Abstract
Copper is a transition metal that exists in different chemical forms (e.g., Cu2+,Cu+, and Cu0) and at high concentrations it is toxic. Here, we investigated the Cu2+-induced toxicity in Drosophila melanogaster, evaluating the survival, locomotion, and the activity of acetylcholinesterase (AChE) and glutathione S-transferase (GST) enzymes. Flies were exposed to Cu2+(0.1–1 mmol CuSO4/kg of diet or approximately 0.1–1 mM Cu2+) and allowed to mate during 24 h. GST and AChE enzymes were evaluated in the larvae and in the head and the body (thorax + abdomen) of the adult male and females flies. The total number of adult females (0.4–1 mM) and males (0.75 and 1 mM) was decreased by CuSO4. The climbing ability was hampered in flies exposed to 1 mM Cu2+. In larvae, Cu2+(0.4–1 mM) increased AChE activity (P < 0.002). In males’ heads, 0.4 mM Cu2+ increased the AChE activity (P < 0.01). In adults’ bodies, Cu2+inhibited the activity in both sexes, but with greater effectiveness in males (0.1 to 1 mM) than in females (1 mM). Regarding GST activity, 0.1 mM Cu2+increased, but 1 mM decrease GST in larvae. In the head of flies, Cu2+decreased the GST activity at intermediate (0.4 mM) and increased GST at the highest concentration (1 mM) in males. In the bodies, the effect of Cu2+was similar. In conclusion, Cu2+exposure in D. melanogaster disrupted locomotion and enzymatic parameters that can be related to changes in AChE and in the detoxifying GST enzyme.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is a transition metal that exists in different ionic forms (e.g., Cu2+ and Cu+) and is essential to all living organisms from bacteria to humans [1]. Indeed, at low concentrations, copper is required for proper cellular metabolism; but at high concentrations, copper can be toxic because of its redox-active properties [2,3,4,5,6,7]. The concentrations of copper in water and soil are approximately 7 and 50 ppm, respectively; and in the atmosphere, Cu ranges from 5 to 200 ng/m3. In humans, the allowed upper limit of the copper concentration is approximately 1.5 mg/L of serum [8]. The majority of copper in healthy cells is found in the prosthetic groups of enzymes or bound to proteins, for instance, cytochrome c oxidase, copper-zinc-superoxide dismutase (Cu, Zn-SOD), and ceruloplasmin [9, 10].
In contrast to the protein-associated Cu, the free copper is potentially toxic and accumulated in the liver and brain [11,12,13]. Of particular importance, copper has been implicated in the pathogenesis of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases [14,15,16]. The presence of free copper in the living cells can accelerate the ROS formation via Fenton or Haber-Weiss reactions that can induce protein and DNA damage, lipid peroxidation, mitochondrial swelling, and cell death [17,18,19].
Copper homeostasis is tightly coordinated by copper chaperones that ensure the suitable distribution of copper to the cells and copper-requiring proteins [20, 21]. Bacteria, yeast (Saccharomyces cerevisiae), and mammalians have homologs of these proteins, which indicate a conserved metabolism of copper in these organisms [22]. In general, the eukaryotes have three different copper chaperones: Atx1p/Atox1 which delivers copper to ATP7 copper-requiring protein transporters, Cox17 which takes copper to the mitochondrial cytochrome c oxidase, and CCS, a copper chaperone that supplies copper to superoxide dismutase 1 [23,24,25].
Cu toxicity can occur after accidental, occupational, or environmental exposure [26]. In addition, human exposure is frequently associated with the use of copper in agriculture [27], by water consumption [28], and in the industrial production of electric conductors [29,30,31]. Toxicological endpoints of copper exposure have been reported for many model organisms, including bacteria [32, 33], yeast [34, 35], nematodes [36, 37], amphibians [38], and human cell lines [39,40,41].
The fruit flies Drosophila melanogaster is an important non-mammalian experimental organism that can be used to assess macroscopic dysfunctions (lifespan, fertility, phenotypic aberrations) induce by metals as well as the molecular pathways that can be involved in the toxic response to the metals [42, 43]. Regarding copper metabolism, D. melanogaster has been exploited as an excellent model to unravel the molecular mechanisms involved in Cu physiology [44, 45]. Of particular importance, D. melanogaster synthesizes five Cu-thionin isoforms (i.e., MtnA, MtnB, MtnC, MtnD, and MtnE) [46] and has numerous genes and detoxification mechanisms analogous to humans [47]. In this view, the activity of the acetylcholinesterase enzyme has been shown to be an efficient and widely used mechanism to evaluate toxicological endpoints involving the nervous system [48, 49].
Since only a few studies have studied comparatively the copper toxicity at different developmental stages in flies [50,51,52,53,54], here we evaluated the toxicity of Cu2+ during the entire life cycle of D. melanogaster (i.e., from larvae to adults), using morphological, behavioral, and biochemical endpoints of toxicity. Particularly, with the demonstration that inbred female flies were more sensitive to Cu2+ than males [7], we have hypothesized and tested here that the exposure during the entire development stages of flies could indicate differences in the biochemical and behavioral responses of male and female adult flies to copper. In fact, the fruit fly D. melanogaster is a holometabolous insect and its development from egg to the adult stage has high energy costs [55]. Furthermore, the sensitivity of developing flies to toxicants can be expected to be higher than that of adults. In this view, previous studies have indicated that CuSO4 (≈ 0.6 to 5 mM) delayed the development of the fruit flies, but the authors have not investigated the potential influence of sex on the morphological and biochemical endpoints of toxicity [56]. As cited above, copper homeostasis in D. melanogaster seems to be modulated by X-linked genes [7], particularly the expression metallothionein-related genes, which are more expressed in male than in female flies [52]. Thus, one would be expected a higher vulnerability of females to copper. However, a similar [52] or higher negative impact of copper in males than females have been reported [52, 53]. In view of these incongruences, it is necessary to further study the influence of sex differences in copper susceptibility in D. melanogaster.
Material and Methods
Chemicals
Copper sulfate pentahydrate (CuSO4·5H2O), 1-chloro-2, 4-dinitrobenzene (CDNB), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and acetylthiocholine iodide were purchased from Sigma (USA). All other reagents were commercial products of the highest purity grade available.
