain and its role in ameliorating cognitive decline and neurodegeneration. This information is essential to understand the nature of estrogen and its translational value for future use to combat neurological disorders in aging female brain.
14.2 Estrogen, Menopause and Brain Aging
Women now live a larger part of their lives in estrogen deprived state (menopause) which predisposes them to cognitive decline, memory disturbances and neurodegeneration . AD is one of the most debilitating diseases of the central nervous system following progressive brain aging and results in the formation of beta-amyloid plaques, neurofibrillary tangles and neuronal loss. Estradiol is biologically most prevalent and active compound of a class of steroids called estrogens (Behl et al. 2000) and exerts potent and wide-ranging effects on the development, growth, differentiation and function of various tissues throughout the body. It is mainly synthesized in the ovaries and to a lesser extent in testes and placenta. Estrogen is known to occur in mammals in several forms, i.e. estrone (E1) , 17b estradiol (E2) , estriol (E3) , and 17a estradiol (17a) . Recent results of experimental and clinical studies have indicated that estrogen acts not only on the hypothalamus-hypophysis axis, but also on other brain regions to name a few like amygdala, hippocampal formation, cerebellum etc., thus influencing brain functions not related to reproductive activity. In these brain areas, estrogen modulates memory, cognition , postural stability, fine motor activity and mood. Age related decline of estrogen in postmenopausal women poses a great risk of cognitive deficits and progressive AD in comparison to the same age group men. Extensive work in the field of steroid hormones and their actions in brain have led us to the remarkable but partial understanding of estrogen’s neuroprotective role in certain brain areas involved in learning , memory and cognition.
Estrogen has been investigated for its neuroprotective effects in a variety of systems and experimental models of neurodegenerative disorders and cerebral ischemia (Dubal et al. 1998; Green and Simpkins 2000). The association between memory and estrogen was first clinically reported when menopausal women frequently expressed complaints of memory disturbance and concentration problems (Sherwin 2000). Indeed alterations in cognitive performance have long been linked with menopause (Neurgaren and Kraines 1965). These clinical observations led to the beginning of scientific human study in the 1980s and thereafter Sherwin and colleagues accomplished a systematic analysis of the influence of estrogen loss and replenishment on memory functions. Results of these investigations established deterioration of verbal memory with estrogen loss and estrogen replacement therapy immediately after menopause restored the memory alterations to premenopausal state (Sherwin 1988) . These findings prompted Fillit and colleagues (1986) to investigate the effect of estrogen replacement therapy on cognitive tasks in women with AD. The results of this small clinical trial proved to be pivotal to establish the role of estrogen in memory mechanisms in female brain. Several in vivo and in vitro studies suggest that estrogen exerts a protective effect in various brain disorders by influencing the inflammatory response . This anti-inflammatory hypothesis also stems from the evidence that menopause, which is characterized by the drastic drop in estrogen levels, results in an increased incidence of inflammatory pathologies of brain and other tissues. Treatment with physiological doses of estrogen before the onset of disease downregulates the expression of inflammatory factors, including cytokines, chemokines and their receptors (Matsuda et al. 2001; Matejuk et al. 2001), apolipoprotein E and other modulators of leukocyte migration (Horsburgh et al. 2002, Vegeto et al. 2003).
Results of basic science, clinical science, and epidemiological analyses need more data to demonstrate and understand the neuroprotective potential of estrogen and certain other compounds with estrogen-like activity against age-related risk factors of developing AD and other neurodegenerative disorders .
