Abstract
Sepsis survivors frequently develop late cognitive impairment. Because little is known on the mechanisms of post-septic memory deficits, there are no current effective approaches to prevent or treat such symptoms. Here, we subjected mice to severe sepsis induced by cecal ligation and puncture (CLP) and evaluated the sepsis-surviving animals in the open field, novel object recognition (NOR), and step-down inhibitory avoidance (IA) task at different times after surgery. Post-septic mice (30 days post-surgery) failed in the NOR and IA tests but exhibited normal performance when re-evaluated 45 days after surgery. Cognitive impairment in post-septic mice was accompanied by reduced hippocampal levels of proteins involved in synaptic plasticity, including synaptophysin, cAMP response element-binding protein (CREB), CREB phosphorylated at serine residue 133 (CREBpSer133), and GluA1 phosphorylated at serine residue 845 (GluA1pSer845). Expression of tumor necrosis factor α (TNF-α) was increased and brain insulin signaling was disrupted, as indicated by increased hippocampal IRS-1 phosphorylation at serine 636 (IRS-1pSer636) and decreased phosphorylation of IRS-1 at tyrosine 465 (IRS-1pTyr465), in the hippocampus 30 days after CLP. Phosphorylation of Akt at serine 473 (AktpSer473) and of GSK3 at serine 9 (GSK3βpSer9) were also decreased in hippocampi of post-septic animals, further indicating that brain insulin signaling is disrupted by sepsis. We then treated post-septic mice with liraglutide, a GLP-1 receptor agonist with insulinotropic activity, or TDZD-8, a GSK3β inhibitor, which rescued NOR memory. In conclusion, these results establish that hippocampal inflammation and disrupted insulin signaling are induced by sepsis and are linked to late memory impairment in sepsis survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is defined as a state of high-grade systemic inflammation caused by infection. One of its major symptoms is sepsis-associated encephalopathy (SAE), a form of acute brain dysfunction [1, 2] which initially manifests with confusion, progressing from mild delirium to coma [1-3]. Sepsis survivors frequently present late cognitive impairment, characterized by deficits in memory and executive functions [4, 5]. Significantly, many patients never fully recover from such deficits [4, 6, 7]. Little is known on the mechanisms underlying long-lasting cognitive impairment after critical illness [1, 5], hampering the development of effective ways to prevent or treat such conditions.
Global cognition scores in sepsis survivors are comparable to scores for patients with mild Alzheimer’s disease (AD) [3, 5], and it has been suggested that sepsis survivors present dementia with particular involvement of the frontal lobe and hippocampus [8]. Disrupted brain insulin signaling is a feature of AD and other tauopathies and has been implicated in cognitive deficits in those disorders [9–15]. Because sepsis is associated with severe deregulation of glucose homeostasis and peripheral insulin resistance [16], we hypothesized that inhibition of brain insulin signaling might be associated with cognitive impairment observed in sepsis survivors. Previous studies from our group showed that activation of tumor necrosis factor α (TNF-α)/c-Jun N-terminal kinase (JNK) signaling triggers inhibitory serine phosphorylation of insulin receptor substrate-1 (IRS-1), a key protein in insulin signaling, centrally implicated in peripheral insulin resistance in diabetes and in brain insulin resistance in dementia [9, 13, 17].
Here, we show that mice surviving sepsis induced by cecal ligation and puncture (CLP) presented cognitive deficits in the novel object recognition (NOR) and step-down inhibitory avoidance (IA) tests, up-regulated hippocampal expression of TNF-α, decreased levels and phosphorylation of cAMP response element-binding protein (CREB), and reduced synaptophysin levels. Of note, CLP also induced hippocampal IRS-1 inhibition, and systemic treatment with an anti-diabetes agent (liraglutide) rescued cognitive function in sepsis-surviving mice. We also demonstrate that hippocampi of sepsis-surviving mice showed aberrant activation of glycogen synthase kinase 3 (GSK3β), a serine kinase that is negatively regulated by insulin signaling, and treatment with the GSK3β inhibitor improved memory performance. Collectively, our results provide the grounds for targeting brain insulin signaling as a potential disease-modifying therapy for late cognitive impairment in sepsis survivors.
Methods
Animals
Two-month-old male Swiss mice, weighing between 25 and 30 g, were used. Animals were housed in groups of five per cage and had free access to food and water, under a 12-h light/dark cycle in a room with controlled temperature and humidity. All procedures followed the Principles of Laboratory Animal Care from the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Federal University of Rio de Janeiro (protocol no. IBqM 054/2012).