Drosophila melanogaster Stock and Culture
Diet preparation was performed following Adedara and collaborators’ [57] method with some adaptations. D. melanogaster wild-type was obtained from the National Species Stock Center (Bowling Green, OH, USA). The flies were maintained on cornmeal medium (1% corn flour, 2% w/v brewer’s yeast, 1% w/v sucrose, 1% w/v powdered milk, 1% w/v agar, and 0.08% v/w nipagin and 93.92% distilled water) at constant temperature and humidity (22–24 °C; 60–70% relative humidity) under 12-h dark/light cycle conditions.
Cu2+ Exposure
Four different copper concentrations were chosen to perform the present study: a non-toxic concentration (0.1 mM), a low toxic (0.4 mM), a middle-toxic (0.75 mM), and a high toxic (1 mM). The doses were selected according to both to the literature data and to pilot studies (data not shown) using the survival pattern observed. Indeed, the decrease in survival rate responses was dose-dependent for all the phases analyzed. Here, we have to emphasize that 0.1 mM did not decrease the survival rates in all the developmental stages studied. Another study from our laboratory, for instance, in an acute exposure of 4 days to 0.5, 1, 3, 5, 10, and 20 mM Cu2+, has found that the concentrations of 3 mM cause the death of 50% of the flies and 1 mM were already toxic [58].
In relation to Cu2+ exposure, basically, a mother solution of CuSO4 (150 mmol/L) was prepared in distilled water and kept on the refrigerator (4–6 °C). This solution was diluted to (in mmol of CuSO4/L) 10.1, 40.4, 75.75, and 101, and then 0.3 ml of these solutions were mixed with 30 g of still hot and liquid diet medium to give the final concentrations of approximately 0.1, 0.4, 0.75, and 1 mmol of Cu2+/kg of diet (0.1–1 mM of CuSO4). The mixture was stirred manually until homogenous solutions were obtained. The 4-day-old adult flies (30 per vial) from the five experimental groups were allowed to mate for 24 h. Then, they were removed, and the development of larvae, pupae, and adults was analyzed for 5, 9, and 13 days of exposure, respectively. Six independent replicates were made for each group.
Drosophila melanogaster Development
The D. melanogaster development was analyzed according to Rand and collaborators [59] with some adaptations. The animals were counted on the fifth (larvae stage), ninth (pupae stage), and thirteenth (adults) days after mating to determine the number of animals that survived. At the adult stage, the males and females were counted separately and expressed in percentage.
Negative Geotaxis
The locomotor activity of copper exposure was assessed using the negative geotaxis assay perform according to Ali and collaborators [60] with some adaptations. The potential neurotoxicity of CuSO4 was assessed using the negative geotaxis assay (climbing test). Ten 4-day-old adult male or female flies from each group were immobilized under mild ice anesthesia for 10 min. Then, they were placed in a vertical glass column (height 10 cm, diameter 1.5 cm) marked with a line in the height corresponding to 6 cm. The flies were allowed to recover anesthesia for 20 min. After that, the animals were tapped to the bottom of the column and, after 6 s, the number of flies that crossed the 6-cm mark was recorded.
Preparation of Larvae Samples
Twenty larvae from each group were anesthetized on ice for 10 min and weighed. The larvae were homogenized in 1 mL potassium phosphate buffer (0.1 M pH 7.4) and centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was used for the biochemical analysis, and protein concentrations were carried out at 280 nm absorbance in NanoDrop 2000 spectrophotometer, using bovine serum albumin (BSA) as a standard. The samples were diluted to 1 mg/ml before the determination of acetylcholinesterase and glutathione S-transferase activities.
Preparation of Adult Fly Samples
Four-day-old adult male or female flies (groups of 20 flies) were anesthetized in ice for 10 min. Then, their heads were separated from the thorax and abdomen (body). The tissues were weighed and homogenized in 1 mL potassium phosphate buffer (0.1 M, pH 7.4) and centrifuged at 10,000g for 10 min at 4 °C. The supernatant was separated from the pellet and protein contents were assess by Lowry (1951) [61]. The samples were diluted to 1 mg/ml before the determination of acetylcholinesterase and glutathione S-transferase activity in both tissues, bodies (thorax and abdomen), and heads, separately.
Head, Total Body (Entire Fly), and Body (Thorax + Abdomen) Weight and Head-to-Total Body Weight Ratio
The head and body (thorax + abdomen) weights of groups of 20 flies were determined in a digital balance with 0.01 mg precision (SHIMADZU AUW220D, Japan). The total body weight was determined by summing the head + the body weights. The head-to-total body weight ratio was calculated as an endpoint of toxicity. The use of this parameter in toxicology can give an idea about the toxicity of a given chemical to specific organs in different species [62, 63].
Determination of Acetylcholinesterase Activity
Determination of AChE activity was carried out according to Ellman (1961) [64]. The reaction mixture contained 20 μL of potassium phosphate buffer (0.1 M, pH 7.4), 20 μL of 25 mM, 5,5′-dithiobisnitrobenzoic acid (DTNB), and 60 μL sample (the total protein content in each well was 60 μg). The reaction was initiated by adding 20 μL of 8 mM acetylthiocholine solution. The reaction was monitored for 60 min (15-s intervals) at 415 nm with a BioRad iMark microplate reader. The enzyme activity was calculated as ηmol of acetylthiocholine hydrolyzed per mg protein per minute, using the cysteine as the source of thiol in the standard curve [57].
Determination of Glutathione S-Transferase Activity
Glutathione S-transferase activity was determined according to Habig (1974) [65] in which the conjugation of 1-chloro-2,4 dinitrobenzene (CDNB) with glutathione (GSH) is determined. The reaction was performed in 120 μL of GST buffer (0.25 M potassium phosphate buffer, pH 7.0, containing 2.5 mM ethylenediaminetetraacetic acid (EDTA), 60 μL of sample (the total protein content in each well was 60 μg), 10 μL of 100 mM GSH, and 10 μL of 25 mM CDNB. The thioether formation was monitored for 8 min (30-s intervals) at 340 nm (25 °C) in a SpectraMax plate reader (molecular devices). The activity was expressed as specific activity (delta A340 nm/min/mg of protein). Here, it was not possible to use the absorption molar coefficient of CDNB-GSH conjugate because the path length of light in the wells of ELISA reader plate is not 1 cm. Here, it was not possible to use the absorption molar coefficient of CDNB-GSH conjugate because the path length of light in the wells of ELISA reader plate is not 1 cm.