14.3 Mechanism of Action of Estrogen
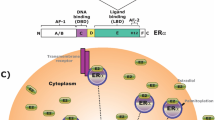
Estrogen produces its actions through binding to specific receptors known as estrogen receptors (ERs). ERs are cis-acting transcription factors which are members of steroid receptors super family (Giguère et al. 1988). ERs contain three main structural and functional domains, a highly conserved DNA-binding domain, C-terminal ligand-binding domain and hyper variable N-terminal transactivation domain (Mangelsdorf et al. 1995). After binding to its ligand, two molecules of ER dimerize and bind to the specific DNA sequence, estrogen response element (ERE) inside the nucleus and lead to the activation of target gene transcription factors (Tsai and O’Malley 1994). The mechanism of action of estrogen has been reviewed considerably following the discovery of the novel member of the steroid receptor super family i.e. ERb (Kuiper et al. 1996) with high degree of sequence homology (95 %) with the classical ERα (Giguère et al.1988). Each receptor shows high affinity binding with estrogen and may bind to each other as homodimers or heterodimers. This receptor-ligand dimer then binds with ERE or associate with AP1 transcription factors c-jun/c-fos to regulate the transcription of responsive genes (Kuiper et al. 1996, 1997; Paech et al. 1997). Several studies have also established that estrogen rapidly (within seconds to a few minutes) influences cellular physiology in different cell types of estrogen responsive tissues via activating a multitude of intracellular signaling mechanisms (Belcher and Zsarnovsky 2001). In addition to regulation of gonadal functions, ERa and ERb are also present in both female and male brains, specifically in brain areas involved in memory and cognition (McEwen 2001). Among the brain regions, major interest is focused on hippocampus and neocortex (Mehra et al. 2005). In rat and mouse, different regions and cell-populations in the brain, including hypothalamus, hippocampus , cerebellum and pituitary appear to express ERa and ERb (Mitchner et al. 1998; Osterlund et al. 1998). While the protein sequences of ERa and ERb are highly homologous, they represent two distinct gene products. In humans, the gene for ERa is located on chromosome 6 while that for ERb is on chromosome 14 (Couse and Korach 1999).
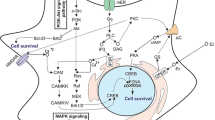
Since estrogen is a are potent neuroprotective hormone , to reveal its exact molecular and cellular mechanisms of action would offer putative drug target points. Gonadal steroids act on both genomic and nongenomic pathways related to nuclear as well as membrane and cytoplasmic receptors, respectively. Extensive research activity is going on worldwide, including our recent research work, to define both genomic and intracellular signaling pathways in order to determine the molecular actions of estrogens. For example: (1) Activation of Src/ERK/CREB/Bcl-2 signaling pathways by estrogen triggers neuroprotective mechanism (Sharma and Mehra 2008), (2) Estradiol (E2) -induced formation of dendritic spines in hippocampus acts via Akt (protein kinase B ) -mediated signaling events (Zhou et al. 1996), and (3) modulation of cyclic AMP response element binding protein (CREB ) pathway (Szegõ et al. 2006; Wappler et al. 2009) provides an insight into the existence of the ‘nongenomic’ action of the ovarian hormone. According to Falkenstein et al. (2000), estrogen rapidly activates adenylate cyclase, ERK 1 and 2, and MAPK pathways . Activation of adenylate cyclase is known to result in increased cAMP concentration and downstream activation of protein kinase A (PKA ) through a well described cAMP-PKA signal transduction pathway (Gu and Moss 1996). Estradiol increases cAMP accumulation in hypothalamic neurons (Weissman et al. 1975) and human neuroblastoma cells (Watters and Dorsa 1998) and exerts cellular effects in neurons consistently with increased cAMP levels (Gu and Moss 1996; Minami et al. 1996) and increased phosphorylation of CREB protein (Gu and Moss 1996; Sharma et al. 2007). Further in this regard, a rapid estrogen induction of phosphorylated CREB was found in the periventricular nucleus (Gu and Moss 1996), preoptic area and the bed nucleus of striaterminalis in rat brain (Zhou et al. 1996). Extensive studies in this research area are being conducted worldwide revealing estrogenic actions in rat hippocampus which occur via nuclear and membrane receptors associated with the synaptic plasticity and neuroprotection . In this course, we have shown that increased phosphorylation of CREB results in i) increased synaptophysin activity, ii) higher Bcl2:Bax ratio (attenuates apoptosi)s (Sharma et al. 2007; Sharma and Mehra 2008) and iii) regulates mitochondrial cellular energy balance which plays a prominent role (Toulmond et al. 1996) in estrogen mediated neuroprotection. Estrogen induced increase in dendritic spine density in the hippocampus has been reported to be dependent on CREB phosphorylation (Segal and Murphy 1998, Sharma et al. 2007). Our planned studies will continue to search for the involvement of E2-induced additional remarkable signaling molecules with a significance to find potent therapeutic targets .