Cecal Ligation and Puncture
Animals were subjected to cecal ligation and puncture (CLP) as previously described [18]. Briefly, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) given intraperitoneally. Under aseptic conditions, a 2-cm midline laparotomy was performed to allow exposure of the cecum and the adjoining intestine. The cecum was ligated with a 4.0-silk suture at its base, below the ileocecal valve, and was perforated two times with an 18-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation sites, returned to the peritoneal cavity, and the laparotomy was closed with a surgical 9-mm clip. CLP and sham-operated animals received subcutaneous injections of sterile isotonic saline (50 mL/kg) immediately and 12 h after surgery, as well as antibiotic therapy. In the sham-operated (control) group, mice were subjected to all surgical procedures, but the cecum was neither ligated nor perforated. Following sepsis induction, the animals were individually assessed in terms of weight, activity in the homecage, general appearance, behavior, and altered breathing 1, 4, 24, 48 h and 1 week post-surgery. Survival rates were 100% in the sham-operated group and 45% in the sepsis group. All sepsis surviving animals were included in the study.
Drug Treatment
All drugs were prepared fresh on the treatment day and given intraperitoneally (i.p.) or subcutaneously (s.c.) in a final injection volume of 0.1 mL for every 10 g of body weight. After surgery, sham and CLP animals were treated with antibiotics (30 mg/kg ceftriaxone, 25 mg/kg clindamycin, s.c.) every 12 h for 3 days. Treatment with liraglutide (Bachem; 50 nmol/kg/day, s.c., during 10 days) or TDZD-8 (Sigma Aldrich; 5 mg/kg/day, i.p., during 5 days) began 20 and 25 days after CLP, respectively.
Behavioral Experiments
Open-field test: The test was performed in an arena measuring 30 cm × 30 cm surrounded by 50-cm high walls. The floor of the arena was divided into nine squares by lines. During each trial (lasting 300 s), the number of lines crossed on the floor of the arena (number of crossings) and the number of rearings (elevation on rear paws, denoting exploratory behavior) were recorded to determine locomotor/exploratory behavior. Animals were evaluated in the open-field task 10, 15, 25, 30, and 45 days after surgery.
Novel object recognition test (NOR): Thirty days after surgery, when no differences in locomotor and exploratory behaviors were found between the CLP and sham groups in the open-field test, the animals were assessed in the novel object recognition (NOR) task. Training and test sessions were performed in an arena measuring 30 × 30 × 45 cm. Test objects were made of glass or plastic and had different shapes, colors, sizes, and textures. During sessions, objects were fixed to the box using tape to prevent displacement caused by exploratory activity of the animals. Preliminary tests showed that none of the objects used in our experiments evoked innate preference. Before training, each animal was submitted to a 5-min long habituation session, in which they were allowed to freely explore the empty arena. Training consisted of a 5-min long session during which animals were placed at the center of the arena in the presence of two identical objects. The time spent exploring each object was recorded by a trained researcher. Sniffing and touching the object were considered exploratory behavior. The arena and objects were cleaned thoroughly between trials with 20% ethanol to eliminate olfactory cues. Two hours after training, the animals were again placed in the arena for the test session, when one of the two objects used in the training session was replaced by a new one. Again, the amount of time spent exploring familiar and novel objects was measured. Results were expressed as percentage of time exploring each object during the training or test session and were analyzed using a one-sample Student’s t test comparing the mean exploration time for each object to the fixed value of 50%. By definition, animals that recognize the familiar object as such (i.e., learn the task) explore the novel object >50% of the total time [13].
Inhibitory avoidance task: For training, mice were placed on a platform measuring 10 cm × 7 cm × 2 cm placed in the center of an inhibitory avoidance box (Insight, São Paulo, Brazil). The box measured 38 cm × 25 cm × 30 cm, and its floor was made of conductive metal grids, spared 1-cm apart, and connected to a power source. When mice stepped down the platform with four paws, they received a 0.7 mA footshock for 2 s. The box and platform were thoroughly cleaned between trials with alcohol 20% to eliminate olfactory cues. Twenty-four hours after the training session, mice were again placed on the platform and time to step down the platform with four paws was recorded. A ceiling time of 300 s was used on test session.
Western immunoblot: Thirty days after surgery, mice were euthanized by decapitation and their hippocampi and frontal cortex were rapidly dissected and frozen in liquid nitrogen. For total protein extraction, the samples were thawed and homogenized in buffer containing 25 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% NP-40 (Invitrogen), 1% sodium deoxicholate, 0.1% SDS, 5 mM EDTA, 1% Triton X-100, and phosphatase and protease inhibitor cocktails (Thermo Scientific Pierce). Protein concentrations were determined using the BCA kit (Thermo Scientific). Samples containing 30 μg protein were resolved in 4–20% polyacrylamide Tris–glycine gels (Invitrogen) and blotted to nitrocellulose membranes at 300 mA for 1 h. Blots were incubated with 5% non-fat milk in Tween-TBS at room temperature for 2 h and incubated overnight at 4 °C with primary antibodies diluted in blocking buffer. Primary antibodies used were anti-IRS-1pSer636 (1:200), anti–IRS-1pTyr465 (1:200), anti-IRS-1 (1:500), anti-PSD-95 (1:2000), and anti-tau pSer396 (1:2000) (all from Santa Cruz Biotecnology); anti-GSK3βpSer9 (1:1000), anti-GSK3β (1:1000), anti-AktpSer473 (1:1000), anti-Akt (1:1000), anti-CREBpSer133 (1:200), anti-CREB (1:500), anti-JNKpT183/Y185 (1:400), and anti-JNK (1:500) (all from Cell Signaling); anti-synaptophysin (1:2000), anti-tau (1:1000), and anti-β-tubulin (1:5000) (all from Abcam); anti-GluA1pSer845 (1:300, Invitrogen) and anti-GluA1 (1:500, Millipore). After overnight incubation, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:10–50,000; Invitrogen) and IRDye800CW- or IRDye680RD-conjugated secondary antibodies (LI-COR; 1:10,000) at room temperature for 2 h. Chemilumescence was developed using SuperSignal West Femto (Thermo Fisher Scientific). Alternatively, fluorescence intensities were quantified in an Odissey CLx apparatus (LI-COR).