Statistical Analysis
All data were expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed by one- or two-way analysis of variance (ANOVA) followed by the Newman-Keulls post hoc test, using the GraphPad Prism5 software. Differences were considered statistically significant among groups when P < 0.05. The details of the two-way ANOVA analysis are available in supplementary files.
Results
Development of Flies Exposed to Cu2+
Copper exposure caused a dose-dependent decrease in the development of larvae, pupae, and adult flies (Fig. 1a–c). In larvae, F(4,25) = 56.03 (P < 0.0001). The means ± SEM of the number of larvae (after 5 days of exposure) were 100.2 ± 6.06, 82.0 ± 0.73, 67.0 ± 2.20, 40.5 ± 4.94, and 33.0 ± 1.93 for the control, 0.1, 0.4, 0.75, and 1 mM CuSO4 groups, respectively. Similar tendency was observed for pupae (F(4,25) = 15.20 (P < 0.0001)) after 9 days of treatment. The means were 96.33 ± 8.08, 69.33 ± 1.11, 66 ± 5.87, 41.33 ± 7.86, and 42.33 ± 2.48 for the control, 0.1, 0.4, 0.75, and 1 mM CuSO4 groups, respectively. The total number of adult flies (male + female) after 13 days of exposure to copper F(4,25) = 23.60 (P < 0.0001) was also reduced in a dose-dependent manner: 87.66 ± 7.59 (control), 67.83 ± 2.78 (0.1 mM CuSO4), 57.0 ± 4.84 (0.4 mM CuSO4), 26.66 ± 6.22 (0.75 mM CuSO4), and 34.33 ± 1.72 (1 mM CuSO4).
Number of larvae (a), pupae (b), adults (c), and males and females (d) after exposure to Cu2+. Larvae, pupae, and adults were determined after 5, 9, and 13 days of treatment, respectively. Data are expressed as the means ± SEM (N = 6) analyzed by one-way ANOVA (a, b, c) or two-way ANOVA (d) followed by Newman-Keuls post hoc test. Asterisk indicates significant differences compared to control (P < 0.05)
In accordance with the results described in the previous paragraph (one-way ANOVA for the total number of adult flies), two-way ANOVA yielded a significant main effect of copper (F(4,50) = 23.60; P < 0.0001). Post hoc comparisons indicated that the number of eclosions in females (0.4–1 mM) and males (0.75 and 1 mM) was decreased by CuSO4 (Fig. 1d). In contrast, the main effect of sex was not significant (P = 0.596), which was a consequence of the similar proportion of male and female hatchlings in all groups (Fig. 1d and Table 1). The means ± SEM of the groups was 40.16 ± 4.11 (males) and 40.47 ± 3.69 (females) in control flies, 31.0 ± 0.73 (males) and 35 ± 0.73 (females) in 0.1 mM CuSO4 group, 28.5 ± 4.24 (males) and 29.6 ± 2.07 (females) in 0.4 mM CuSO4 group, 19.16 ± 2.24 (males) and 15.5 ± 4.08 (females) in 0.75 mM CuSO4 group, and 9.00 ± 0.36 (males) and 17.0 ± 0.63 (females) in 1 mM CuSO4 group.
Weight of Larvae Exposed to Cu2+
The means (± SEM) of the weight (in mg) of the larvae were 3.34 ± 0.09 (control), 3.10 ± 0.04 (0.1 mM CuSO4), 2.89 ± 0.03 (0.4 mM CuSO4), 2.20 ± 0.05 (0.75 mM CuSO4), and 1.88 ± 0.12 (1.0 mM). Statistical analysis indicated a significant reduction in the larvae weight in the animals exposed to Cu2+ (0.4–1 mM) (F(4,25) = 60.62, P < 0.0001), when compared to control group (Fig.2).
Acetylcholinesterase Activity in the Larvae Treated with Cu2+
One-way ANOVA indicated a significant effect of copper (F(4,25) = 9.11; P = 0.0001) in the activity of larval AChE. The means (± SEM) of AChE activity in the larvae exposed to 0.4 (3.15 ± 0.31), 0.75 (3.23 ± 0.14), and 1 mM Cu2+ (3.05 ± 0.55) were significantly higher than in the control group (1.90 ± 0.10). The concentration of 0.1 mM Cu2+ (1.23 ± 0.10) did not show significant differences when compared to the control group (Fig. 3).
Glutathione S-Transferase Activity in the Larvae Treated with Cu2+
One-way ANOVA has shown a significant effect of copper (F(4,25) = 38.52; P < 0.001) in larvae GST. The GST activity was increased significantly in the group exposed to 0.1 mM Cu2+ (0.47 ± 0.01) but exhibited a decrease in the group exposed to1 mM Cu2+(0.20 ± 0.01) when compared to the control group (0.28 ± 0.01) (Fig. 4). However, the groups exposed to 0.4 mM Cu2 (0.25 ± 0.02) and 0.75 mM Cu2 (0.207 ± 0.014) did not show significant differences compared to the control.
Weight of the Adult Flies Exposed to Cu2+
In adult flies, two-way ANOVA yielded a significant main effect of copper in heads (F(4,50) = 16.09; P < 0.0001; Fig.5a) in both males and females. In the flies exposed to 0.75 (0.57 ± 0.02 for males and 0.61 ± 0.02 for females) and at 1 mM of Cu2+ (0.59 ± 0.024 for males and 0.57 ± 0.003 for females), there was a decrease in the mean of the head weight when compared to the control group (1.07 ± 0.11 in males and 1.09 ± 0.04 in females).