14.4 Estrogen and Neuroprotection
Estrogen mediated neuroprotection has been described in several neuronal culture models of neuronal toxicity including that of the serum deprivation (Gollapudi and Oblinger 1999), b amyloid-induced toxicity (Behl et al. 1997; Gridley et al. 1997) and excitotoxicity systems (Weaver et al. 1997; Singer 1999). It has also been demonstrated in experimental models of oxidative stress (Moosmann and Behl 1999). Estrogen has a large number of cellular effects including the modulation of apoptotic factors, which has been shown to enhance neuronal survival (Green and Simpkins 2000). The Bcl-2 family of proteins are important modulators of neuronal apoptosis and include both inhibitors (Bcl-2 and Bcl-XL) as well as promoters (Bax and Bad). A marked increase in the Bcl-2 immunoreactivity has been observed in NT2 neuronal cell cultures (Singer et al. 1998) and in the neurons of the arcuate nucleus of rat following 17a estradiol administration (Gollapudi and Oblinger 1999). Similarly, 17b estradiol increased i) Bcl-XL mRNA levels in PC 12 cells transfected with ERa (Gollapudi and Oblinger 1999) and ii) Bcl-XL immunoreactivity in primary rat hippocampal neurons (Pike 1999). 17b estradiol exposure also caused a marked decrease in the levels of pro-apoptotic protein BAD in ERa transfected PC 12 cells (Gollapudi and Oblinger 1999). The expression of a member of Bcl-2 binding proteins family (BNIP) was significantly reduced by treatment with 17b estradiol in neural cell culture (Balcredito et al. 2001). It may be postulated that the activation of cAMP signaling and/or increased CREB phosphorylation could contribute to the neuroprotective effects of estradiol in these above mentioned studies as activation of cAMP pathway is associated with decreased susceptibility of neuronal cells to apoptotic signals (Kobayashi and Shinozawa 1997). Further, activation of cAMP-PKA-CREB pathway has been postulated to be associated with enhanced neuronal survival following an increase in the expression of anti-apoptotic protein Bcl-2. Recent studies of Wappler et al. (2010) have reported that a single high dose of estradiol (4 mg/kg b.w.) pre-treatment results in significant neuroprotection against hippocampal cell loss in ischemic gerbils of various age groups . All relevant behavioral tests of hippocampal memory functions offered evidence of improved memory in these animals. Wappler and colleagues (2011) have also reported that a single pre-treatment dose of estradiol exerts varying effects on hippocampal synaptic plasticity in different age groups at both shorter (4 postoperative days) and longer (25 postoperative days) time points. It increased hippocampal synaptic density in both age groups, however, this increase was more pronounced in younger animals. Thus it is clear from these studies that with aging there is a lower tendency of synaptogenesis .
In our laboratory, to understand the neuroprotective potential of estrogen and other estrogen-like compounds, we have systematically examined the protein chemistry of various brain areas by using immunohistochemical and proteomic approaches. The functional outcome has been assessed with behavioral tests of experimental learning and memory paradigms in ovx and aging female rats. Our experimental results have so far revealed that estrogen decline following ovariectomy in adult female rats leads to degenerative changes in hippocampal neurons. Following ovariectomy or aging, there appeared cytoplasmic vacuolation and Nissl substance was unevenly distributed, rarefied and juxtaposed against the cell membrane in the pyramidal neurons of the CA3-CA1 (Fig. 14.1b). Nuclear changes observed with DAPI staining also confirmed the juxtamembranous condensation of chromatin material in hippocampal neurons of OVX and aged animals (Fig. 14.2b, c). Estradiol therapy (0.1 mg/kg body wt. for 30 days) given to ovx rats seemed to reverse the effects of ovariectomy, such that the uniformity of Nissl staining patterns, position of nucleoli and cytoplasmic consistency were seen to be very much like that in the ovary-intact hippocampus (Fig. 14.1e). Nuclear morphology of hippocampal neurons of E2 treated ovx rats was more or less comparable to that of the ovary intact rat hippocampal neurons (Fig. 14.2d). These results were further substantiated by results on the expression of anti-apoptotic Bcl2 and pro-apoptotic Bax proteins in the hippocampii of various experimental groups. Hormonal depletion (ovx or aging) resulted in downregulation of Bcl-2 and increased Bax expression in CA1-CA3 and dentate gyrus, while E2 treatment to ovx rats resulted in the reversal (near to ovary intact levels) of these markers of cell death and survival (Fig. 14.3a). Hormone treatment led to an increased Bcl-2:Bax ratio indicative of a shift towards neuronal survival . Neuronal cell death in various subfields of hippocampus was further confirmed by TUNEL assay where percentage of TUNEL positive neurons was increased following ovariectomy or aging, while E2 therapy decreased the percent TUNEL positive neurons (Fig. 14.3b).
