Abstract
Emerging evidence indicates that certain microRNAs (miRNAs) play important roles in epileptogenesis. MiR-219 is a brain-specific miRNA and has been shown to negatively regulate the function of N-methyl-d-aspartate (NMDA) receptors by targeting Ca2+/calmodulin-dependent protein kinase II (CaMKII)γ. Herein, we found that the level of miR-219 was decreased in both the kainic acid (KA)-induced epilepsy model and in cerebrospinal fluid specimens of epilepsy patients. Importantly, silencing of miR-219 by its antagomir in vivo resulted in seizure behaviors, abnormal cortical electroencephalogram (EEG) recordings in the form of high-amplitude and high-frequency discharges, and increased levels of CaMKIIγ and an NMDA receptor component, NR1, in a pattern similar to that found in KA-treated mice. Moreover, treatments with the miR-219 agomir in vivo alleviated seizures, abnormal EEG recordings, and decreased levels of CaMKIIγ and NR1 in KA-treated mice. Furthermore, treatment with MK-801, an antagonist of NMDA receptors, significantly alleviated abnormal EEG recordings induced by miR-219 antagomir. Together, these results demonstrate that miR-219 plays a crucial role in suppressing seizure formation in experimental models of epilepsy through modulating the CaMKII/NMDA receptor pathway and that miR-219 supplement may be a potential anabolic strategy for ameliorating epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are small (~22 nt) noncoding transcripts that regulate expression of protein-coding mRNAs at the posttranscriptional level [1]. Dysregulation of miRNAs has been proposed to contribute to neurological injuries and neurodegenerative diseases, including epilepsy [2–4]. Epilepsy is a common, serious neurologic disorder characterized by recurring unprovoked seizures that result from abnormal firing of populations of neurons in the brain [5]. A series of profiling studies have identified bidirectional spatiotemporal changes of certain miRNAs accompanying epilepticus [6–12]. However, detailed mechanisms underlying the change of these miRNAs and their functional effects during epilepsy have yet to be elucidated.
MiR-219 is a conserved miRNA expressed in both rodent and human brains but not in other tissues [13, 14]. Several studies have demonstrated that miR-219 plays a critical role in regulating neural precursor differentiation, especially oligodendrocyte differentiation during development [15–17]. In addition, miR-219 has been found to negatively regulate the function of N-methyl-d-aspartate (NMDA) receptors by targeting Ca2+/calmodulin-dependent protein kinase II (CaMKII)γ, an integral downstream responder to NMDA-mediated Ca2+ signaling [18]. Given the importance of NMDA receptors in mediating neuronal activity/firing that is altered in epilepsy, herein we explored the correlation between miR-219 and epilepsy.
Materials and Methods
Reagents
Kainic acid (KA), pilocarpine, and MK-801 were purchased from Sigma-Aldrich. Antibodies against CaMKIIγ, NR1, and GAPDH were from Cell Signaling Technology. RNAiso for Small RNA kit and SYBR® PrimeScript miRNA RT-PCR Kit were from Takara, Japan. MiR-219 agomir, miR-219 antagomir, scrambled control miRNA, and oligonucleotide primers for mouse or human U6, miR-219, and miR-19a genes were all from RiboBio (Guangzhou, China).
Cerebrospinal Fluid (CSF) Samples
CSF specimens from eight temporal lobe epilepsy (TLE) patients (5 males and 3 females, mean age 27.6 years, range 15 to 45 years) and six patients with temporal vascular malformation but no history of epilepsy or other neurological diseases (as controls, 4 males and 2 females, mean age 28.3 years, range 16 to 48 years) were collected from Affiliated Zhongshan Hospital of Xiamen University and Affiliated Chenggong Hospital of Xiamen University. The protocol of this study complied with the guidelines for the conduct of research involving human subjects as established by the National Institutes of Health and the Ethics Committee on Human Research at Xiamen University.
Cell Culture and Treatments
Hippocampal neurons were isolated from E15.5 C57BL/6 mice as previously described [19]. HT22 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal bovine serum (FBS). N2a cells were maintained in a 1:1 mixture of DMEM and Opti-MEM containing 5 % FBS. For treatments, cells were incubated with a medium containing 50 or 100 μM KA for 24 h.