Weights of the heads (in mg) (a), bodies (thorax + abdomen; in mg) (b), and the head-to-body weight ratio (c) of 4-day-old adult flies after 13 days of Cu2+ exposure. Data represent the mean ± SEM (N = 6) analyzed by two-way ANOVA followed by Newman-Keuls post hoc test. Asterisk indicates significant differences compared to control (P < 0.05)
A similar tendency was observed for the total body weight (Fig. 5b). The results obtained in the two-way ANOVA exhibited a main effect of copper (F(4,50) = 4.21; P < 0.0001). In accordance, the flies exposed to 0.75 (1.59 ± 0.07 in males and 1.49 ± 0.005 in females) and at 1 mM of Cu2+ (1.55 ± 0.05 in males and 1.53 ± 0.05 in females) have a reduction in the body weight means when compared to control means (2.75 ± 0.13 in males and 2.79 ± 0.19 in females).
There were no significant differences between male and females weights. The head-to-body weight ratio is being shown in Fig. 5c.
Climbing Behavior (Negative Geotaxis)
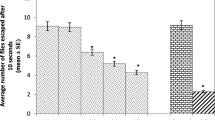
Two-way ANOVA yielded a significant main effect of copper (F(4,50) = 9.17; P < 0.001). Flies of both sexes exposed to 1 mM of Cu2+ (76.66 ± 2.10 in males and 75.0 ± 2.23 in females; data expressed as mean ± SEM of the percentage of flies that reached the 6-cm line within 6 s) exhibited a reduction in climbing ability when compared to the control group (91.66 ± 4.01 in males and 90 ± 3.65 in females (Fig. 6).
Acetylcholinesterase Activity in the Adult Flies Treated with Cu2+
In the adult flies, the AChE activity was measured separately in the head and body (thorax + abdomen). In the heads (Fig. 7a), two-way ANOVA yielded a significant main effect of copper (F(4,50) = 60.23; P < 0.0001), of sex (F(4,50) = 7.503; P < 0.0001), and copper × sex interaction (F(4,50) = 7.816; P < 0.0001). The head AChE activity was higher in females than in males (P < 0.0001) and cooper did not modify the AChE in females. However, in the head of males, 0.4 mM Cu2+ (48.37 ± 3.37) increased the enzyme activity when compared to the control group (35.56 ± 3.30). The means ± SEM of the head AChE activity in ηmol acetylthiocholine hydrolyzed/min/mg protein were 35.56 ± 3.30 (males) and 53.96 ± 2.55 (females) for the control group, 33.17 ± 1.71 (males) and 47.05 ± 1.32 (females) in 0.1 mM of Cu2+, 48.37 ± 3.37 (males) and 44.81 ± 2.21 (females) in 0.4 mM of Cu2+, 39.59 ± 0.41 (males) and 49.08 ± 1.16 (females), and 29.03 ± 0.36 (males) and 43.57 ± 2.47 (females).
AChE activity of 4-day-old adult flies after 13 days of Cu2+ exposure in the heads (a) and bodies (thorax + abdomen) (b) of 4-day-old adult flies. Data represent means ± SEM (N = 6) analyzed by two-way ANOVA followed by Newman-Keuls post hoc test. Asterisk indicates significant differences compared to control (P < 0.05)
In the bodies, two-way ANOVA yielded a significant main effect of sex (F(4,50) = 38.72; P < 0.0001), of copper (F(4,50) = 61.92; P < 0.0001), and copper × sex interaction (F(4,50) = 18.63; P < 0.0001). In contrast to the head, the AChE in the body of males was higher than in females (P < 0.0001). Copper inhibited the activity both in females and males, but with greater effectiveness in males than in females (Fig. 7b). The means (± SEM) of the AChE activity (in ηmol/min/mg protein) were 18.40 ± 0.66 (males) and 10.54 ± 0.41 (females) for the control group, 9.19 ± 1.64 (males) and 9.26 ± 0.41 (females) in the groups treated with 0.1 mM of Cu2+, 11.66 ± 0.76 (males) and 7.79 ± 0.64 (females) in 0.4 mM of Cu2+, 7.17 ± 0.27 (males) and 6.93 ± 1.53 (females) in the groups treated with 0.75 mM of Cu2+, and 5.90 ± 0.59 (males) and 6.33 ± 0.62 (females) in the groups treated with 1 mM Cu2+.
Glutathione S-Transferase Activity in Adult Flies Treated with Cu2+
In the heads (Fig. 8a), two-way ANOVA yielded significant main effects of Cu+2 (F(4,50) = 5.16; P = 0.0015), sex (F(4,50) = 6.97; P = 0.011), and Cu+2 × sex interaction (F(4,50) = 20.86; P < 0.0001). In both sexes, CuSO4 decreased the GST activity at intermediate concentrations (0.4 mM); and at the highest concentration (1 mM), Cu2+ increased the activity in the heads of males. The means (± SEM) of the GST activity (in delta absorbance at 340 nm or deltaA340/min/mg protein) were 0.095 ± 0.006 (males) and 0.088 ± 0.01 (females) for the control group, 0.107 ± 0.009 (males) and 0.074 ± 0.01 (females) in the flies treated with 0.1 mM of Cu2+, 0.05 ± 0.001 (males) and 0.065 ± 0.006 (females) in the flies treated with 0.4 mM of Cu2+, 0.085 ± 0.009 (males) and 0.09 ± 0.0009 (females) in the flies treated with 0.75 mM of Cu2+, and 0.144 ± 0.09 (males) and 0.105 ± 0.004 (females) in the flies treated with 1 mM of Cu2+.
Glutathione S-transferase activity of 4-day-old adult flies after 13 days of Cu2 exposure in the heads (a) and bodies (thorax + abdomen) (b). Data represent means ± SEM (N = 6) analyzed by two-way ANOVA followed by Newman-Keuls post hoc test. Asterisk indicates significant differences compared to control (P < 0.05)
In the bodies (thorax + abdomen) (Fig. 8b), two-way ANOVA revealed a significant main effects of copper (F(4,50) = 23, 57, P < 0.0001), sex (F(4,50) = 64.08; P < 0.0001), and copper × sex interaction (F(4,50) = 4.045; P = 0.0064). As observed in the heads, low and intermediate concentrations of Cu2+ decreased the GST in males and females bodies (though the effect was more pronounced in males). In addition, the highest concentration increased the body GST activity in females and males (but the effect tended to be robust in females). The means (± SEM) of the GST activity (delta A340/min/mg protein) were 0.201 ± 0.009 (males) and 0.252 ± 0.01 (females) for the control group, 0.126 ± 0.003 (males) and 0.228 ± 0.015 (females) in flies treated with 0.1 mM of Cu2+, 0.210 ± 0.006 (males) and 0.227 ± 0.015(females) in flies treated with 0.4 mM of Cu2+, 0.171 ± 0.002 (males) and 0.213 ± 0.016 (females) in flies treated with 0.75 mM of Cu2+, and 0.243 ± 0.001 (males) and 0.304 ± 0.013 (females) in flies treated with 1 mM of Cu2+.