Cresyl violet stained coronal sections of a Ovary intact adult, b OVX adult, c OVX aged (24 months), d OVX + E2 aged (24 months), e OVX+E2 adult rat hippocampus CA1 neuronalcytoarchitecture. Note the cytoplasmic vacuolation and reduced Nissl staining (b, c, d) following ovariectomy and aging and reversal of ovariectomy-induced changes (e) following E2 therapy. (SO stratum oriens; SR stratum radiatum; SP stratum pyramidale; Scale bars 25 µm)
High power photomicrographs of DAPI stained coronal sections of a Ovary intact adult; b OVX adult; c aged (24 months), and d OVX + E2 adult rat hippocampus CA1 showing the nuclear morphology of CA1 pyramidal neurons. Certain cardinal features of apoptosis like juxtamembranous condensation and clumping of chromatin material (arrows), membrane ‘blebbing’ etc., were displayed by the nuclei of some of the pyramidal neurons of experimental groups. (SO stratum oriens; SR stratum radiatum; SP stratum pyramidale; Scale bars 25 µm (b) and 50 µm (a, c, d))
Bar diagrams depicting a the number of Bcl-2 and Bax positive neurons/mm2 and b percentage of apoptotic cells (TUNEL positive) in CA1-3 of hippocampus from various experimental groups. Ovariectomy or aging decreased the number of Bcl-2 positive neurons which led to a shift towards apoptotic side. 17b-estradiol (E2) treatment increased the Bcl-2 positive neurons which led to the reversal of Bcl-2:Bax ratio towards neuronal survival. Percentage of TUNEL positive cells was also increased following ovariectomy or aging. A decrease in percent apoptotic cells can be seen following E2 therapy
Also, the synaptic activity in estrogen deficit (ovx or naturally aged) rat hippocampus was significantly decreased as observed with the expression of presynaptic nerve terminal marker synaptophyysin (syp). E2 replenishment resulted in the upregulation of synaptophysin activity in ovx rat hippocampus. We have also investigated the p-CREB expression in the hippocampus of these experimental animals and observed a decrease in CREB phosphorylation after ovariectomy or aging, while increased CREB phosphorylation was observed in E2 supplemented ovx rats (Sharma et al. 2007). These observations are consistent with other findings where p-CREB is reported to have increased dendritic spine density (Murphy and Segal 1997) and enhanced neuronal survival in hippocampus (Jin et al. 2001). Our studies have further been substantiated by behavioral assessment of hippocampal learning and memory through Morris-water maze task performed by these experimental animals. Ovx rats had a poor performance on recall task monitored as latency to find hidden platform . Aged rats also performed in the same way and did not show any significant difference in platform latency time vis-à-vis ovx animals. However, E2 treated ovx rats had a better performance to find the platform and latency scores were significantly lower and almost comparable to those of ovary intact controls (Fig. 14.4).
Recently, these molecular expression studies have also been accomplished by our group in ovx rats followed by estrogen supplementation of one month and kept until aging . The results of this group were compared with a group of naturally aging controls and ovariectomized aged controls. Animals of all the three groups were sacrificed when they attained the age of 24 months. Though degenerative changes in neuronal cytoarchitecture were visible in hippocampaii of all the three aged groups (Fig. 14.1c, d and 14.2c), but the changes were certainly more pronounced in the ovx aged group (Fig. 14.1c). The synaptophysin, p-CREB and Bcl-2 expression was greatly downregulated in ovx aged rats than in their naturally aging counterparts or the ones with one month E2 therapy in adulthood (Fig. 14.3a). However, this treatment schedule did not seem to be of great help in fully restoring the synaptic activity and neuronal health. Results of the ovx aged control group explain the view that abrupt cessation of estrogen production may have an adverse outcome on age-related neurodegeneration and AD type dementia and that short-term estrogen replenishment after ovariectomy may not support the hormone`s neuroprotective effects for a longer period in due course of aging. These findings raise a need to formulate a long-term estrogen therapy to obtain consistent neuroprotective effects of the hormone throughout aging of the female brain . These observations from the experimental animal model of postmenopausal aging are summarized in Table 14.1 .