Animals and Treatments
ICR and C57BL/6 mice, which are commonly used for epilepsy study, weighing 20–25 g and at 6 to 8 weeks of age, were provided by the Experimental Animal Center of Xiamen University. All animal procedures were in strict accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Xiamen University.
For treatments, mice were subjected to i.c.v. injection of KA (40 μg/kg) to the right lateral cerebral ventricle (0.5 mm posterior to the bregma, 1 mm lateral to the midsagittal suture, and 2–2.5 mm below the dura matter) using a 5-μl Hamilton syringe. In some experiments, 6 h after i.c.v. injection of KA, mice were subjected to another i.c.v. injection of miR-219 agomir, miR-219 antagomir, or scrambled control miRNA at 10 nmol/kg. Mice were then sacrificed at indicated time periods after treatments, and cortical and hippocampal tissues of these mice were carefully isolated for quantitative real-time PCR (qRT-PCR) assays to study miR-219 levels or Western blotting assays to detect CaMKIIγ and NR1 levels.
The NMDA receptor antagonist MK-801 (0.5 mg/kg) was administered intraperitoneally into mice immediately after i.c.v. injection of miR-219 antagomir or scrambled control miRNA at 10 nmol/kg. Mice were then subjected to electroencephalogram (EEG) recording at 24 h after treatments.
EEG Recording and Behavior Observation
In some experiments, mice treated as described above were subjected to EEG recording 24 h after the final i.c.v. injection, following the method described previously [20]. Briefly, polyamide-insulated stainless steel monopolar microelectrodes (0.1 mm diameter; Plastics One Inc. Roanoke, VA) were implanted bilaterally into the frontal area of both hemispheres (−1.5 mm bregma, 1.8 mm lateral). In addition, an insulated, 50-μm-diameter stainless steel wire (California Fine Wire) was implanted 1.7 mm below the surface of the brain. The reference electrode was placed in the cerebellum. Data were analyzed by Nicolet1.0 software. In addition, seizure induced in treated mice was observed and staged I–V according to an adjusted version of the Racine scale [21].
Reverse Transcription and qRT-PCR
Total RNA was isolated from tissues or cells using RNAiso for Small RNA kit (Takara, Japan) according to the manufacturer’s protocol. Total RNA was dissolved in nuclease-free water and stored at −80 °C. Reverse transcription and qRT-PCR were performed using a SYBR® PrimeScript miRNA RT-PCR Kit (Takara, Japan) according to the manufacturer’s protocol. The set of mouse or human U6 primers was used as an internal control for each specific gene amplification. The relative levels of expression were quantified and analyzed by using 7500 Fast software V2.0.6 (Invitrogen). The real-time value for each sample was averaged and compared using the CT method, where the amount of target RNA (2−ΔΔCT) was normalized to the endogenous mouse or human U6 reference (ΔCT).
Western Blotting
Western blotting was performed as a standard procedure. All cells were lysed in RIPA buffer, and total protein concentrations were determined with a BCA Protein Assay Kit (Thermo Scientific). Total protein (50 μg) was loaded for each sample into 10 % SDS-PAGE. Gels were transferred onto PVDF membranes (Millipore). Mouse CaMKIIγ-, NR1-, or GAPDH-specific primary antibody was incubated overnight at 4 °C followed by an HRP-conjugated secondary antibody. The immunoreactive proteins were detected by using enhanced chemiluminescence (ECL, Thermo) and images were analyzed using the ImageJ1.42. Data were normalized to GAPDH.
Statistical Analysis
All data were analyzed by using GraphPad Prism 5 statistical software. Data were compared by Student’s t tests (two groups) or by one-way analysis of variance (ANOVA) post Tukey’s multiple comparison test (more than two groups). A P value less than 0.05 was considered to be statistically significant. Data in all figures are expressed as mean ± standard error of the mean (SEM).
Results
MiR-219 Level is Decreased in KA-Induced Seizure Models and in CSF Specimens of TLE Patients
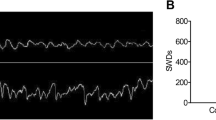
To study potential involvement of miR-219 in epilepsy, we first generated an animal model of epilepsy by unilateral i.c.v. injection of KA into the ICR mouse brain. Treated mice indeed exhibited generalized tonic-clonic seizures reaching grade VI~V with Racine standards. EEG recordings at 24 h after injection identified high-amplitude, high-frequency discharges in KA-injected mice when compared with vehicle-injected mice (Fig. 2a–c).
