Abstract
Inhibitory synaptic receptors are dysfunctional in epileptic brains, and agents that selectively target these receptors may be effective for the treatment of epilepsy. MicroRNAs interfere with the translation of target genes, including various synaptic proteins. Here, we show that miR-203 regulates glycine receptor-β (Glrb) in epilepsy models. miR-203 is upregulated in the hippocampus of epileptic mice and human epileptic brains and is predicted to target inhibitory synaptic receptors, including Glrb. In vitro transfection, target gene luciferase assays, and analysis of human samples confirmed the direct inhibition of GLRB by miR-203, and AM203, an antagomir targeting miR-203, reversed the effect of miR-203. When intranasal AM203 was administered, AM203 reached the brain and restored hippocampal GLRB levels in epileptic mice. Finally, intranasal AM203 reduced the epileptic seizure frequency of mice. Overall, this study suggests that GLRB expression in the epileptic brain is controlled by miR-203, and intranasal delivery of AM203 showed therapeutic effects in chronic epilepsy mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is the most common and serious neurological disease, affecting 65 million people worldwide [1]. Although the number of available anti-epileptic drugs is increasing, one third of patients remain resistant to current medications [2], and there is an urgent need for more effective treatments based on novel mechanisms of action [3]. Epilepsy is associated with aberrant excitatory neurotransmission due to dysfunctional inhibitory ion channels and neurotransmitters such as gamma-aminobutyric acid (GABA) and glycine signals [2]. Binding of glycine to post-synaptic glycine receptors leads to the opening of an anion channel, resulting in an influx of Cl− ions, which hyperpolarizes the post-synaptic membrane and reduces neuronal firing [4]. Inhibition or mutations of this receptor cause neurons to be over-excited, resulting in pain, cramping, and hyperekplexia [5]. Several anti-convulsive drugs, such as topiramate and alpidem, interact with glycine receptors [6, 7].
MicroRNAs (miRNAs) are 21- to 23-nucleotide RNAs that post-transcriptionally regulate gene expression by promoting the degradation of target mRNAs or by repressing their translation [8]. miRNAs are implicated in a variety of neurological diseases, such as stroke [9], Parkinson’s disease [10], and Alzheimer’s disease [11]. In epilepsy, changes in miRNA expression have been reported in the brains of mice with pilocarpine- and kainate-induced status epilepticus (SE) [12–16] and in the temporal lobe of patients with epilepsy [17, 18]. In acute SE mice, silencing miR-34a, miR-132, or miR-184 produces neuroprotection [13, 14, 19, 20]. However, miRNA-based therapeutic agents targeting chronic epilepsy have not yet been developed.
Inhibitory neurotransmission in the central nervous system is tightly controlled [21–23]; therefore, we suspected that excess suppression of target mRNAs by miRNAs participates in the pathogenesis of epilepsy. To investigate this possibility, we analyzed miRNA expression in the human epileptic hippocampus as well as in mice with pilocarpine-induced chronic epilepsy, which is characterized by spontaneous recurrent seizures (SRS). We then studied the gene-miRNA interactions and the in vivo therapeutic effect of an antagomir against an overexpressed target miRNA.
Methods
Generation of Chronically Epileptic Mice
This study was approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital, South Korea, and all experiments have been performed in accordance with the approved guidelines and regulations. The committee is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Every effort was made to limit the number of animals used. ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the Basel declaration were considered when planning the experiments.
Male C57BL/6J mice (22 to 25 g, 5 weeks old) were treated with methylscopolamine (1 mg/kg i.p., Sigma-Aldrich, St. Louis, MO, USA). After 30 min, the mice received a single i.p. injection of pilocarpine (330 mg kg−1, Sigma-Aldrich) to induce SE. The onset of SE was defined as the beginning of continuous tonic-clonic behavioral seizures after several discontinuous convulsive seizures (Racine scale ≥ IV) [24]. The mice were injected intraperitoneally with diazepam (5 mg/kg, Samjin Pharmaceuticals, Seoul, South Korea) 40 min after the onset of SE to terminate prolonged seizures. After SE, all animals were fed a 5 % glucose solution for 2 days until they began to consume normal food pellets. Control mice were wild-type littermates of SRS mice and only received methylscopolamine. Chronic epilepsy was defined as frequent attacks of SRS (4–5 on Racine’s scale) 60 days after SE.