Wappler et al. (2010) have also investigated neuroprotective efficacy of a single high dose of estradiol (4 mg/kg b.w.) pre-treatment in young (4 months), middle-aged (9 months) and old (18 months) female gerbils following 10 min global brain ischemia . In this study, both apoptotic and necrotic cells were quantified and a battery of behavioral tests were done for functional assessment of efficacy of a single dose of estradiol in ischemic gerbil hippocampus. Estradiol pre-treatment ensued in significant neuroprotection against hippocampal cell loss in all experimental age groups. Estrogen improved memory performances in all the behavioral tests at each of the experimental ages. However, age-related differences were observed in behavioral tests. It is thus obvious that age of the experimental animals may affect the outcome of E2 therapy following brain ischemia .
14.5 Neurodegeneration, Immune Response and Estrogen
Acute and chronic inflammation in brain areas is supposed to play an important role in a number of brain functions including learning and memory and also in age related neurodegenerative processes underlying the progress of brain disorders. Brain aging and neurodegeneration are closely related to immune processes originating either in the central nervous system or invading the brain from the periphery. Microglia and astrocytes in the brain also produce different interleukins and cytokines, which respond to injury and degenerative pathologies. Many of the inflammatory cytokines generally associated with the peripheral immune system are found and produced within the central nervous system (Pan et al. 1998;Toulmond et al. 1996). Neurons can also endogenously produce cytokines in the brain apart from their primary originating source of immune system (Gahring et al 1996). The production of cytokines in CNS can be stimulated by peripheral cytokines. In the CNS, cytokines instigate their effects through both the cross-talk with their receptors (expressed by both glial and neuronal cells) and modulation of neurotransmitter receptor function. For instance, IL-1 modulates GABA receptors and enhances the inhibitory response. Synaptic plasticity is modulated by both IL-1 and IL-6 through inhibiting long-term potentiation. Moreover, TNF-a has been reported to modulate the neuronal response of NMDA activated glutamate receptors (GluR). Excessive NMDA receptor activation results in neuronal death through excitotoxicity . Neuronal cell death in ischemia, trauma, and neurodegenerative diseases is largely a result of excitotoxicity. Antioxidants, growth factors and certain cytokines protect against excitotoxicity, either through directly modulating receptor function or indirectly through inhibiting key metabolic steps subsequent to GluR activation. Further, TNF-a receptor deficient mice have greater vulnerability to ischemic brain damage following arterial occlusion, indicating the neuroprotective role of TNF-a in brain (Carlson et al 1999). Post-menopausal decline of estrogen has been postulated to lead to elevation of proinflammatory cytokines and estrogen replacement therapy restores the cytokine to normal levels in blood serum (Porter 2000; Fahlman 2000). Although mechanisms responsible for this beneficial effect of estrogen have not been completely elucidated, certain studies suggest that estrogen replacement therapy (ERT) lowers the production of proinflammatory cytokines such as IL-6 in postmenopausal women (Saucedo 2002). In rodents also, estropausal aging has been associated with increased secretion of pro-inflammatory cytokines such as interleukin-6 (Ye and Johnson 2001; Godbout and Johnson 2004) and decreased secretion of anti-inflammatory cytokines, such as interleukin-10 (Ye and Johnson 2001). The mechanism of neuroprotective effects augmented by estrogen may include anti-inflammatory responses and may change i) the levels of various cytokines (interferong, tumor necrosis factora , different types of interleukins) and ii) the function of microglia as the only immune competent cells housed in the central nervous system. Varying levels of cytokines in brain areas between ovx state, aging and following hormone therapy will serve as an approach to systematically address inflammatory pathophysiological changes in age related neurodegenerative disorders . Comparison of various signaling molecules, receptor proteins and cytokines in specified brain areas in the course of aging and followed by estrogen and other drug therapies may provide evidence and explanations of changes in behavioral, cognitive and motor functions. This will serve as an approach to systematically address pathophysiological changes at the protein level in age related neurodegenerative disorders .