Discussion
The fruit fly D. melanogaster has been widely used as an alternative model to study copper metabolism and its dyshomeostasis [23, 25, 52, 53, 57, 66, 67]. In accordance with other studies [7, 51, 55, 57, 68], here, we have observed that copper decreases the development and the survival rate of larvae, pupae, and adults of D. melanogaster. From 0.4 to 1 mM, Cu2+ caused a concentration-dependent decrease in the number of larvae, pupae, and adults, whereas the dose of 0.1 mM Cu2+ did not cause overt toxicity. Literature data have indicated that exposure of D. melanogaster to 50 μg/mL (≈ 0.8 mM) copper sulfate exhibited around 20% developmental loss in the larval stage and delayed the time to pupation by 10–18 h. In fact, the effect of copper was dependent on the concentration and exposure to concentrations higher than 2.5 mM for long periods (from egg to adult period) caused a drastic reduction in survival [7, 55].
Adequate food intake is essential for life and regulated by complex metabolic mechanisms and specific neural circuits [68, 69]. Our results indicated a severe effect of Cu2+ (0.4 to 1 mM) in larvae weight gain when compared to the control group. However, in the adults, only the two highest doses (0.75 and 1 mM of Cu2+) caused a decrease in body weight. The loss of weight may be associated with an aversion to the diets containing a high content of copper or to a direct toxic effect of accumulated copper in critical metabolic processes [57, 70].
Copper toxicity can cause locomotor dysfunctions [57, 71,71,73] and the negative geotaxis can be used to determine locomotor deficits in flies [57, 74, 75]. The deleterious effects of Cu2+ in the locomotor ability of flies can be explained by copper-induced dysfunctions in the nervous system as observed in vertebrates [76,76,78]. The behavioral results obtained here are in agreement with previous studies from our laboratory with D. melanogaster, where exposure to 1 and 3 mM of CuSO4 for 4 days caused a significant inhibition of total AChE activity and an impairment in the climbing ability (negative geotaxis) of adult flies. Here, we observed that both male and female flies exposed to 1 mM of CuSO4 for 13 days had an impairment in climbing ability and inhibition of body AChE (abdomen + thorax), but not in the head. Since in the previous study [57], the activity was determined in the entire flies, we may suggest that the effects of Cu2+ in AChE in the thorax + abdomen was more related to AChE inhibition in the ganglia than in the brain (head). The abdominal ganglia mediate the larval locomotion and are intrinsically associated with aminergic and cholinergic transmission [79]; consequently, an increase or a decrease in AChE can disrupt the locomotion and other behaviors of larvae and adult flies.
As discussed above, previous studies have indicated that climbing behavior can be impaired in flies exposed to 0.5–15 mM of copper [57, 80]. Here, in the adult flies, the effect of Cu2+ in the activity of AChE varied depending on the region used (head or bodies (thorax + abdomen)), on the concentration of CuSO4, and on the sex of the flies. As a role, the intermediate concentrations of CuSO4 increase the AChE activity in males’ head and did not modify the head AChE activity in the females. Furthermore, the AChE activity in the females’ head was higher than that of males. In contrast, the body (thorax + abdomen) AChE activity was higher in males than in females, and the enzyme was inhibited in both sexes, but with higher potency in males. The sexual and body region differences in enzyme activities may be related to sexually dimorphic responses to endogenous or xenobiotics stressors in flies [81,81,82,83,84,85,86,87,89], which have been linked to complex neurochemical, metabolic, and hormonal differences in male and female flies [81, 82, 86, 89].
The inhibition of AChE activity might be explained by copper-induced protein unfolding and, subsequently, wrong aggregation. In addition, metals have a capacity to bind to functional groups of proteins, such as imidazole, sulfhydryl, and carboxyl groups, leading to a decrease in AChE activity. Although the method of Elmann’s is not appropriate to determine the in vitro effect of metals that can oxidized thiol groups [90], Cu2+ can interact and inhibit AChE directly [90]. Thus, the protein-metal interaction can compromise the AChE catalytic activity and, consequently, can cause stable enzyme dysfunction. However, the concentration of copper required to inhibit the AChE or ChE in vitro is high (10 and 20 mmol/L) and in vivo, the exposure to low concentrations (0.06 mg/L or ≈ 1 μM) of Cu2+ for 2 days caused an increase in AChE activity in fish [91]. Thus, after in vivo exposure to Cu2+, the effects observed here in larvae and flies, as well as in previous studies with adult flies [57], were possibly indirect.
In relation to GST, the activity increased in larvae exposed to 0.1 mM Cu2+ but decrease in Cu2+ 1 mM. The inhibition of GST by metals may have occurred via the production of ROS that can interact directly with the enzyme, or can indirectly deplete its substrate (glutathione—GSH), and/or downregulation the expression of GST genes [92]. However, the GST activity increased in the heads of adult male flies, and in the bodies of males and females flies exposed to 1 mM Cu2+, when compared to the control. In addition, female flies presented a higher activity in bodies GST when compared to males. Thus, in adults, copper may have induced the expression of GST genes. Similarly, studies performed in fish have shown that glutathione S-transferase activity was significantly increased in the kidney, liver, and gills when exposed to copper nanoparticles (100 μg/L or ≈ 1.6 μM) when compared to control [93, 94]. In this view, D. melanogaster, such as humans, has orthologue glutaredoxins enzymes that regulate the redox activity on chaperones copper bound sites. The glutaredoxins enzymes, in accordance with our results, have shown a dose-dependent increase in activity and expression when exposed to copper [95].