In Vivo Electrophysiology and Video Monitoring of SRS
In vivo electroencephalography and surgery were performed as previously described [25]. For electroencephalographic monitoring, the mice were subjected to electroencephalographic surgery 2 weeks before the injection of pilocarpine. For surgery, the animals were anesthetized by intraperitoneal injection of 1 % ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg). The surgery was performed using a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Electroencephalograms were obtained with tungsten electrodes (0.005 in., 2 MΩ), which were positioned onto the right hemisphere at AP −1.8 mm, L 2.1 mm, and DV 0.8–1.0 mm (cortex) from the bregma with grounding over the cerebellum. The electrical activities were recorded after amplification (×1200), bandpass filtering from 0.1 to 70 Hz, and digitization at a 400 Hz sampling rate (AS 40) with a digital electroencephalography system (Comet XL; Astro-Med, Inc., Warwick, RI, USA). Electrophysiological data were analyzed offline using PSG Twin 4.2 (Astro-Med, Inc.). Electroencephalographic signals in chronically epileptic mice were continuously recorded for 14 days beginning 60 days after SE. After repeated intranasal treatments with AM203 or vehicle (48 h interval), the signals were continuously recorded for another 14 days. Continuous EEG recordings were combined with video monitoring. In the electro-video monitoring, SRS was defined as convulsive seizures (Racine stage 4–6)24 along with epileptic spikes on electroencephalography. Data from all 14 days were averaged for both the before and after treatment portions of the experiment.
miRNA Microarray
The mice were deeply anesthetized and decapitated 60 days after pilocarpine-induced SE. The brains were immediately removed, and the hippocampus and whole cortex were dissected from the remaining brain structures. Total RNA was isolated from the hippocampi and cortices with TRIzol (Invitrogen, Carlsbad, CA, USA). We used RNA samples that met the quality control standard (260/280 > 1.8, 260/230 > 2.0, 28S/18S rRNA ratio > 1.6, and RNA integrity number > 8.0). The miRNA expression profiles were examined with a mouse miRNA microarray 8 × 15 K kit (detects 567 mouse miRNAs) according to the manufacturer’s instructions (Agilent Technologies, Inc., Santa Clara, CA, USA). Data were normalized and analyzed using GeneSpring GX, version 7.3.1 (Agilent Technologies) according to the manufacturer’s instructions. Values less than 0.01 were set to 0.01. Seven samples (three normal and four epileptic) were tested on each microarray chip. Upregulation was defined as a >2-fold increase in expression vs. control and a P value vs. control of <0.05. Downregulation was defined as a >2-fold decrease in expression vs. control and a P value vs. control of <0.05.
Human Brain Samples
Frozen human hippocampus samples were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders (Baltimore, MD, USA). The protocol was exempted from institutional review board reviews in accordance with the exemption criteria of the Institutional Review Board of Seoul National University Hospital. Epilepsy and control tissues (n = 6 per group, 1 g per sample) were matched for postmortem interval, age, and sex. Each of the 0.5 g tissues were used for RNA extraction and protein analysis.
Real-Time PCR of miRNA
Real-time PCR was performed using TaqMan® MicroRNA Assays (Life Technologies, Carlsbad, CA, USA). All real-time reactions were performed in triplicate on an ABI PRISM 7000 sequence detection system (Ambion-Applied Biosystems, Foster City, CA, USA). The relative expression was calculated using the comparative threshold cycle, and miRNA levels were normalized according to snoRNA202 (mouse) or U6 levels (human) measured using an endogenous snoRNA detection kit (Ambion-Applied Biosystems).
Prediction of miRNA Target Genes
The mature miRNA sequences were obtained from miRBase (http://www.mirbase.org). Potential miRNA target sites in the mouse gene 3′-UTR were identified using TargetScan (http://www.targetscan.org) [26], PicTar (http://pictar.mdc-berlin.de/) [27], and microT (http://diana.cslab.ece.ntua.gr/microT/) [28].
In Vitro Transfection
Neuro-2a cells (American Type Culture Collection, Manassas, VA, USA) were transfected with 50 nM of miR-203 duplex (or scrambled miRNA duplex; Bioneer, Daejon, South Korea) and 50 nM of AM203 (2′-O-methylated-5′-cua gug guc cua aac auu uca c-3′) using Lipofectamine 2000. For Western blotting, cell homogenates were obtained 48 h after transfection and were serially processed for Western blotting using antibodies against GLRB (Santa Cruz Biotechnology, Santa Cruz, CA, USA), GABRA1 (Santa Cruz), GABRA5 (Santa Cruz), GABRB2 (Santa Cruz), GABRB3 (Santa Cruz), SLC1A2 (Santa Cruz), and β-actin (Santa Cruz).