14.6 Selective Estrogen Receptor Modulators (SERMs)
It is now known that most of the cellular effects of E2 are mediated through its binding to ERs . Furthermore, the action of E2 is inhibited by certain synthetic chemical analogs as they avidly bind to the ERs and thereby preventing their activation. This class of chemical compounds (anti-estrogens) has been successfully used in the treatment and prevention of breast cancer in women (Halbreich and Kahn 2000). Among the list of anti-estrogens, tamoxifen (TAM) is the most widely and commonly used therapeutic agent. However, its anti-estrogenic property is not seen in uterus and bone; instead, it behaves as an agonist in these tissues. This bimodal characteristic of TAM can be explained by the fact that it selectively modulates ER in the body. Such a drug that mimics the activity of the natural hormone, 17β-estradiol in some tissues (e.g. skeletal tissue) while opposing the action of the hormone in other tissues (e.g. breast tissue) is known as Selective Estrogen Receptor Modulator (SERM ). TAM has been widely employed in the treatment of breast cancer, but its bio-character in brain remains largely unknown, particularly in context of its neuroprotective role. Before one takes a look at the biological effects of SERMs , it is imperative to know why an estrogen-agonist behaves as an agonist and why an estrogen-antagonist behaves as an antagonist following its binding to ligand-binding domains of ER. The most important information regarding this phenomenon has been made available from the 3-Dimensional (3D) crystallographic studies (Littleton-Kearney et al. 2002). However, factors that make the ER to adopt certain tissue specific structural differences, thereby exhibiting mixed agonist/antagonist responses following the coupling of TAM to ER still remains elusive. Besides being used as a therapeutic agent for breast cancer, the biological character of TAM in the central nervous system is not yet fully understood. Recently, the role of TAM as a neuroprotectant in experimental model systems of neurodegeneration and ischemic insults has received a lot of attention. It has been shown that TAM administration prevents neuronal loss in the hilar region of the hippocampus following cytotoxic insults (kainic acid induction) to OVX rats (Ciriza et al. 2004). Moreover, partial reversal of hippocampal damage (revealed by bilateral cell count) was observed in four vessels occluded (global ischemia) male Wistar rats following TAM administration (Bagetta et al. 2004 ). The same bio-character of TAM has been observed in our experiments on ovx rats where synaptic plasticity and neuroprotection was restored (Sharma et al. 2007, Sharma and Mehra 2008). Furthermore, TAM has still not been investigated in brain aging especially at different stages of menopause (viz. perimenopause, middle age and aged). It can also provide greater understanding on the role of chronic E2 or TAM therapy influencing hippocampal function.
14.7 Where to Go
Extensive review of the estrogenic actions in female brain areas involved with learning, memory and cognition has so far provided understanding of its mechanism of action in female brain. Age associated effects of this hormone are still not clear as to how it could behave postmenopausally where no therapy is initiated at perimenopause phase. These assumptions may put some light on the varying effects of diverse doses of estrogen at different stages during aging. Future studies should be planned to explore the effects and mechanism of action of different doses of various estrogenic ligands, because of their varying potency, in middle aged (perimenopausal) and aged (postmenopause) female brain. This approach will further help to identify designer estrogenic ligands of tissue specific nature and strength and their targeted delivery where outweighing risk factors will be insignificant for institution of a better estrogen therapy for age related neurological disorders in post-menopausal women .