RNA extraction and quantitative real-time PCR analysis: Hippocampal tissue was homogenized in 1 mL Trizol (Invitrogen) and RNA extraction was performed according to the manufacturer’s instructions. Purity and integrity of RNA were determined by the 260/280 nm absorbance ratio and by agarose gel electrophoresis. Only preparations with ratios >1.8 and no signs of RNA degradation were used. One microgram of RNA was used for cDNA synthesis using the Super-Strand III Reverse Transcriptase kit (Invitrogen). Expression of genes of interest was analyzed by qPCR on an Applied Biosystems 7500 RT-PCR system using the Power SYBR kit (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or actin was used as endogenous controls. TNF-α and CREB were amplified using the following primers: for TNF-α, forward: 5′-CCCTCACACTCAGATCATCTTCT-3′, reverse: 5′-GCTACGACGTGGGCTACAG-3′; for CREB, forward: 5′-CCAGCCATCAGTTATCCAGTCTC-3′, reverse: 5′-GAATCAGTTACACTATCCACAGACTCCT-3′. Cycle threshold (Ct) values were used to calculate fold changes in gene expression using the 2−DCt method. In all cases, reactions were performed in 15 μl reaction volumes.
Data analysis: Data from the open-field task and molecular analyses are expressed as means ± SEM, and statistical significances were determined by paired samples Student’s t test, one-way ANOVA followed by Bonferroni, or Mann-Whitney, as indicated in figure legends. Results from the NOR test are expressed as percentage of time spent exploring each object (novel or familiar) during the training or test session and were analyzed using a one-sample t test comparing the mean exploration time for each object with the fixed value of 50%. Animals that recognize the familiar object as such (i.e., learn the task) explore the novel object >50% of the total time.
Results
Sepsis Survivors Show Late Aversive and Recognition Cognitive Impairment
We initially examined locomotor and exploratory behaviors in post-septic mice by testing them in open-field sessions at different time points after CLP. Locomotor and exploratory behaviors were impaired for up to 25 days after CLP and were fully recovered after 30 days (Fig. 1a, b). Thirty days after CLP, when animals had fully recovered from sepsis-induced locomotor deficit, they were tested in the NOR paradigm. Sepsis-surviving mice failed in the NOR test when evaluated late after the acute clinical signals of sepsis (30 days after surgery) (Fig. 1c). Sepsis survivors recovered the ability to learn the NOR task when retrained 45 days after surgery (Fig. 1d). As expected, sham-operated mice had no locomotor and exploratory deficits and learned the NOR task at both 30 and 45 days post-surgery (Fig. 1c, d). In addition, when evaluated in an aversive and hippocampal-dependent task, post-septic mice also showed late long-term memory impairment (Fig. 1e).
Post-septic mice show impaired aversive and recognition memory. Two-month-old male Swiss mice were subjected to cecal ligation and puncture (post-septic) or were sham operated (Sham). Animals were tested in an open-field arena 15, 25, 30, and 45 days after surgery and the numbers of crossings (a) and rearings (b) were measured. At days 30 (c) and 45 (d) post-sepsis, mice were trained and tested in the novel object recognition (NOR) task. The percentages of time spent exploring the familiar (old) or the novel (new) objects in the test session were measured. e A different group of mice was trained in the step-down inhibitory avoidance task and, 24 h after, step-down latencies were recorded in a test session. In a–d, bars represent means ± S.E.M. *p < 0.05, one-sample Student’s t test. In e, bars represent median ± interquartile range, *p < 0.05 in Mann-Whitney test (n = 10 mice/group)
Cognitive Impairment in Post-Septic Mice Is Accompanied by Synapse Damage
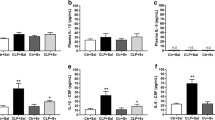
We next investigated markers of synapse structure and function in the hippocampus of sepsis survivors. We found that mRNA (Fig. 2a) and protein (Fig. 2b) levels of CREB, a transcription factor that plays key roles in synaptic plasticity, learning, and memory [19], were markedly decreased in sepsis-surviving mice 30 days after CLP. Further, levels of CREB phosphorylated at Ser133 were reduced in the hippocampus of post-septic mice (Fig. 2c). We also analyzed levels of synaptic markers in sepsis-surviving mice 30 days after surgery. We found CLP induced a decrease in hippocampal levels of synaptophysin, a pre-synaptic marker, but had no effect on levels of PSD-95, a post-synaptic scaffolding protein (Fig. 2d, e). AMPA receptors (AMPARs) are central to glutamatergic synaptic plasticity in the hippocampus [20], and trafficking of AMPARs into post-synaptic densities is dependent on phosphorylation of the GluA1 subunit [21]. Compared to control mice, GluA1pSer845 levels were significantly decreased in the hippocampus of sepsis survivors (Fig. 2f). Results suggest that hippocampal synapse integrity and function are impaired in sepsis-surviving mice, which could contribute to cognitive impairment.