Conclusion
In conclusion, the exposure of D. melanogaster to Cu2+ from the egg to the adult period disrupted the locomotor behavior of adults and enzymatic parameters in larvae and adults. In fact, the development was delayed in a dose-dependent manner. The weight of larvae and adult flies was decreased at the two highest doses of CuSO4, and the locomotor activity was negatively affected by the highest dose tested. These effects may be related to the changes observed in AChE and GST activities. In short, further studies are necessary to investigate the mechanisms involved in copper-induced toxicity at specific stages of D. melanogaster development.
References
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Bahadorani S, Mukai S, Egli D, Hilliker AJ (2010) Overexpression of metal-responsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol Aging 31:1215–1226
Mercer SW, Wang J, Burke R (2017) In vivo modeling of the pathogenic effect of copper transporter mutations that cause Menkes and Wilson diseases, motor neuropathy, and susceptibility to Alzheimer’s disease. J Biol Chem 292(10):4113–4122
Schlichting D, Sommerfeld C, Müller-Graf C, Selhorst T, Greiner M, Gerofke A, Ulbig E, Gremse C, Spolders M, Schafft H, Lahrssen-Wiederholt M (2017) Copper and zinc content in wild game shot with lead or non-lead ammunition – implications for consumer health protection. PLoS One 12(9):e0184946
Alaraby M, Hernández A, Marcos R (2017) Copper oxide nanoparticles and copper sulphate act as antigenotoxic agents in drosophila melanogaster. Environ Mol Mutagen 58(1):46–55
Eskici G, Axelsen PH (2012) Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry 51:6289–6311
Ballinski MA, Woodruff R (2017) Differential sexual survival of Drosophila melanogaster on copper sulfate. Genetica 145:131–137
Barceloux DG (1999) Copper. J Toxicol. Clin Toxicol 37(2):217–230
Montes S, Rivera-Mancia S, Diaz-ruiz A, Tristan-Lopez L, Rios C (2014) Copper and copper proteins in Parkinson’s disease. Oxidative Med Cell Longev 147251
Osredkar J, Sustar N (2011) Copper and zinc, biological role and significance of copper/zinc imbalance. Journal of Clinical Toxicology S:3
Squitti R, Ventriglia M, Barbati G, Cassetta E, Ferreri F, Dal Forno G, Ramires S, Zappasodi F, Rossini PM (2007) ‘Free’ copper in serum of Alzheimer’s disease patients correlates with markers of liver function. J Neural Transm 114:1589–1594
Kieffer DA, Medici V (2017) Wilson disease: at the crossroads between genetics and epigenetics - a review of the evidence. Metabolic Brain Disease Vol. 20, No. 4
Kitzberger R, Madl C, Ferenci P (2005) Wilson disease metabolic brain disease 20, No 4, 295–302
Southon A, Burke R, Camakaris J (2013) What can flies tell us about copper homeostasis? Metallomics 5:1346–1356
Strausak D, Mercer JFB, Dieter HH, Stremmel W, Multhaup G (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 55(2):175–185
Bandmann O, Heinz KW, Kaler SG (2015) Wilson’s disease and other neurological copper disorders. Lancet Neurol 14:103–113
Behzadfar L, Abdollahi M, Sabzevari O, Hosseini R, Salimi A, Naserzadeh P, Sharifzadeh M, Pourahmad J (2017) Potentiating role of copper on spatial memory deficit induced by beta amyloid and evaluation of mitochondrial function markers in the hippocampus of rats. Metallomics 9:969–980
Letelier ME, Lepe AM, Fáundez M, Salazar J, Maríın R, Aracena P, Speisky H (2005) Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact 151:71–82
Letelier ME, Sánchez-Jofré S, Peredo-Silva L, Cortés-Troncoso J, Aracena-Parks P (2010) Mechanisms underlying iron and copper ions toxicity in biological systems: pro-oxidant activity and protein-binding effects. Chem Biol Interact 188:220–227
Barresi V, Trovato-Salinaro A, Spampinato G, Musso N, Castorina S, Rizzarelli E, Condorelli DF (2016) Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation ofSLC31A1, SCO1, andCOX11in colorectal cancer. FEBS Open Bio. 6(8):794–806
Hatori Y, Lutsenko S (2013) An expanding range of functions for the copper chaperone/antioxidant protein Atox1. 19(9):945–957. Antioxid Redox Signal
Peña MMO, Lee J, Thiele DJ (1999) A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 129:1251–1260
Balamamurugan K, Egli D, Hua H, Rajaram R, Seisenbacher G, Georgiev O, Schaffner W (2007) Copper homeostasis in Drosophila by complex interplay of import, storage and behavioral avoidance. EMBO J 21; 26(4):1035–1044
Markossian KA, Kurganov BI (2003) Copper chaperones, intracellular copper trafficking proteins. Function, structure, and mechanism of action. Biochem Mosc 68(8):827–837
Hua H, Günter V, Georgiev O (2011) Distorted copper homeostasis with decreased sensitivity to cisplatin upon chaperone Atox1 deletion in Drosophila. Biometals 24:445–453
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Ottesen R, Gorham S, Pettengill J, Rideout S, Evans P, Brown E (2015) The impact of systemic and copper pesticide applications on the phyllosphere microflora of tomatoes Andrea. J Sci Food Agric 95(5):1116–1125
Georgopoulos PG, Roy A, Yonone-Lioy MJ, Opiekun RE, Lioy PJ (2011) Envinronmetal copper: its dynamics and human exposure issues. J Toxicol Environ Health, Part B: Crit Rev 4(4):341–394
Carmona ER, Inostroza-Blancheteau C, Obando V, Rubio L, Marcos R (2015) Genotoxicity of copper oxide nanoparticles in Drosophila melanogaster. Mutat Res 791:1–11
Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30:499–511,7
Mashock MJ, Zanon T, Kappell AD, Petrella LN, Andersen EC, Hristova KR (2016) Copper oxide nanoparticles impact several toxicological endpoints and cause neurodegeneration in Caenorhabditis elegans. PLoS One 11(12):e0167613