References
Baron-Cohen S, Knickmeyer RC, Belmonte MK (2005) Sex differences in the brain: implications for explaining autism. Science 310:819–823
Bagetta G, Chiappetta O, Amantea D, Iannone M, Rotiroti D, Costa A, Nappi G, Corasaniti MT (2004) Estradiol reduces cytochrome c translocation and minimizes hippocampal damage caused bytransient global ischemia in rat. Neurosci Lett 368:87–91
Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ (1999) Predictors of skeletal muscle mass in elderly men and women. Mech Aging Dev 107(2):123–136
Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F (1997) Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol 51(4):535–41
Behl C, Moosmann B, Manthey D, Heck S (2000) The female sex hormone oestrogen as neuroprotectant: activities at various levels. Novartis Found Symp 230:221–234
Belcher SM, Zsarnovszky A (2001) Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther 299(2):408–414
Belcredito S, Vegeto E, Brusadelli A, Ghisletti S, Mussi P, Ciana P, Maggi A (2001) Estrogen neuroprotection: the involvement of the Bcl-2 binding protein BNIP2. Brain Res Brain Res Rev 37(1–3):335–342
Cahill L (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484
Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC (1999) Inflammatory cytokines IL-1a, IL-1b, IL-6, and TNF- a impart neuroprotection to an excitotoxin through distinct pathways1. J Immunol 163(7):3963–3968
Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM (2004) Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol 61:209–221
Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, and Standaert DG (2007) Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis 26:606–614
Cosgrove KP, Mazure CM, and Staley JK (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855
Couse JF, Korach KS (1999) Estrogen receptor null mice: what have we learned and where will they lead us. Endocr Rev 20(3):358–417
Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, et al. (2006) Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol 16:415–420
Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM (1998) Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18(11):1253–1258
Fahlman MM, Boardley D, Flynn MG, Bouillon LE, Lambert CP, Braun WA (2000) Effects of hormone replacement therapy on selected indices of immune function in postmenopausal women. Gynecol Obstet Invest 50(3):189–193
Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M (2000) Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev 52(4):513–556
Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J (1986) Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology 11:337–345
Gahring LC, Carlson NG, Kulmar RA, Rogers SW (1996) Neuronal expression of tumor necrosis factor a in the murine brain. Neuroimmunomodulation 3:289
Giguère V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331(6151):91–94
Godbout JP, Johnson RW (2004) Interleukin-6 in the aging brain. J Neuroimmunol 147(1–2):141–144
Gollapudi L, Oblinger MM (1999) Estrogen and NGF synergistically protect terminally differentiated, ERalpha-transfected PC12 cells from apoptosis. J Neurosci Res 1;56(5):471–481
Green PS, Simpkins JW (2000) Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci 18:347–358
Gridley KE, Green PS, Simpkins JW (1997) Low concentrations of estradiol reduce beta-amyloid (25–35)-induced toxicity, lipid peroxidation and glucose utilization in human SK-N-SH neuroblastoma cells. Brain Res 778(1):158–165
Gu Q, Moss RL (1996) 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16(11):3620–3629
Halbreich U, Kahn LS (2000) Selective oestrogen receptor modulators--current and future brain and behaviour applications. Expert Opin Pharmacother 1:1385–1398
Henderson VW (2008) Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol 51(3):618–626
Horsburgh K, Macrae IM, Carswell H (2002) Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab 22(10):1189–1195
Jin K, Mao XO, Simon RP, Greenberg DA (2001) Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci 16(1):49–56
Kobayashi Y, Shinozawa T (1997) Effect of dibutyrylcAMP and several reagents on apoptosis in PC12 cells induced by a sialoglycopeptide from bovine brain. Brain Res 778(2):309–317
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 93(12):5925–5930
Littleton-Kearney MT, Ostrowski NL, Cox DA, Rossberg MI, Hurn, PD (2002) Selective estrogen receptor modulators: tissue actions and potential for CNS protection. CNS Drug Rev 8:309–330
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83(6):835–839
Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H (2001) 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res 65(6):529–542
Matsuda J, Vanier MT, Saito Y, Suzuki K, Suzuki K (2001)Dramatic phenotypic improvement during pregnancy in a genetic leukodystrophy: estrogen appears to be a critical factor. Hum Mol Genet 10(23):2709–2715
McArthur S, McHale E, and Gillies GE (2007a) The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology 32:1462–1476
McCarthy MM and Konkle AT (2005) When is a sex difference not a sex difference? Front Neuroendocrinol 26:85–102
McEwen BS (2001) Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol 91(6):2785–2801