KA-injected and vehicle-injected control ICR mice were sacrificed at days 1, 3, 7, 10, and 14 after treatments. Levels of miR-219 in hippocampal and cortical regions of these mice were determined by qRT-PCR. The results showed that KA treatments resulted in a significant decrease of miR-219 levels in the hippocampus at days 1, 3, 7, and 10 after treatments (Fig. 1a). The reduction of miR-219 levels recovered as time passed by, and there was no significant difference of miR-219 levels between KA-treated and control mice at day 14 after treatments (Fig. 1a). Similarly, miR-219 levels in the cortex were lower in KA-treated samples than in controls, though differences were not statistically significant (Fig. 1b). The reduction of miR-219 levels were also verified in the hippocampus in seizure induced by pilocarpine (supplemental data Fig. 1).
miR-219 level is decreased in kainic acid-induced seizure models and in CSF specimens from TLE patients. ICR mice were i.c.v. injected with kainic acid (KA) or vehicle control (Ctrl) and sacrificed at days 1, 3, 7, 10, and 14 after treatments. a Hippocampal and b cortical regions were dissected and used for RNA extraction and reverse transcription. MiR-219 levels were quantified by qRT-PCR and normalized to those of U6 before comparison. N = 6, **: p < 0.01. ns not significant. c Hippocampus and cortex of C57/BL6 mice subjected to i.c.v. injection of KA or control (Ctrl) for 24 h were dissected and used for analyzing miR-219 levels by qRT-PCR. N = 6, **p < 0.01. ns not significant. d Primary hippocampal neurons, e HT22, and f N2a cells were treated with various amounts of KA for 24 h. Cells were then subjected to RNA extraction, reverse transcription, and qRT-PCR to detect miR-219 levels. N = 3, ***p < 0.001. g CSF specimens from TLE patients (n = 8) and temporal vascular malformation patients (n = 6) were analyzed for miR-219 levels. ***p < 0.001
In C57BL/6 mice, we also observed a significant decrease of miR-219 levels in the hippocampus and a trend of reduction (though not statistically significant) of miR-219 levels in the cortex upon KA treatments (Fig. 1c). Furthermore, the effect of KA treatments on reducing miR-219 levels was confirmed in primary hippocampal neurons (Fig. 1d), in HT22 (Fig. 1e), and in N2a cell lines (Fig. 1f).
Moreover, we examined miR-219 expression in CSF specimens of TLE patients. The qRT-PCR analysis showed a dramatic reduction of miR-219 levels in CSF specimens from TLE patients when compared with those from temporal vascular malformation patients who had no history of epilepsy (Fig. 1g).
Inhibition of MiR-219 Induces Seizures and Abnormal EEG and Increases CaMKIIγ and NR1 Levels
Since the level of miR-219 was markedly decreased in KA-induced models and in TLE patients, it is reasonable to extrapolate that depletion of miR-219 might induce seizure in normal mice. An antagomir targeting miR-219 was i.c.v. injected into the mouse brain, and the level of miR-219 was confirmed to be downregulated 24 h after injection (Fig. 2d). Hippocampal level of an unrelated miRNA, miR-19a, was not altered by the injection of the miR-219 antagomir (Fig. 2e). Importantly, mice treated with the miR-219 antagomir exhibited staring eyes, beard trembling, and repeated washing face action, accompanied by head and facial tics, and gradually developed into an involuntary movement of the arms. Such seizures reached grade II~III with Racine standards. Consistently, these mice showed abnormal EEG recordings (Fig. 2a) in the form of high-amplitude (Fig. 2b) and high-frequency discharges (Fig. 2c).