Luciferase Assay
We used transfection-ready luciferase reporter constructs bearing the 3′-UTR sequences of human Glrb (#1 from Origene, Rockville, MD, USA; #2 from SwitchDB, Menlo Park, CA, USA), Gabra1 (SwitchDB), or Gabrb3 (SwitchDB). HeLa cells were plated in RPMI media containing 10 % fetal bovine serum at 5 × 104 cells per well in 24-well plates. After 24 h, the cells were transfected with 50 nM of miRNA-203 duplexes (or scrambled miRNA duplexes; Bioneer) and 83 ng/ml of each luciferase expression vector using Lipofectamine 2000 (Life Technologies). Firefly luciferase activity was assessed after 24 h using the Luciferase Reporter 1000 Assay System (Promega, Madison, WI, USA). The relative ratios of firefly luciferase activity were used for analysis.
Western Blotting for Human Samples
Homogenates of brain tissues were prepared and serially processed for Western blotting using antibodies against GLRB (Santa Cruz Biotechnology), GABRA1 (Santa Cruz Biotechnology), and GABRB3 (Santa Cruz Biotechnology). Immunoreactive proteins were visualized with enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA) and digitally scanned using a GS-700 scanner (Bio-Rad, Hercules, CA, USA). The optical densities of each band relative to the measured values of β-actin bands were determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Treatment of Chronically Epileptic Mice with Antagomirs
Intranasal administration of antagomirs was conducted as previously described [11]. For intranasal administration of antagomirs, anesthetized mice were placed in a supine position with the head in an upright position. AM203 (5 nmol in 24 μL of 0.1 % v/v diethylpyrocarbonate-treated distilled water; Bioneer) was administered by pipette in 4-μL drops (a total of six fractions), alternating between each nostril every 2 min. Epilepsy-control mice received an equal volume of PBS, and epilepsy-scrambled mice received an equal amounts of scrambed antagomir (2′-O-methylated-5′-aag gca agc uga ccc uga agu u-3′; Bioneer).
ELISA-Based Measurement of AM203 in Brain Tissues
The measurement was performed as previously described [29]. AM203 (2.5 nM in 24 μL of 0.1 % v/v diethylpyrocarbonate-treated distilled water) was administered once in normal C57BL/6J mice. At each time point (1 h, 1 day, and 7 days; n = 4 for each time point), the brains were obtained by deep anesthesia and decapitation. The hippocampus and cortex were separated and frozen at −80 °C. Nucleic acid was extracted from the test samples with phenol:chloroform:isoamyl alcohol (25:24:1; Sigma-Aldrich). To construct a standard curve, serially diluted AM203 was added to the normal brain tissues, and nucleic acid was extracted. A 3′-biotinylated locked nucleic acid (LNA) capture probe (5′-GGACCACTAG-3′-Biotin, all nucleotides were LNAs, Exiqon, Vedbaek, Denmark) was added [0.5 pmol/well in 100 μL of 5× SSCT buffer (1× SSCT buffer: 150 nM sodium chloride, 15 mM sodium citrate, and 0.05 % v/v Tween-20)] to a streptavidin-coated 96-well plate (Thermo Fisher Scientific Inc., Waltham, MA, USA). The plate was incubated for 30 min at room temperature and then washed three times with 2× SSCT. Brain nucleic acid extracts (100 μL in 2× SSCT) from test samples or standard samples were added and incubated for 30 min at 37 °C. Then, the plate was washed three times with 2× SSCT. A 5′-DIG-conjugated LNA detection probe (DIG-5′-GTGAAATGTT-3′, all nucleotides were LNAs, Exiqon) was added (0.5 pmol/well in 100 μL of 5× SSCT), and the plate was incubated for 30 min at room temperature. The plate was then washed three times with 2× SSCT, and 100 μL of anti-DIG antibodies conjugated with alkaline phosphatase (Roche, Basel, Switzerland) was added. Next, the plate was incubated for 40 min at room temperature and was washed five times with 2× SSCT. A chemiluminescent ELISA Substrate (POD, Roche) was added to the plate, and the plate was incubated for 20 min in room temperature. After adding the stop solution (100 μL of 1 M H2SO4), the chemiluminescence was measured by a SPECTRAMAX 190 (Molecular Device, Sunnyvale, CA, USA).
Western Blotting and Correlation Analysis Between miR-203 and Target Protein Expressions
In animal studies, anesthetized mice were decapitated, and the each brain regions (hippocampus and cortex) were immediately removed. Human brain samples were serially processed for Western blotting. Homogenates of brain tissues were serially processed for Western blotting using antibodies against GLRB (Santa Cruz Biotechnology), GABRA1 (Santa Cruz Biotechnology), and GABRB3 (Santa Cruz Biotechnology). Immunoreactive proteins were visualized with enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA) and digitally scanned using a GS-700 scanner (Bio-Rad, Hercules, CA, USA). The optical densities of each band relative to the measured values of β-actin bands were determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
To visualize the tissue distribution of GLRB at 7 days after the treatment, mice (n = 2 per the treatment groups; normal, epilepsy-PBS, epilepsy-scrambled antagomir, and epilepsy-AM203) were deeply anesthetized and perfused through the heart with 10 ml of cold saline and 10 ml of 4 % paraformaldehyde in 0.1 M PBS. Three sequential coronal tissue sections of hippocampus from each mouse (500-μm interval, approximately −1.5 to −2.5 mm antero-posterior from the bregma) were stained with antibody against GLRB (Santa Cruz Biotechnology).