Cognitive impairment is accompanied by synapse damage in post-septic mice. Two-month-old male Swiss mice were subjected to cecal ligation and puncture (post-septic) or were sham operated (Sham). qPCR or Western blot analysis were performed in hippocampal extracts 30 days after CLP. a CREB mRNA levels analyzed by qPCR; b total CREB; c pCREB (pSer133); d synaptophysin (SYP); e PSD-95; f GluA1pSer845 normalized by total GluA1. Graphs show densitometric data normalized by β-tubulin (e–e). Bars represent means ± S.E.M.; *p < 0.05; #p = 0.0850 (c) or p = 0.0816 (d); Student’s t test (n = 5–10 animals/group). Lanes were run in the same gel but were non-contiguous
Sepsis-Surviving Mice Show Hippocampal Inflammation and Impaired Insulin Signaling
Because the hippocampus plays a major role in memory processing and TNF-α is a key inflammatory mediator implicated in hippocampus-dependent memory deficits in dementia [9, 13], we evaluated hippocampal expression of TNF-α 30 days after CLP. TNF-α mRNA levels were significantly increased in the hippocampus of sepsis-surviving animals (Fig. 3a), showing that local production of TNF-α is up-regulated in post-septic brains late after the acute clinical signals of sepsis. Increased TNF-α levels can lead to activation of stress kinases such as JNK and IKK, which in turn cause serine phosphorylation and inhibition of IRS-1 [9, 13]. Indeed, we found that JNK was activated in the hippocampus of CLP mice 30 days after surgery (Fig. 3b). Because serine phosphorylation of IRS-1 has been implicated in brain insulin resistance in dementia, we examined IRS-1pSer levels in the hippocampi of sepsis-surviving mice. Hippocampal levels of IRS-1pSer636 were increased in post-septic mice compared to sham-operated mice (Fig. 3c). Further, hippocampi from sepsis-surviving mice showed decreased phosphorylation of IRS-1 at tyrosine residue 465 (IRS-1pTyr465), an essential step in physiological insulin signaling (Fig. 3d).
Cognitive impairment is accompanied by hippocampal inflammation and impaired insulin signaling in post-septic mice. Two-month-old male Swiss mice were subjected to cecal ligation and puncture (post-septic) or were sham operated (Sham). qPCR or Western blot analysis were performed in hippocampal (a–g) or cortical (h) extracts 30 days after CLP. a TNF-α mRNA levels analyzed by qPCR; bJNKpT183/Y185 normalized by total JNK; c IRS-1pSer636 normalized by total IRS-1; d IRS-1pTyr465 normalized by total IRS-1; e Akt-pSer473 normalized by total Akt; f GSK3βpSer9 normalized by total GSk3β; g tau-pSer396 normalized by total tau; h IRS-1pSer636 normalized by total IRS-1 in cortical tissue of mice. Bars represent means ± S.E.M.; *p < 0.05; #p = 0.0615 (e), p = 0.0748 (g), #p = 0.0513; Student’s t test (n = 5–9 animals/group). Lanes were run in the same gel but were non-contiguous
Insulin signaling activates Akt through a well-defined mechanism mediated by the IRS1/PI3K pathway. Activated Akt phosphorylates glycogen synthase kinase 3β (GSK3β) at serine residue 9 (GSK3βpSer9), inhibiting its activity. GSK3β is a serine kinase that plays key roles in a number of physiological processes as well as in the pathogenesis of dementia [22] and in insulin resistance [23]. Thus, we investigated the impact of sepsis on hippocampal phosphorylation of Akt and GSK3β. Levels of Akt phosphorylated at serine residue 473 (AktpSer473) and of GSK3β phosphorylated at serine residue 9 (GSK3βpSer9) were both decreased in the hippocampi of post-septic animals compared to control mice (Fig. 3e, f). An important target of GSK3β in the brain is tau, a microtubule-stabilizing protein whose hyperphosphorylation has been implicated in several neurodegenerative diseases collectively known as tauopathies [24]. In agreement with an over-activation of GSK3β (revealed by reduced GSK3βpSer9 levels), increased levels of tau phosphorylated at serine 396 (tau-pSer396), a residue that is hyperphosphorylated in Alzheimer’s disease and other tauopathies, were found in the hippocampi of sepsis-surviving mice (Fig. 3g). In addition to the hippocampus, other brain regions play essential roles in memory processing. We therefore examined levels of IRS-1pSer636 in the cortex of post-septic mice, as a representative protein of insulin signaling disruption. We also found increased levels of IRS-1pSer636 in the cortex of sepsis-surviving mice when compared to sham-operated animals (Fig. 3h). Collectively, these findings support the notion that impaired brain insulin signaling, increased GSK3β activity, and tau hyperphosphorylation accompany late memory impairment in sepsis.