Silencing of miR-219 induces seizure-like EEG and increases CaMKIIγ and NR1 levels. ICR mice were i.c.v. injected with vehicle control (Ctrl), KA, negative control (NC) antagomir, or miR-219 antagomir and subjected to following tests at 24 h after the injection. a Mice were analyzed for EEG and representative images were shown. The b amplitude and c spike frequency of seizure EEG were quantified for comparison. d Mice were sacrificed and hippocampal tissues were analyzed for miR-219 levels by qRT-PCR. e Hippocampal level of an unrelated miRNA, miR-19a, was not significantly altered by injection of antagomir miR-219. f Hippocampal tissue lysates were analyzed for CaMKIIγ and NR1 protein levels by Western blotting. Protein levels were quantified by densitometry for comparison. N = 6, *p < 0.05; **p < 0.01; ***p < 0.001
Previous reports demonstrated that miR-219 negatively regulates the function of NMDA receptors by targeting CaMKIIγ, an integral downstream responder to NMDA-mediated Ca2+ signaling [18]. To gain further insight into the mechanism by which miR-219 regulates seizure activity, we next investigated whether the expression of CaMKIIγ and NR1, a key component of NMDA receptors, can be affected by miR-219 in experimental seizure. Indeed, miR-219 antagomir treatments, as well as KA treatments, dramatically increased the protein levels of CaMKIIγ and NR1 (Fig. 2f).
Supplement of MiR-219 Protects Against Epileptogenesis
Since a reduction of miR-219 causes epilepsy-like phenotypes, we next studied whether supplement of miR-219 using its agomir exerts protective effects in experimental models of epilepsy. We injected miR-219 agomir or control agomir into KA-treated mice. Injection of miR-219 agomir alleviated the reduction of hippocampal miR-219 levels upon KA treatments (Fig. 3d) and had no effect on miR-19a level (Fig. 3e). Importantly, supplement of miR-219 markedly reduced seizure severity (the onset and frequency of convulsions) and alleviated abnormal EEG recording (Fig. 3a) in the form of high amplitude (Fig. 3b) and high frequency (Fig. 3c) in KA-treated mice. Moreover, protein levels of CaMKIIγ and NR1 were also reduced by miR-219 agomir treatments (Fig. 3f).
Supplement of miR-219 alleviates KA-induced seizure-like EEG and CaMKIIγ and NR1 level change. ICR mice were i.c.v. injected with Agomir-219 or Agomir negative control (Ctrl) alone, or with Agomir-219 or Agomir negative control (Agomir-NC) at 6 h after KA injection. Twenty-four hours later, mice were subjected to following experiments. a Mice were analyzed for EEG and representative images were shown. The b amplitude and c spike frequency of seizure EEG were quantified for comparison. d Mice were sacrificed and hippocampal tissues were analyzed for miR-219 levels by qRT-PCR. e Hippocampal level of miR-19a was not significantly altered by injection of agomir miR-219. f Hippocampal tissue lysates were analyzed for CaMKIIγ and NR1 protein levels by Western blotting. Protein levels were quantified by densitometry for comparison. N = 6, *p < 0.05; **p < 0.01; ***p < 0.001
NMDA Receptor Antagonist Treatments Alleviate Seizures Induced by MiR-219 Antagomir
To further determine whether reduction of miR-219 causes epilepsy through the CaMKII/NMDA receptor pathway, we intraperitoneally administered MK-801, a noncompetitive antagonist of NMDA receptors, or saline (as control) into mice treated with a miR-219 antagomir. We found that seizure induced in MK-801 co-treated mice reached only grade I~II with Racine standards, whereas that in control mice reached grade II~III. Furthermore, treatments with MK-801 markedly alleviated abnormal EEG recording induced by miR-219 antagomir (Fig. 4a), in the form of high amplitude (Fig. 4b) and high frequency (Fig. 4c).
MK-801 treatments alleviate seizures induced by miR-219 antagomir. ICR mice were intraperitoneally administered with MK-801 or saline, immediately after i.c.v. injection of miR-219 antagomir or negative control miRNA at 10 nmol/kg. Twenty-four hours later, mice were subjected to EEG recording. a Mice were analyzed for EEG and representative images were shown. The b amplitude and c spike frequency of seizure EEG were quantified for comparison. N = 6, *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The brain-specific expression pattern of miR-219 [13] indicates that the major physiological function of miR-219 is associated with neural activity. Indeed, miR-219 has been shown to regulate oligodendrocyte differentiation and myelination [15–17]. In this study, we find that the level of miR-219 is decreased in the brain of KA-induced epilepsy model mice. A more dramatic change of miR-219 in the hippocampus than in the cortex suggests that hippocampus is more sensitive to KA than cortex does. Consistently, cells treated with KA have reduced levels of miR-219. Moreover, the level of miR-219 is also decreased in CSF of TLE patients. KA treatment has been well used to reproduce the pathophysiological events of epileptogenesis and the behavioral state in TLE [22]. In addition, other chemicals have also been widely used to induce seizure, such as pilocarpine, pentylenetetrazol, and penicillin [23]. When we treated mice with pilocarpine to induce seizure in mice, we also found a significant decrease of miR-219 in the hippocampus (Supplementary Figure 1). Importantly, we find that inhibition of miR-219 by its antagomir can induce seizure behaviors and seizure-like EEG. Together, these findings demonstrate that miR-219 plays an important role in epileptogenesis.