Statistical Analysis
Heat maps for miRNA expression were generated using the z-score, which was calculated as follows: z-score = [(raw value for the expression of each miRNA − mean value for all miRNA expression)/standard deviation of all miRNA expression]. Student’s t test was used to compare the two groups, and Kruskal–Wallis analysis of variance was used for comparisons between three or more groups. When P values from the Kruskal–Wallis test were <0.05, the Mann–Whitney U test was used for post hoc inter-group comparisons. A two-tailed P value below 0.05 indicated statistical significance. In human sample study, bivariate correlations in target protein expressions (optical density) and miR-203 levels measured by real-time PCR were assessed by the Pearson correlation coefficients, and P < 0.05 was considered to indicate statistical significance.
Results
Upregulation of miR-203 in the Epileptic Brain
We first examined miRNA expression in chronically epileptic mice 60 days after pilocarpine-induced SE using a microarray (n = 3 for normal and 4 for epileptic mice). Compared with normal mice, the hippocampus of chronically epileptic mice showed increased expression of 11 miRNAs and reduced expression of four miRNAs (Fig. 1a, Supplementary Table 1). In the cortex of chronically epileptic mice, ten miRNAs were increased, and none were reduced (Fig. 1b, Supplementary Table 1). Among these ten miRNAs, five (miR-199b, miR-203, miR-223, miR-451, and miR-455) were upregulated in both the hippocampus and cortex (Supplementary Table 1).
miR-203 upregulation in epileptic brains. miRNA expression in the hippocampus (a) and cortex (b) from mice with spontaneous recurrent seizures was analyzed by microarray. The hippocampus of epileptic mice showed increased expression of 11 miRNAs and reduced expression of four miRNAs (a). In the cortex of chronically epileptic mice, 10 miRNAs were increased (b). Panels show only the up and downregulated miRNAs as defined by a >2-fold change in expression and a P value vs. control of <0.05. Colors indicate the z-score, with red indicating higher expression (higher z-score) and green indicating lower expression (n = 3 for normal and 4 for epileptic mice). The arrows indicate miR-203. c Real-time PCR showed that miR-203 was significantly upregulated in the hippocampus of chronically epileptic mice (n = 6 per group). The expression of miR-203 is shown relative to the expression of sno202 RNA. d Real-time PCR using human hippocampal tissues showed that epilepsy patients have an increased level of miR-203 in the hippocampus compared to the control subjects (n = 6 per group). The expression of miR-203 is shown relative to the expression of U6 RNA. e Neuro2a cells were transfected with scrambled miRNA, miR-203, or AM203 (antagomir-203), and possible target proteins were analyzed. f miR-203 reduced GLRB, GABRA1, and GABRB3 expression, and AM203 neutralized the effect of miR-203 as detected by Western blotting (e) and densitometry (f) (n = 3 per group). *P < 0.05 vs. normal (c), control (d), or the other transfection groups (f)
To find a reproducible target in various epilepsy models, we compared the current expression profile with previous studies of rat and human epilepsy. Of the differentially expressed miRNAs in the current profile, only miR-203 was reproducibly upregulated in both human and rat temporal lobe epilepsy (TLE) [14, 17, 18]. miR-223 and miR-451 were upregulated in several reports regarding human TLE in the hippocampus [18]; however, these miRNAs were not upregulated in other reports of human and rat models [14, 17]. Thus, we focused on miR-203 in our subsequent studies. Real-time PCR analysis confirmed the microarray results that showed that miR-203 is significantly upregulated in the hippocampus of chronically epileptic mice (n = 6 per group, P < 0.05, Fig. 1c). In the cortex of epileptic mice, miR-203 was not upregulated in the real-time PCR analysis, indicating that the changes in microarray were not significant enough (Fig. 1c).