Boosting Insulin Signaling Prevents Cognitive Impairment in Sepsis Survivors
In order to investigate whether drugs that target the insulin signaling pathway could ameliorate sepsis-associated cognitive impairment, CLP-mice were treated with liraglutide, a GLP-1 receptor agonist known to up-regulate insulin-triggered pathways [25]. Remarkably, s.c. treatment with liraglutide for 10 days beginning on day 20 post-CLP rescued cognitive impairment in post-septic mice (Fig. 4a). To further determine if hippocampal over-activation of GSK3β was involved in the development of sepsis-associated cognitive defects, a different group of mice was treated with daily i.p. injections of the selective GSK3β inhibitor, TDZD-8, which rescued performance in the NOR task (Fig. 4b). These results support the notion that disruption of brain insulin signaling in post-septic mice is an important factor underlying late cognitive impairment and that boosting insulin signaling could be further investigated as a therapeutic alternative for this devastating condition.
Cognitive impairment is rescued by normalizing insulin signaling in post-septic mice. Two-month-old male Swiss mice were subjected to cecal ligation and puncture (post-septic) or were sham operated (Sham). Beginning on days 20 and 25 after surgery, respectively, mice were treated with a liraglutide (50 nmol/kg/day, for 10 days, s.c.) or b TDZD-8 (5 mg/kg/day, for 5 days, i.p.) or vehicle. At day 30 post-sepsis, mice were trained in the novel object recognition (NOR) task. The percentages of time spent exploring the familiar (old) or the novel (new) objects in the test session were measured. Bars represent means ± S.E.M. *p < 0.05, one-sample Student’s t test (n = 10 mice/group)
Discussion
Sepsis-induced brain dysfunction has received increasing attention in recent years, as convincing evidence shows that infectious diseases and systemic inflammation are risk factors for delirium, cognitive impairment, and neurodegenerative diseases [1, 3–6, 26, 27]. Even though sepsis survivors frequently show cognitive deficits that persist for months or years, seriously compromising patient recovery and independence, the mechanisms underlying this memory dysfunction are unclear.
Early cognitive impairment has been described in animal models of sepsis [18, 28, 29]. Barichello and colleagues evaluated cognitive function of sepsis-surviving mice 10 days after CLP surgery, using an aversive behavior paradigm [18]. The model described here significantly extends those findings by recapitulating late cognitive impairment seen in post-septic patients, after acute inflammation has ceased, which has translational importance for this condition. In agreement with previous studies, we found that sepsis-surviving mice show impaired locomotor/exploratory activities in an open-field task, which lasts for up to 25 days after CLP. Most experimental studies to date have assessed sepsis-induced cognitive deficits within this time frame and therefore are susceptible to interference from locomotor deficit [18, 30–32]. Moreover, the majority of studies have evaluated fear memories, which is not the type of memory mainly affected in sepsis-surviving patients [18, 30, 32]. Here, we followed post-septic mice until they no longer showed impaired locomotor/exploratory activities and trained them in an aversive memory task and also in the NOR test, evaluating memories that require integrity of the hippocampus and other brain regions [33, 34]. Post-septic mice showed impaired recognition and aversive memory 30 days after CLP. Remarkably, this is the first animal model of sepsis with cognition-related translational impact, since it recapitulates late cognitive effects seen in sepsis-surviving humans.
Even though some authors suggest that many sepsis survivors never fully recover cognitive functions [4, 7], some studies consider this impairment to be at least partially reversible [6]. Of note, it is not known whether sepsis induces or worsens a pre-existing neurodegenerative process, as information concerning the previous cognitive status of patients admitted to intensive care units is most often lacking. It is possible that previous cognitive status of patients may account substantially for the link between sepsis and long-lasting cognitive impairment. Under our conditions, recognition memory deficit seen late after CLP was transient, as mice had normal performance in the NOR test when re-trained 45 days after induction of sepsis. Results show that CLP in mice followed by cognitive evaluation in the novel object recognition task provides a suitable model to study the late, reversible cognitive impairment caused by sepsis, as seen in humans.