One study reported that miR-219 can negatively regulate the function of NMDA receptors by directly targeting CaMKIIγ [18]. Here we confirm that inhibition of miR-219 by its antagomir can increase levels of CaMKIIγ and NR1, a core subunit of NMDA receptors. CaMKIIγ is a component of the CaMKII enzyme that is of central importance in neuronal signaling. CaMKII is highly enriched at excitatory synapses and mediates long-term potentiation (LTP) not only through modulating AMPA receptors but also through modulating NMDA receptors [24]. CaMKII can phosphorylate NMDA receptor subunits and modulate NMDA receptor trafficking and activity state [25, 26]. In addition, CaMKII can directly interact with NMDA receptors for LTP mediation [27]. On the other hand, NMDA receptor activity reciprocally regulates CaMKII phosphorylation and activation [28]. Alteration of both CaMKII and NMDA receptors has been identified in epilepsy. For example, CaMKII is activated and autophosphorylated in epilepsy [29], whereas NMDA receptors are activated during the epileptogenic stimuli and NMDA receptor activation promotes limbic epileptogenesis [30]. Inhibition of NMDA receptor activity by its noncompetitive antagonist MK-801 has been shown to prevent seizure [31]. Here we also found that co-treatment with MK-801 can dramatically alleviate seizure severity induced by miR-219 antagomir. Therefore, our results suggest that miR-219 participates in epilepsy through modulating the CaMKII/NMDA receptor pathway.
Despite progress in understanding the contribution of miRNAs to epileptogenesis, few studies have identified specific miRNAs that may serve as target for disease treatment. One study reported that silencing miR-134 produces neuro-protective and seizure-suppressive effects [11]. Another recent work found that a reduction of miR-128 expression in postnatal neurons causes increased motor activity and fatal epilepsy in mice, whereas that an increased miR-128 expression in adult neurons protects mice against abnormal motor activities associated with chemically induced Parkinson’s disease and seizures [6]. In our study, we demonstrate that treatment with miR-219 agomir alleviates seizure, abnormal EEG, and increased CaMKIIγ and NR1 levels in KA-induced experimental model of epilepsy. These results suggest that like silencing of miR-134 and supplement of miR-128, supplement of miR-219 in epilepsy may provide another anabolic strategy for ameliorating epilepsy.