The sequence of miR-203 is conserved in the mouse (mmu-miR-203) and human (hsa-miR-203) genomes [26]. To reproduce the upregulation of miR-203 in human epilepsy, we measured the miR-203 level in human hippocampus samples from six epilepsy patients and six control subjects of similar age and sex (Supplementary Table 2, Supplementary Fig. 1). Because human autopsy samples undergo postmortem degradation of RNA [11], epilepsy and control samples were matched according to the postmortem intervals. This is why we could not use epilepsy surgery samples, and only limited clinical information was available. Nevertheless, real-time PCR of these samples confirmed that miR-203 is upregulated in the hippocampus of epilepsy patients compared with the controls (P < 0.05, Fig. 1d), reproducing previous reports [17, 18].
miR-203 Decreases the Glycine Receptor-β Subunit Protein Level
Due to the consistent upregulation of miR-203 in the epileptic hippocampus, we searched for miR-203 target genes using target prediction programs [26–28]. Among the possible target 3′ UTR loci, we selected the loci that were conserved between the mouse and human as well as targets that have been previously implicated in epilepsy. In this manner, the programs predicted that miR-203 may bind to the 3′-untranslated regions of Glrb (glycine receptor, β subunit), Gabra1 (GABA A receptor, subunit alpha1), Gabra5 (GABA A receptor, subunit alpha5), Gabrb2 (GABA A receptor, subunit beta 2), Gabrb3 (GABA A receptor, subunit beta 3), and Slc1a2 (glutamate transporter member 2) (Supplementary Fig. 2).
To investigate the true target genes of miR-203 among the in silico-predicted possible targets, we transfected miR-203 or antagomir-203 (AM203, an antagomir targeting miR-203) into Neuro-2a cells. Western blotting of target proteins showed that miR-203 decreased the GLRB, GABRA1, and GABRB3 protein levels only (n = 3 per group, all P < 0.05), while the other target proteins were not changed by miR-203 (Fig. 1e, f, Supplementary Fig. 3). In addition, the decrease in GLRB, GABRA1, and GABRB3 proteins in miR-203-transfected cells was antagonized by co-transfection with AM203 (n = 3 per group, all P < 0.05; Fig. 1e, f).
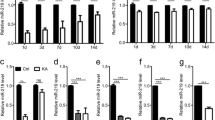
To investigate whether the observed changes in the three target genes are relevant to human epilepsy, we measured the protein levels in human hippocampus samples and correlated the protein levels with the miR-203 levels in human tissue. The results showed that GLRB and GABRA1 are reduced in our hippocampus samples (n = 6 per group, P < 0.01 and P < 0.05, respectively, Fig. 2a, b). However, only the GLRB level was well-correlated with the miR-203 level (P = 0.015, Fig. 2c), while the GABRA1 and GABR3 levels were not correlated (P = 0.236 and 0.414, respectively, Fig. 2d, e), suggesting that Glrb is a more relevant target gene involved in human epilepsy. To confirm the direct interaction between Glrb and miR-203, we transfected HeLa cells with miR-203 and luciferase vectors bearing the 3′ untranslated regions (UTR) of Glrb, Gabra1, or Gabr3. We used two different vectors for Glrb (#1 and #2). miR-203 reduced the luciferase signal of the Glrb vectors only (n = 8 per group, P < 0.01), confirming that miR-203 directly inhibits the translation of Glrb (Fig. 2f). Thus, we selected Glrb as the target gene of miR-203 in the epileptic brain.
Glrb as the target of miR-203. a The protein expression of GLRB, GABRA1, and GABRB3 was analyzed in hippocampal tissues from control and epileptic brains (n = 6 per group). b Epileptic subjects showed reduced GLRB and GABRA1 levels compared with the control subjects as detected by Western blotting (a) and densitometry (b). In a correlation analysis between miR-203 and the target protein levels, miR-203 was well correlated with GLRB levels (c) and did not correlate with the GABRA1 (d) or GABRB3 levels (e), suggesting that GLRB expression is dependent on miR-203 expression. Red dots indicate epilepsy samples and black dots show control samples. f In support of this finding, miR-203 reduced the luciferase activity of the vectors bearing the 3′-UTR region of Glrb, while miR-203 had no effect on the vectors bearing the 3′-UTRs of Gabr1 or Gabrb3 (n = 8 per group). We used two different vectors for Glrb (#1 and #2). *P < 0.05 and **P < 0.01 compared to the control
AM203 Increases GLRB Protein Levels and Reduces SRS in Mice with Chronic Epilepsy
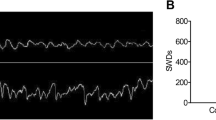
We have previously shown that an intranasally delivered antagomir reaches the brain by the olfactory bulb pathway and sufficiently inhibits hippocampal miRNAs [11]. To perform AM203 intranasal delivery, we first tested whether AM203 reaches the brain through the intranasal route. We treated normal mice with 2.5 nmol of AM203 intranasally (Fig. 3a) and measured the levels in the cortex and hippocampus at 1 h, 1 day, and 7 days after the delivery (n = 4 per group at each time point). The AM203 level peaked at 1 h and persisted for 7 days in both the cortex and hippocampus (Fig. 3b). This result suggests that intranasal administration successfully delivers AM203 to the brain.