Synapse loss has been found to be a better correlate of memory impairment than other neurodegeneration markers in some forms of dementia, including Alzheimer’s disease [35]. Our previous studies have shown that synaptophysin levels serve as a suitable marker of synapse damage and correlates well with cognitive impairment in mice [36, 37]. A recent study showed decreased levels of synaptophysin and PSD-95 in the hippocampus and cortex of septic mice 24 h and 3 days after CLP [31]. Weberpals and colleagues also showed that a single intraperitoneal injection of bacterial lipopolysaccharide (LPS) (5 mg/kg) induces long-term cognitive deficits and changes in synaptic key proteins (PSD-95 and synaptophysin) in the brain of wild-type mice, at least 2 months after sepsis induction [29]. Herein, we found that CLP still exerted a deleterious impact on hippocampal pre-synaptic terminals 30 days after surgery, as demonstrated by decreased synaptophysin levels compared to control animals. On the other hand, hippocampal levels of PSD-95, a major scaffolding protein in the post-synaptic density, were comparable in sepsis-surviving mice and in sham-operated animals 30 days after surgery.
Several studies have established the relevance of cAMP-responsive element binding protein (CREB) in hippocampal memory-related processes [19]. During memory consolidation, CREB undergoes phosphorylation at serine residue 133 and binds to the CRE promotor region as a consequence of calcium influx in the post-synaptic terminal [38]. Here, we show that post-septic mice show decreased hippocampal expression of CREB and lower levels of CREBpSer133 compared to control animals. AMPAR phosphorylation/trafficking is another important consequence of glutamatergic post-synaptic activation in the hippocampus. Phosphorylation at specific sites following neuronal activation determines AMPAR translocation to synapses [20]. Here, we found decreased levels of GluA1pSer845 in the hippocampus of mice 30 days after CLP. Altogether, these findings suggest that sepsis compromises plasticity mechanisms in hippocampal post-synaptic terminals.
Previous studies have shown increased levels of IL-1β and other pro-inflammatory cytokines in the hippocampus of rodents following sepsis [28, 31, 39–41]. When evaluated at longer periods after sepsis, increased levels of pro-inflammatory cytokines have been found in cerebrospinal fluid or in whole-brain homogenates of CLP-induced sepsis animals [42, 43]. In addition, wild-type mice or rats submitted to LPS-induced sepsis also show a prolonged increase in pro-inflammatory cytokines in the hippocampus [29, 41]. Current results demonstrate increased hippocampal expression of TNF-α 30 days after CLP, suggesting that even after the acute phase of peripheral inflammation has ceased, production of inflammatory mediators persists in post-septic brains. This local production of pro-inflammatory mediators may contribute to the persistent cognitive impairment seen in our model.
Recent findings suggest that several types of dementia, including AD, are characterized by defective brain insulin signaling [15], which is apparently linked to cognitive dysfunction [10–12]. Additional studies have implicated increased brain TNF-α levels in impaired insulin signaling and memory deficits [9, 13]. We thus hypothesized that insulin signaling could be disrupted in the brains of sepsis-surviving mice and could underlie sepsis-induced cognitive impairment. Indeed, we found that hippocampi of post-septic mice show over-activation of TNF-α/JNK signaling, increased levels of IRS-1pSer636 and decreased levels of IRS-1pTyr465, hallmarks of insulin resistance in both periphery and CNS [10, 11, 44].
In the insulin signaling pathway, Akt activation occurs as a key step downstream from IRS-1 activation [45–47]. Under physiological conditions, activation of Akt by phosphorylation at serine and threonine residues leads to inactivation of GSK3β via serine phosphorylation [23, 48]. In type 2 diabetes (T2D), for example, defective insulin signaling leads to reduced levels of AktpSer473 and consequent over-activation of GSK3β. Increasing evidence suggests that GSK3β plays a key role not only in diabetes but also in dementia [49, 50]. Consistent with defective insulin signaling following CLP, levels of AktpSer473 and GSK3βpSer9 were decreased in the hippocampus of post-septic mice. We also found that the hippocampus of post-septic mice had elevated levels of phosphorylated tau protein, a major target of GSK3β. Our results also suggest that disrupted insulin signaling is not restricted to the hippocampus, since findings on IRS-1pSer636 levels were reproduced in the cortex of post-septic mice. Collectively, these findings support the notion that sepsis-surviving mice show impaired brain insulin signaling, similar to AD brains and other dementias.
Previous studies have shown that liraglutide and exenatide, GLP-1 receptor agonists used to treat T2D patients, prevent cognitive impairment related to impaired brain insulin signaling in several models [9, 13, 51–53]. Significantly, clinical trials are under way to determine the clinical relevance of these drugs in AD. To determine whether normalizing insulin signaling could prevent sepsis-induced cognitive impairment, mice submitted to CLP were systemically treated with liraglutide or TDZD-8, a GSK3β inhibitor. Interestingly, treatment with liraglutide or TDZD-8 recovered cognition in sepsis-surviving mice 30 days after CLP. These findings improve our understanding of the mechanisms underlying late cognitive impairment in sepsis survivors, and provide new targets for the development of treatments for this condition.