Mehra RD, Sharma K, Nyakas C, Vij U (2005) Estrogen receptor alpha and beta immunoreactive neuron in normal adult and aged female rat hippocampus: a qualitative and qunatitative study. Brain Research 1056:22–35
Mitchner NA, Garlick C, Ben-Jonathan N (1998) Cellular distribution and gene regulation of estrogen receptors alpha and beta in the rat pituitary gland. Endocrinology 139(9):3976–3983
Moosmann B, Behl C (1999) The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A 96(16):8867–8872
Morley JE (2001) Androgens and aging. Maturitas 38(1): 61–71
Murphy DD, Segal M (1997) Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci 94(4):1482–1487
Neurgaren BL, Kraines RJ (1965) Menopausal symptoms in women of various ages. Psycho Med 27:266–273
Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL (1998) Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res 54(1):175–180
Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277(5331):1508–1510
Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ (1998) Tumor necrosis factor-a: a neuromodulator. Endocrinology 139(9):3976–3983
Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem 72(4):1552–1563
Pike CJ, Rosario ER, Nguyen TV (2006) Androgens, aging, and Alzheimer’s disease. Endocrine 29(2): 233–241
Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB (2001) Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol 36(2):311–326
Rosario ER, Pike CJ (2008) Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res Rev 57(2): 444–453
Saucedo R, Rico G, Basurto L, Ochoa R, Zárate A (2002) Transdermal estradiol in menopausal women depresses interleukin-6 without affecting other markers of immune response. Gynecol Obstet Invest 53(2):114–117
Segal M, Murphy DD (1998) CREB activation mediates plasticity in cultured hippocampal neurons. Neural Plast 6(3):1–7
Sharma K, Mehra RD. (2008) Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res 1204:1–15
Sharma K, Mehra RD, Dhar P, Vij U (2007) Chronic exposure to estrogen and tamoxifen regulates synaptophysin and phosphorylated cAMP response element-binding (CREB) protein expression in CA1 of ovariectomized rat hippocampus. Brain Res 1132(1):10–19
Sherwin BB (1988) Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357
Sherwin BB (2000) Oestrogen and cognitive function throughout the female lifespan. Novartis Found. Symp 230:188–196
Singer CA, Rogers KL, Dorsa DM (1998) Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport 9(11):2565–2568
Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM (1999) The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci 19(7):2455–2463
Swaab DF (2004) Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol 19:301–312
Szegõ EM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM (2006) Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci 26(15):4104–4110
Tsai MJ, O’Malley BW (1994) Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486
Toulmond S, Parnet P, Linthorst AC (1996) When cytokines get on your nerves: cytokine networks and CNS pathologies. Trends Neurosci 19:409
Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A (2003) Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A 100(16):9614–9619
Wappler EA, Szilágyi G, Gál A, Skopál J, Nyakas C, Nagy Z, Felszeghy K (2009) Adopted cognitive tests for gerbils: Validation by studying aging and ischemia. Physiol Behav 97:107–114
Wappler EA, Felszeghy K, Szilágyi G, Gál A, Skopál J, Mehra RD, Nyakas C, Nagy Z (2010) Neuroprotective effects of estrogen treatment on ischemia-induced behavioural deficits in ovariectomized gerbils at different ages. Behav Brain Res 209(1):42–48
Wappler EA, Felszeghy K, Varshney M, Mehra RD, Nyakas C, Nagy Z (2011) Brain plasticity following ischemia: effect of estrogen and other cerebroprotective drugs. In: Balestrino M (ed) Brain ischemia: Book 2. InTech – Open Access Publisher, ISBN 979–953–307–734–4
Watters JJ, Dorsa DM (1998) Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci 18(17):6672–6680
Weaver CE Jr, Park-Chung M, Gibbs TT, Farb DH (1997) 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res 761(2):338–341
Weissman BA, Daly JW, Skolnick P (1975) Diethylstilbestrol-elicited accumulation of cyclic AMP in incubated rat hypothalamus. Endocrinology 97(6):1559–1566
Wilson CA and Davies DC (2007) The control of sexual differentiation of the reproductive system and brain. Reproduction 133:331–359
Ye SM, Johnson RW (2001) An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 9(4):183–192
Zhou Y, Watters JJ, Dorsa DM (1996) Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137(5):2163–2166
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Mehra, R.D., Varshney, M.K., Kumar, P. (2012). Estrogen-Mediated Neuroprotection: Hope to Combat Neuronal Degeneration and Synaptic Plasticity Post-menopause. In: Thakur, M., Rattan, S. (eds) Brain Aging and Therapeutic Interventions. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5237-5_14
Download citation
DOI: https://doi.org/10.1007/978-94-007-5237-5_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5236-8
Online ISBN: 978-94-007-5237-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)