Baek YW, Na Y (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Grey B, Steck TR (2001) Concentrations of copper thought to be toxic to Escherichia coli can induce the viable but nonculturable condition. Appl Environ Microbiol 11: 5325–5327
Kasemets K, Suppi S, Kunnis-Beres K, Kahru A (2013) Toxicity of CuO nanoparticles to yeast Saccharomyces cerevisiae BY4741 wild-type and its nine isogenic single-gene deletion mutants. Chem Res Toxicol 26(3):356–367
Greco MA, Hrab DI, Magner W, Daniel JK (1990) Cu, Zn superoxide dismutase and copper deprivation and toxicity in Saccharomyces cerevisiae. J Bacteriol 172:317–325
Boyd WA, Williams PL (2003) Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ Toxicol Chem 11:2768–2774
Yu ZY, Zhang J, Yin DQ (2012) Toxic and recovery effects of copper on Caenorhabditis elegans by various food-borne and water-borne pathways. Chemosphere 11:1361–1367
Bazar MA, Quinn MJ, Mozzachio K, Bleiler JA, Archer CR, Phillips CT, Johnson MS (2009) Toxicological Responses of Red-Backed Salamanders (Plethodon cinereus) to Soil Exposures of Copper. Arch Environ Arch Environ Contam Toxicol 57:116–122
Semisch A, Ohle J, Witt B, Hartwig A (2014) Cytotoxicity and genotoxicity of nano and microparticulate copper oxide: role of solubility and intracellular bioavailability. Part Fibre Toxicol 11:10
Merker K, Hapke D, Reckzeh K, Schmidt H, Lochs H, Grune T (2005) Copper related toxic effects on cellular protein metabolism in human astrocytes. Biofactors 24(1–4):255–261
Tchounwou PB, Newsome C, Williams J, Glass K (2008) Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Metal Ions Biol Med 10:285–290
Song L, Connolly M, Fernández-Cruz ML, Vijver MG, Fernández M, Conde E, de Snoo GR, Peijnenburg WJ, Navas JM (2014) Species-specific toxicity of copper nanoparticles among mammalian and piscine cell lines. Nanotoxicology 8(4):383–393
Vecchio G (2015) A fruit fly in the nanoworld: once again Drosophila contributes to environment and human health. Nanotoxicology 9:135–137
Demir E, Turna F, Vales G, Kaya B, Creus A, Marcos R (2013) In vivo genotoxicity assessment of titanium, zirconium and aluminium nanoparticles, and their microparticulated forms, in Drosophila. Chemosphere 93:2304–2310
Zhou H, Cadigan KM, Thiele DJ (2003) A copper-regulated transporter required for copper acquisition, pigmentation, and specific stages of development in Drosophila melanogaster. J Biol Chem 278(48):48210–48218
Hwang JEC, De Bruyne M, Warr CG, Burke R (2014) Copper overload and deficiency both adversely affect the central nervous system of Drosophila. Metallomics 6(12):2223–2229
Pérez-Rafael S, Kurz A, Guirola M, Capdevila M, Palaciosa O, Atrian S Is MtnE, the fifth Drosophila metallothionein, functionally distinct from the other members of this polymorphic protein family. Metallomics 4:342–349
Abolaji AO, Kamdem JP, Lugokenski TH, Nascimento TK, Waczuk EP, Farombi EO, Loreto ÉL, Rocha JB (2014) Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic Biol Med 82:204–205
Abolaji AO, Babalola OV, Adegoke AK, Farombi EO (2017) Hesperidin, a citrus bioflavonoid, alleviates trichloroethylene-induced oxidative stress in Drosophila melanogaster. Environ Toxicol Pharmacol 55:202–207
Calap-Quintana P, González-Fernández J, Sebastiá-Ortega N, Lorens JV, Moltó, M. D (2017) Drosophila melanogaster models of metal-related human diseases and metal toxicity Int J Mol Sci, 18 (7), art. no. 1456
Massadeh A, Al-Momani F, Elbetieha A (2008) Assessment of heavy metals concentrations in soil samples from the vicinity of busy roads: influence on Drosophila melanogaster life cycle. Biol Trace Elem Res 122:292–299
Egli D, Yepiskoposyan H, Selvaraj A, Balamurugan K, Rajaram R, Simons A, Multhaup G, Mettler S, Vardanyan A, Georgiev O, Schaffner W (2006) A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol Cell Biol 26(6):2286–2296
Han X, Geller B, Moniz K, Das P, Chippindale AK, Walker VK (2014) Monitoring the developmental impact of copper and silver nanoparticle exposure in Drosophila and their microbiomes. Sci Total Environ 487:822–829
Christie NT, David G, Gosslee DG, Bate LC, Jacobson KB (1983) Quantitative aspect of metal ion content and toxicity in Drosophila. Toxicology 26:295–312
Lenaerts C, Cools D, Verdonck R, Verbakel L, Broeck JV, Marchal E (2017) The ecdysis triggering hormone system is essential for successful moulting of a major hemimetabolous pest insect, Schistocerca gregária. Sci Rep 7:46502
Ding L, Wang Y (2006) Effect of copper on the development, protein and esterase isozymes of Drosophila melanogaster. Integrative Zool 2:73–77
Adedara IA, Klimaczewski CV, Barbosa NBV, Farombi EO, Souza DO, Rocha JBT (2015) Influence of diphenyl diselenide on chlorpyrifos-induced toxicity in Drosophila melanogaster. J Trace Elem Med Biol 32:52–59
Klimaczewski CV, Ecker A, Piccoli B, Aschner M, Barbosa NV, Rocha JBT (2018) Peumus boldus attenuates copper-induced toxicity in Drosophila melanogaster. Biomed Pharmacother 97:1–8
Rand MD, Montgomery SL, Prince L, Vorojeikina D (2014) Developmental toxicity assays using the Drosophila mode. Curr Protoc Toxicol 1.12:1-1.12.20
Ali YO, Escala W, Ruan K R. Zhai RG (2011) Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments (Jove) 49
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32:448–466
Chernoff N, Hill DJ, Chorus I, Diggs DL, Huang H., King D., Lang J. R, Le T.-T., Schmid J. E, Travlos GS, Whitley EM, Wilson RE Wood CR (2018) Cylindrospermopsin toxicity in mice following a 90- d oral exposure. J Toxicol Environ Health, Part A, 1528–7394 (Print) 1087–2620 (Online)
Ellman GL (1959) Tissue sulphydril groups. Arch Biochem Biophys 82(1):70–77