The current study highlights the impact of sepsis on hippocampal insulin signaling, synapse structure and function and demonstrates that late cognitive impairment in sepsis-surviving mice is associated with up-regulation of brain TNF-α, disruption of insulin signaling cascades, and plasticity-associated molecules. Moreover, late cognitive impairment after sepsis was reversed when insulin signaling was pharmacologically boosted in mice. Our results suggest that stimulation of brain insulin signaling could provide a novel neuroprotective approach to prevent cognitive impairment in sepsis survivors. We note that liraglutide is a drug already approved for treatment of T2D, and that identifying a similar pathogenic mechanism of insulin resistance contributing to sepsis-induced cognitive impairment may open new avenues for rapid implementation of clinically approved anti-diabetic drugs as therapeutics in post-septic patients.
References
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8(10):557–566. doi:10.1038/nrneurol.2012.183
Lamar CD, Hurley RA, Taber KH (2011) Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. The Journal of neuropsychiatry and clinical neurosciences 23(3):237–241. doi:10.1176/appi.neuropsych.23.3.237
Widmann CN, Heneka MT (2014) Long-term cerebral consequences of sepsis. The Lancet Neurology 13(6):630–636. doi:10.1016/S1474-4422(14)70017-1
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA : the journal of the American Medical Association 304(16):1787–1794. doi:10.1001/jama.2010.1553
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14):1306–1316. doi:10.1056/NEJMoa1301372
Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, 3rd, Canonico A, Merkle K, Cannistraci CJ, Rogers BP, Gatenby JC, Heckers S, Gore JC, Hopkins RO, Ely EW, Visions Investigation VISNS (2012) The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Critical care medicine 40 (7):2022–2032. doi:10.1097/CCM.0b013e318250acc0
Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, Mormann F, Weide J et al (2013) Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry 84(1):62–69. doi:10.1136/jnnp-2012-302883
Sharshar T, Bozza F, Chretien F (2014) Neuropathological processes in sepsis. The Lancet Neurology 13(6):534–536. doi:10.1016/S1474-4422(14)70064-X
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated abeta oligomers. J Clin Invest 122(4):1339–1353. doi:10.1172/JCI57256
De Felice FG (2013) Connecting type 2 diabetes to Alzheimer’s disease. Expert Rev Neurother 13(12):1297–1299. doi:10.1586/14737175.2013.864824
De Felice FG (2013) Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest 123(2):531–539. doi:10.1172/JCI64595
de la Monte SM (2009) Insulin resistance and Alzheimer’s disease. BMB Rep 42(8):475–481
Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J et al (2013) TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s beta-amyloid oligomers in mice and monkeys. Cell Metab 18(6):831–843. doi:10.1016/j.cmet.2013.11.002
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122(4):1316–1338. doi:10.1172/JCI59903
Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY, Louneva N, Lee VM et al (2014) Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol 128(5):679–689. doi:10.1007/s00401-014-1328-5
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–1367. doi:10.1056/NEJMoa011300
Clarke JR, Lyra ESNM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, Katashima CK, Razolli D et al (2015) Alzheimer-associated a beta oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO molecular medicine 7(2):190–210. doi:10.15252/emmm.201404183
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33(1):221–223 discussion 262-223
Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR (1998) Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1(7):595–601. doi:10.1038/2830
Huganir RL, Nicoll RA (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80(3):704–717. doi:10.1016/j.neuron.2013.10.025
Oh MC, Derkach VA, Guire ES, Soderling TR (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281(2):752–758. doi:10.1074/jbc.M509677200
Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8(9):663–672. doi:10.1038/nrn2194
McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24(8):1571–1583. doi:10.1038/sj.emboj.7600633
Utton MA, Vandecandelaere A, Wagner U, Reynolds CH, Gibb GM, Miller CC, Bayley PM, Anderton BH (1997) Phosphorylation of tau by glycogen synthase kinase 3beta affects the ability of tau to promote microtubule self-assembly. The Biochemical journal 323(Pt 3):741–747
Ryan GJ, Hardy Y (2011) Liraglutide: once-daily GLP-1 agonist for the treatment of type 2 diabetes. J Clin Pharm Ther 36(3):260–274. doi:10.1111/j.1365-2710.2010.01180.x
Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD (2010) Critical care and the global burden of critical illness in adults. Lancet 376(9749):1339–1346. doi:10.1016/S0140-6736(10)60446-1
Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE et al (2010) Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 38(7):1513–1520. doi:10.1097/CCM.0b013e3181e47be1
Sui DM, Xie Q, Yi WJ, Gupta S, Yu XY, Li JB, Wang J, Wang JF et al (2016) Resveratrol protects against sepsis-associated encephalopathy and inhibits the NLRP3/IL-1beta Axis in microglia. Mediat Inflamm 2016:1045657. doi:10.1155/2016/1045657
Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schafers M et al (2009) NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience 29(45):14177–14184. doi:10.1523/JNEUROSCI.3238-09.2009