Therapeutic effects of intranasal AM203 administration. a Anesthetized mice were placed in a supine position with the head in an upright position, and solutions were administered by pipette in 4-μL drops (a total of six fractions), alternating between each nostril every 2 min. S.-T.L drew the figure. b After one intranasal delivery, the AM203 levels peaked at 1 h and were maintained for 7 days in the cortex and hippocampus (n = 4 per group at each time point). c, d Intranasal delivery of AM203 in chronically epileptic mice (epilepsy-AM203, treatments on days 0 and 2) increased the hippocampal GLRB level compared with the control treatment (epilepsy-control) as detected by Western blotting (c, cropped gels. The full-length blot of hippocampus is presented in supplementary Fig. 2) and densitometry (d) on day 10 (n = 3 per group). In contrast, AM203 treatment had little effect on GLRB expression in the cortex. e To determine if AM203 has a therapeutic effect in an epilepsy model, we performed electroencephalographic monitoring of chronically epileptic mice for 14 days before and after the treatment with intranasal AM203 or PBS (control) at a 48-h interval. f Representative electroencephalography traces show SRS in chronically epileptic mice, and the long non-ictal period after the treatment with intranasal AM203. g SRS quantification revealed that intranasal AM203 reduced the number of SRS episodes by 71.6 % compared with the previous treatment (n = 7 per group). **P < 0.01. SE status epilepticus, SRS spontaneous recurrent seizures
To determine if intranasal AM203 is therapeutically effective for chronic epilepsy, we treated chronically epileptic mice with 5 nmol of AM203 or PBS (control) intranasally on day 0 and day 2. Western blotting of hippocampal tissues collected on day 10 showed that the GLRB protein level was lower in chronically epileptic mice than in normal mice (n = 3 per group, P < 0.01) and that intranasal AM203 increased the GLRB level (n = 3 per group, P < 0.01, Fig. 3c, d). In contrast, little amount of GLRB protein was present in the cortex, and AM203 did not detectably alter its expression (Fig. 3c, d). When we visualized the tissue distribution of GLRB in each treatment group, the GLRB expression was diffuse throughout the whole hippocampal sections (Supplementary Fig 4). Meanwhile, the epileptic mice showed similar levels of GABRA1 and GABRB3 in the hippocampus compared with the normal mice, and AM203 treatment did not increase the protein levels of GABRA1 and GABRB3 (Supplementary Fig. 5). Thus, the results showed that AM203 enhances GLRB expression, the target protein in epileptic mice.
To investigate whether this target gene modulation has therapeutic relevance, we next performed electroencephalographic monitoring of chronically epileptic mice for 14 days, treated them with intranasal AM203 or PBS (control) at 48-h intervals, and monitored the mice for another 14 days (n = 7 per group, Fig. 3e). The frequency of spontaneous recurrent seizure (SRS) episodes was similar before and after treatment in mice administered PBS (mean number of episodes/14 days ± standard deviation = 36.3 ± 13.3 before treatment vs. 32.7 ± 9.9 after treatment; P = 0.69; Fig. 3f, g). In contrast, the seizure frequency was significantly reduced by AM203 treatment (36.6 ± 15.5 episodes/14 days before treatment vs. 10.4 ± 10.5 episodes/14 days after treatment; P = 0.003; Fig. 3f, g). This result showed that AM203 has an anti-epileptic effect in chronically epileptic mice.
Discussion
This study showed that miR-203 is increased in the hippocampus of mice with pilocarpine-induced chronic epilepsy and human epileptic brains and targets the Glrb gene. Intranasal administration of the antagomir of miR-203, AM203, restored GLRB expression and reduced the frequency of seizures in chronically epileptic mice, suggesting that an miR-203-GLRB axis is involved in the production of seizures, and AM203 may be a candidate for anti-epileptic drug development.
Because single miRNA represses the production of hundreds of proteins [26], we should note that GLRB is not the only source of the anti-epileptic effect of AM203; multiple proteins and pathways may be involved, and GABRA1, GABRB3, and other genes might be still one of them. Especially, it has been previously suggested that miR-203 can inhibit GABRA1 expression [30]. In addition, it would be possible that miR-203 affects various target genes over time during epileptogenesis. Deep sequencing of Argonaute-bound RNAs before and after the AM203 treatment might help to understand more about the therapeutic mechanism of AM203. Nevertheless, the modulation of the glycine receptor by miR-203 in our data provides an interesting insight into the direct or indirect role of the glycine system in epilepsy. The successful anti-epileptic effect of AM203 in vivo implies that blocking miR-203 shifts the gene expression in the direction of potentiating inhibitory synapses, such as glycinergic or GABAergic synapses. Cellular electrophysiologic analysis might provide physio-pathologic changes by AM203 in further studies.