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Navarro JA, Schneuwly S (2017) Copper and zinc homeostasis: lessons from Drosophila melanogaster. Front Genet 8:223
Balamurugan K, Schaffner W (2006) Copper hoeostasis in eukaryotes: teetering on a tightrope. Biochim Biophys Acta 1763:737–746
Momani FA, Massadeh AM (2005) Effect of different heavy-metal concentrations on Drosophila melanogaster larval growth and development biological trace element research 108 (1–3)-0271
Garlapow ME, Huang W, Yarboro MT, Peterson KR, Mackay TFC (2015) Quantitative genetics of food intake in Drosophila melanogaster. PLoS One 10(9):0138129
Ferri A, Duffard R, Stürtz N, Duffard AME (2003) Iron, zinc and copper levels in brain, serum and liver of neonates exposed to 2,4-dichlorophenoxyacetic acid. Neurotoxicol Teratol 25:607–613
Siddique YH, Haidari M, Khan W, Fatima A, Jyoti S, Khanam S, Naz F, Rahul, Ali F, Singh BR, Beg T, Mohibullah, Naqvi AH (2015) Toxic potential of copper-doped ZnO nanoparticles in Drosophila melanogaster (Oregon R). Toxicol Mech Methods 25(6):425–432
Zhang T, Xu L, Wu J, Wang W, Mei J, Ma X, Liu J (2015) Transcriptional responses and mechanisms of copper-induced dysfunctional locomotor behavior in zebrafish embryos. Toxicol Sci 148(1):299310
Abbaoui A, Hiba OE, Gamrani H (2016) Copper poisoning induces neurobehavioral features of Parkinson’s disease in rat: alters dopaminergic system and locomotor performance. Abstracts/Parkinsonism and related disorders 22 e149ee192
Nichols CD, Becnel J, Pandey UB (2012) Methods to assay Drosophila behavior. J Vis Exp 61:3795
Madabattula ST, Strautman JC, Bysice AM, O’Sullivan JA, Androschuk A, Rosenfelt C, Doucet K, Rouleau G, Bolduc F (2015) Quantitative analysis of climbing defects in a Drosophila model of neurodegenerative disorders. J Vis Exp 100:52741
Sun Q, Ying M, Ma Q, Huang Z, Zou L, Liu J, Zhuang Z, Yang X (2016) Proteomic analysis of hippocampus in mice following long-term exposure to low levels of copper. Toxicol Res 5:1130–1139
Cristóvão JS, Santos R, Gomes CM (2016) Metals and neuronal metal binding proteins implicated in Alzheimer’s disease Oxidative Medicine and Cellular Longevity 9812178
Sun Y, Zhang G, He Z, Wang Y, Cui J, Li Y (2016) Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae. Int J Nanomedicine 11:905–918
Pauls D, Essen AV, Lyutova R, Giesen LV, Rosner R, Wegener C, Sprecher SG (2015) Potency of transgenic effectors for neurogenetic manipulation in Drosophila larvae. Genetics 199(1):25–37
Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C (2011) Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study Parkinsonism. Biometals 24:1045–1057
Pomatto LCD, Wong S, Tower J, Davies KJA (2018) Sex-specific adaptive homeostasis in D. melanogaster depends on increased proteolysis by the 20S proteasome: data-in-brief. Data Brief 17:653–661
Pomatto LCD, Wong S, Tower J, Davies KJA (2017) Sexual dimorphism in oxidant-induced adaptive homeostasis in multiple wild-type D. melanogaster strains. Arch Biochem Biophys 636:57–70
Signor SA, Abbasi M, Marjora P, Nuzhdin SV (2017) Conservation of social effects (Ψ) between two species of Drosophila despite reversal of sexual dimorphism. Ecol Evol 7(23):10031–10041
Chandegra B, Tang JLY, Chi H, Alic N (2017) Sexually dimorphic effects of dietary sugar on lifespan, feeding and starvation resistance in Drosophila. Aging 9(12):2521–2528
De Nobrega AK, Lyons LC (2016) Circadian modulation of alcohol-induced sedation and recovery in male and female Drosophila. J Biol Rhythm 31(2):142–160
Gruntenko NE, Karpova EK, Burdina EV, Adonyeva NV, Andreenkova OV, Alekseev AA, Rauschenbach IY (2016) Probable mechanism of sexual dimorphism in insulin control of Drosophila heat stress resistance. Physiol Entomol 41(1):59–66
Neckameyer WS, Nieto-Romero AR (2015) Response to stress in Drosophila is mediated by gender, age and stress paradigm. Stress 18(2):254–266
Smith LA, Habib I, Shirkey S, Talon B, Milne A, Nadolski J (2011) Sexual dimorphism in the effect of a taurine supplemented diet on life span in adult Drosophila melanogaster. Int J Zool Res 7(1):34–48
Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE (2010) Sexual dimorphism in the fly brain. Curr Biol 20(18):1589–1601
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2004) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 1; 10(5):360–375
Lima D, Roque GM, Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51
Roling JA, Baldwin WS (2006) Alterations in hepatic gene expression by trivalent chromium in Fundulus heteroclitus. Mar Environ Res 62:122–127
Gupta YR, Sellegounder D, Kannan M, Deepa S, Senthilkumaran B, Basavaraju Y (2016) Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): biochemical, histological and proteomic approaches. Aquac Fish 1:15–23
Carvalho CS, Bernusso VA, Araújo HSS, Espíndola ELG, Fernandes MN (2012) Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 89:60–69
Mercer SW, Burke R (2016) Evidence for a role for the putative Drosophila hGRX1 orthologue in copper homeostasis. Biometals 29:705–713
Funding
This study received financial support from the Institutional Scholarship Program (PIBIC), Brazilian National Council for Scientific and Technological Development (CNPq), Coordination of Improvement of Higher Level Personnel (CAPES), Financier of Studies and Projects (FINEP), and Foundation of Support to the State of Rio Grande do Sul research (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Halmenschelager, P.T., da Rocha, J.B.T. Biochemical CuSO4 Toxicity in Drosophila melanogaster Depends on Sex and Developmental Stage of Exposure. Biol Trace Elem Res 189, 574–585 (2019). https://doi.org/10.1007/s12011-018-1475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1475-y