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Long-term cognitive impairment in sepsis survivors. Crit Care Med 33(7):1671
Moraes CA, Santos G, de Sampaio e Spohr TC, D’Avila JC, Lima FR, Benjamim CF, Bozza FA, Gomes FC (2015) Activated microglia-induced deficits in excitatory synapses through IL-1beta: implications for cognitive impairment in sepsis. Mol Neurobiol 52(1):653–663. doi:10.1007/s12035-014-8868-5
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32(2):342–349. doi:10.1097/01.CCM.0000109454.13145.CA
Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM (2010) Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A 107(6):2652–2657. doi:10.1073/pnas.0915059107
Dere E, Huston JP, De Souza Silva MA (2007) The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31(5):673–704. doi:10.1016/j.neubiorev.2007.01.005
Shankar GM, Walsh DM (2009) Alzheimer’s disease: synaptic dysfunction and abeta. Mol Neurodegener 4:48. doi:10.1186/1750-1326-4-48
Figueiredo CP, Bicca MA, Latini A, Prediger RD, Medeiros R, Calixto JB (2011) Folic acid plus alpha-tocopherol mitigates amyloid-beta-induced neurotoxicity through modulation of mitochondrial complexes activity. Journal of Alzheimer’s disease : JAD 24(1):61–75. doi:10.3233/JAD-2010-101320
Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, Mota-Sales AP, Saraiva LM et al (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. The Journal of neuroscience : the official journal of the Society for Neuroscience 33(23):9626–9634. doi:10.1523/JNEUROSCI.0482-13.2013
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35(4):605–623
Annane D, Sharshar T (2015) Cognitive decline after sepsis. The Lancet Respiratory medicine 3(1):61–69. doi:10.1016/S2213-2600(14)70246-2
Comim CM, Cassol-Jr OJ, Constantino LS, Felisberto F, Petronilho F, Rezin GT, Scaini G, Daufenbach JF et al (2011) Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 36(2):304–311. doi:10.1007/s11064-010-0320-2
Fu HQ, Yang T, Xiao W, Fan L, Wu Y, Terrando N, Wang TL (2014) Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One 9(8):e106331. doi:10.1371/journal.pone.0106331
Singer BH, Newstead MW, Zeng X, Cooke CL, Thompson RC, Singer K, Ghantasala R, Parent JM et al (2016) Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One 11(2):e0149136. doi:10.1371/journal.pone.0149136
Steckert AV, Comim CM, Mina F, Mendonca BP, Dominguini D, Ferreira GK, Carvalho-Silva M, Vieira JS et al (2013) Late brain alterations in sepsis-survivor rats. Synapse 67(11):786–793. doi:10.1002/syn.21686
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63(7):2262–2272. doi:10.2337/db13-1954
Leney SE, Tavare JM (2009) The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. J Endocrinol 203(1):1–18. doi:10.1677/JOE-09-0037
Zaid H, Antonescu CN, Randhawa VK, Klip A (2008) Insulin action on glucose transporters through molecular switches, tracks and tethers. The Biochemical journal 413(2):201–215. doi:10.1042/BJ20080723
Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M (2005) A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes 54(5):1340–1348
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378(6559):785–789. doi:10.1038/378785a0
DaRocha-Souto B, Coma M, Perez-Nievas BG, Scotton TC, Siao M, Sanchez-Ferrer P, Hashimoto T, Fan Z et al (2012) Activation of glycogen synthase kinase-3 beta mediates beta-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol Dis 45(1):425–437. doi:10.1016/j.nbd.2011.09.002
Kremer A, Louis JV, Jaworski T, Van Leuven F (2011) GSK3 and Alzheimer’s disease: facts and fiction. Front Mol Neurosci 4:17. doi:10.3389/fnmol.2011.00017
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B et al (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. The Journal of neuroscience : the official journal of the Society for Neuroscience 29(28):9078–9089. doi:10.1523/JNEUROSCI.1071-09.2009
Long-Smith CM, Manning S, McClean PL, Coakley MF, O’Halloran DJ, Holscher C, O’Neill C (2013) The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-beta plaque and glial pathology in a mouse model of Alzheimer’s disease. Neruomol Med 15(1):102–114. doi:10.1007/s12017-012-8199-5
McClean PL, Holscher C (2014) Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 76(Pt A):57–67. doi:10.1016/j.neuropharm.2013.08.005
Acknowledgements
This work was supported by grants from Brazilian funding agencies Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (F.S.N., C.F.B., F.G.D. F, S.T. F, J.R.C., C.P.F.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (R.L.F., F.B.A., C.F.B., A.F.D.B., F.G.D.F., S.T.F., , J.R.C., C.P.F.), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (J.D.O, D.F.E., C.P.F.). We thank Maíra Oliveira, Katia Laia, and Mariangela M. Viana for the technical support, and Ana Claudia Rangel for the competent lab and project management.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fernanda S. Neves and Patrícia T. Marques contributed equally to this work.
Electronic supplementary material
Supplementary Figure 1
(TIFF 4607 kb)
Supplementary Figure 2.
(TIFF 265 kb)
Rights and permissions
About this article
Cite this article
Neves, F.S., Marques, P.T., Barros‑Aragão, F. et al. Brain-Defective Insulin Signaling Is Associated to Late Cognitive Impairment in Post-Septic Mice. Mol Neurobiol 55, 435–444 (2018). https://doi.org/10.1007/s12035-016-0307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0307-3