We previously showed that an intranasally delivered antagomir reaches the brain and is effective in reducing symptoms in a mouse model of Alzheimer’s disease [11]. Although most systemically administered therapeutics cannot cross the blood–brain barrier, intranasal administration can be used to transport therapeutics to the olfactory bulb, perivascular flow, cerebrospinal fluid, and to the hippocampus and cortex [31], minimizing systemic exposure. Human trials have shown that intranasal administration of insulin improves attention and functional status in Alzheimer’s disease patients [32]. Given the current results, further studies using larger animals are warranted to develop the intranasal delivery method of AM203.
The functions of the other miRNAs that were altered in the chronically epileptic mice remain to be determined. The expression of five miRNAs (miR-199b, miR-203, miR-223, miR-451, and miR-455) was altered in both the hippocampus and cortex. Of these miRNAs, miR-199b induces cellular senescence by reducing SIRT1 expression [33], miR-223 is associated with inflammatory responses and immune regulation [34], miR-451 increases MDR1/p-glycoprotein expression [35], and miR-455 is overexpressed in activated macrophages [36]. In addition, miR-21, an miRNA upregulated only in the epileptic hippocampus, is increased after traumatic injury to the hippocampus [37]. miR-132 expression, which was upregulated in the epileptic hippocampus, is increased by neuronal activity via the transcriptional activation of the cAMP response element-binding protein [38] and, in the pilocarpine-induced mouse model of epilepsy, is elevated by neuronal hyperactivity during recurrent seizures [13]. miR-99b-3p, which was downregulated in the epileptic hippocampus, is associated with modulation of neuronal apoptosis [16]. In addition to the miRNAs changed in our study, several other miRNAs, such as miR-146a [15], have been changed in other epilepsy studies. Moreover, given that miRNAs show dynamic changes at different time point after SE [16], multiple miRNAs can be involved in the overall epilepsy pathogenesis. Diversity of epilepsy animal models, different time points at analysis, and even hippocampal structures analyzed could influence the miRNA expression profile.
In this study, we used human brain samples from autopsy brains, which contain degradated miRNAs. Although snap-frozen samples from epilepsy surgery might provide high quality samples for the miRNA analysis, surgical samples have no appropriate normal controls. Thus, we used postmortem tissues instead in this study, and only little clinical information was available for the tissues. Nevertheless, the upregulation of miR-203 in human epilepsy brain has been reproduced already in other studies [17, 18], and we could confirm the upregulation again here.
The reproducible upregulation of miR-203 in epileptic brains suggests that AM203 may help manage the disease. Before AM203 clinical trials are employed, its efficacy and safety must be confirmed in several other epilepsy models and primates. Although antagomir-based drugs are likely to be very safe, as confirmed in a recent human trial [39], the interaction between AM203 and other genes in systemic organs must be analyzed. In addition, the functions of the other miRNAs that were altered in the epileptic mice remain to be determined. Nevertheless, this study not only identified a novel miRNA-based mechanism that regulates GLRB expression in mice with chronic epilepsy but also showed that AM203 deserves further study as a possible therapeutic agent for the treatment of epilepsy.