References
Kosik KS (2006) The neuronal microRNA system. Nat Rev Neurosci 7:911–920
Dogini DB, Avansini SH, Vieira AS, Lopes-Cendes I (2013) MicroRNA regulation and dysregulation in epilepsy. Front Cell Neurosci 7:172
Eacker SM, Dawson TM, Dawson VL (2009) Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10:837–841
Henshall DC (2013) Antagomirs and microRNA in status epilepticus. Epilepsia Suppl 6:17–19
Jimenez-Mateos EM, Henshall DC (2013) Epilepsy and microRNA. Neuroscience 238:218–229
Tan CL, Plotkin JL, Ven MT, von Schimmelmann M, Feinberg P, Mann S, Handler A, Kjems J, Surmeier DJ, O’Carroll D, Greengard P, Schaefer A (2013) MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 342(6163):1254–1258
Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F (2013) Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci 50(2):291–297
Manna I, Labate A, Mumoli L, Pantusa M, Ferlazzo E, Aguglia U, Quattrone A, Gambardella A (2013) Relationship between genetic variant in pre-microRNA-146a and genetic predisposition to temporal lobe epilepsy: a case-control study. Gene 516(1):181–183
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L, Li Y, Xiao B (2012) MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci 13:115
Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP, Stallings RL, Henshall DC (2011) miRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol 179(5):2519–2532
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, Sano T, O’Tuathaigh C, Waddington JL, Prenter S, Delanty N, Farrell MA, O’Brien DF, Conroy RM, Stallings RL, DeFelipe J, Henshall DC (2012) Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18(7):1087–1094
Sano T, Reynolds JP, Jimenez-Mateos EM, Matsushima S, Taki W, Henshall DC (2012) MicroRNA-34a upregulation during seizure induced neuronal death. Cell Death Dis 3:e287
Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K (2007) MicroRNA modulation of circadian-clock period and entrainment. Neuron 54:813–829
Lukiw WJ (2007) Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 18(3):297–300
Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA (2010) Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron 65(5):597–611
Hudish LI, Blasky AJ, Appel B (2013) miR-219 regulates neural precursor differentiation by direct inhibition of apical par polarity proteins. Dev Cell 27(4):387–398
Ebrahimi-Barough S, Kouchesfehani HM, Ai J, Mahmoodinia M, Tavakol S, Massumi M (2013) Programming of human endometrial-derived stromal cells (EnSCs) into pre-oligodendrocyte cells by overexpression of miR-219. Neurosci Lett 537:65–70
Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C (2009) MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A 106(9):3507–3512
Zhu W, Zheng H, Shao X, Wang W, Yao Q, Li Z (2010) Excitotoxicity of TNFalpha derived from KA activated microglia on hippocampal neurons in vitro and in vivo. J Neurochem 114:386–396
Buckmaster PS, Lew FH (2011) Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci 31(6):2337–2347
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Tchekalarova J, Pechlivanova D, Itzev D, Lazarov N, Markova P, Stoynev A (2010) Diurnal rhythms of spontaneous recurrent seizures and behavioural alterations of Wistar and spontaneously hypertensive rats in kainate model of epilepsy. Epilepsy Behav 17(1):23–32
Kandratavicius L, Balista PA, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Bueno-Junior LS, Leite JP (2014) Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat 10:1693–1705
Coultrap SJ, Bayer KU (2012) CaMKII regulation in information processing and storage. Trends Neurosci 35(10):607–618
Suen PC, Suen PC, Wu K, Xu JL, Lin SY, Levine ES, Black IB (1998) NMDA receptor subunits in the postsynaptic density of rat brain: expression and phosphorylation by endogenous protein kinases. Brain Res Mol Brain Res 59:215–228
Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M (2002) Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36:507–519
Sanhueza M, Lisman J (2013) The CaMKII/NMDAR complex as a molecular memory. Mol Brain 6:10
Zhou X, Zheng F, Moon C, Schlüter OM, Wang H (2012) Bi-directional regulation of CaMKIIα phosphorylation at Thr286 by NMDA receptors in cultured cortical neurons. J Neurochem 122(2):295–307
Perlin JB, Churn SB, Lothman EW, DeLorenzo RJ (1992) Loss of type II calcium/calmodulin-dependent kinase activity correlates with stages of development of electrographic seizures in status epilepticus in rat. Epilepsy Res 11:111–118
McNamara JO, Huang YZ, Leonard AS (2006) Molecular signaling mechanisms underlying epileptogenesis. Sci STKE 356: re12. Review
Deutsch SI, Burket JA, Cannon WR, Jacome LF (2011) Selective mGluR5 antagonism attenuates the stress-induced reduction of MK-801’s antiseizure potency in the genetically inbred Balb/c mouse. Epilepsy Behav 21(4):352–355
Acknowledgments
We thank Zhengli Li for the helpful discussion of the manuscript. This work was supported by grants from the Natural Science Research Foundation of China (81100842, 81225008, 81271895, 81161120496, 91332112, and 91332114) and by the Natural Science Foundation of Fujian Province of China (No. 2012J01418). This work was also supported by the Foundation of Xiamen Science and Technology Bureau (No. 2011S0351).
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Honghua Zheng and Rong Tang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 97 kb)
Rights and permissions
About this article
Cite this article
Zheng, H., Tang, R., Yao, Y. et al. MiR-219 Protects Against Seizure in the Kainic Acid Model of Epilepsy. Mol Neurobiol 53, 1–7 (2016). https://doi.org/10.1007/s12035-014-8981-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8981-5