References
Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA (2011) Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52(Suppl 7):2–26
Moshe SL, Perucca E, Ryvlin P, Tomson T (2015) Epilepsy: new advances. Lancet 385:884–898
Galanopoulou AS, Buckmaster PS, Staley KJ, Moshé SL, Perucca E, Engel J Jr, Löscher W, Noebels JL et al (2012) Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia 53:571–582
Dutertre S, Becker CM, Betz H (2012) Inhibitory glycine receptors: an update. J Biol Chem 287:40216–40223
Davies JS, Chung SK, Thomas RH, Robinson A, Hammond CL, Mullins JG, Carta E, Pearce BR et al (2010) The glycinergic system in human startle disease: a genetic screening approach. Front Mol Neurosci 3:8
Trapani G, Latrofa A, Franco M, Carrieri A, Cellamare S, Serra M, Sanna E, Biggio G et al (2003) Alpidem analogues containing a GABA or glycine moiety as new anticonvulsant agents. Eur J Pharm Sci 18:231–240
Mohammadi B, Krampfl K, Cetinkaya C, Wolfes H, Dengler R, Bufler J (2005) Interaction of topiramate with glycine receptor channels. Pharmacol Res 51:587–592
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK et al (2010) MicroRNAs induced during ischemic preconditioning. Stroke 41:1646–1651
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Lee ST, Chu K, Jung KH, Kim JH, Huh JY, Yoon H, Park DK, Lim JY et al (2012) miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol 72:269–277
Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G et al (2010) Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 30:92–101
Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T et al (2011) miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol 179:2519–2532
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L et al (2012) MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci 13:115
Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA (2010) Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci 31:1100–1107
Sun Z, Yu JT, Jiang T, Li MM, Tan L, Zhang Q, Tan L (2013) Genome-wide microRNA profiling of rat hippocampus after status epilepticus induced by amygdala stimulation identifies modulators of neuronal apoptosis. PLoS One 8:e78375
Kan AA, van Erp S, Derijck AA, de Wit M, Hessel EV, O’Duibhir E, de Jager W, Van Rijen PC et al (2012) Genome-wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol Life Sci 69:3127–3145
McKiernan RC, Jimenez-Mateos EM, Bray I, Engel T, Brennan GP, Sano T, Michalak Z, Moran C et al (2012) Reduced mature microRNA levels in association with dicer loss in human temporal lobe epilepsy with hippocampal sclerosis. PLoS One 7:e35921
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, Sano T, O’Tuathaigh C et al (2012) Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18:1087–1094
McKiernan RC, Jimenez-Mateos EM, Sano T, Bray I, Stallings RL, Simon RP, Henshall DC (2012) Expression profiling the microRNA response to epileptic preconditioning identifies miR-184 as a modulator of seizure-induced neuronal death. Exp Neurol 237:346–354
Harvey RJ, Yee BK (2013) Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov 12:866–885
Weltzien F, Puller C, O’Sullivan GA, Paarmann I, Betz H (2012) Distribution of the glycine receptor beta-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J Comp Neurol 520:3962–3681
Treiman DM (2001) GABAergic mechanisms in epilepsy. Epilepsia 42(Suppl 3):8–12
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Jeon D, Chu K, Lee ST, Jung KH, Kang KM, Ban JJ, Kim S, Seo JS et al (2011) A cell-free extract from human adipose stem cells protects mice against epilepsy. Epilepsia 52:1617–1626
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I et al (2005) Combinatorial microRNA target predictions. Nat Genet 37:495–500
Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, Hatzigeorgiou A (2004) A combined computational-experimental approach predicts human microRNA targets. Genes Dev 18:1165–1178
Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201
Zhao C, Huang C, Weng T, Xiao X, Ma H, Liu L (2012) Computational prediction of MicroRNAs targeting GABA receptors and experimental verification of miR-181, miR-216 and miR-203 targets in GABA-A receptor. BMC Res Notes 5:91
Dhuria SV, Hanson LR, Frey WH 2nd (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–1673
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR et al (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70:440–448
Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E (2010) miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2:415–431
Sonkoly E, Stahle M, Pivarcsi A (2008) MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 18:131–140
Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM (2008) Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 76:582–588
Monk CE, Hutvagner G, Arthur JS (2010) Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One 5:e13669
Redell JB, Zhao J, Dash PK (2011) Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. J Neurosci Res 89:212–221
Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR (2010) Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20:492–498
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK et al (2013) Treatment of HCV infection by targeting microRNA. N Engl J Med 368:1685–1694
Acknowledgments
This work was supported by a grant from the Korean Health Technology R&D Project (HI14C25020000, HI13C1558, HI12C1544) of the Ministry of Health and Welfare, and the Mid-career Researcher Program (NRF-2014R1A2A1A11052709) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning, Republic of Korea. Daejong Jeon was supported by a grant from the Korean Health Technology R&D Project (HI12C0035) of the Ministry of Health and Welfare, Republic of Korea. Sang Kun Lee was supported by Seoul National University Hospital (0320140160).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital, South Korea, and all experiments have been performed in accordance with the approved guidelines and regulations. The committee is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Every effort was made to limit the number of animals used. ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the Basel declaration were considered when planning the experiments.
Conflict of Interest
Dr. Soon-Tae Lee, Dr. Daejong Jeon, Dr. Kon Chu, Dr. Keun-Hwa Jung, Dr. Manho Kim, Dr. Jae-Kyu Roh, and Dr. Sang Kun Lee have a patent for the use of antagomir-203 in epilepsy. The remaining authors have no conflicts of interest.
Additional information
Soon-Tae Lee and Daejong Jeon contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2432 kb)
Rights and permissions
About this article
Cite this article
Lee, ST., Jeon, D., Chu, K. et al. Inhibition of miR-203 Reduces Spontaneous Recurrent Seizures in Mice. Mol Neurobiol 54, 3300–3308 (2017). https://doi.org/10.1007/s12035-016-9901-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9901-7