Abstract
Parkinson’s disease (PD) is an aging-associated neurodegenerative disease with a characteristic feature of α-synuclein accumulation. Point mutations (A53T, A30P) that increase the aggregation propensity of α-synuclein result in familial early onset PD. The abnormal metabolism of α-synuclein results in aberrant level changes of α-synuclein in PD. In pathological conditions, α-synuclein is degraded mainly by the autophagy-lysosome pathway. Triptolide (T10) is a monomeric compound isolated from a traditional Chinese herb. Our group demonstrated for the first time that T10 possesses potent neuroprotective properties both in vitro and in vivo PD models. In the present study, we reported T10 as a potent autophagy inducer in neuronal cells, which helped to promote the clearance of various forms of α-synuclein in neuronal cells. We transfected neuronal cells with A53T mutant (A53T) or wild-type (WT) α-synuclein plasmids and found T10 attenuated the cytotoxicity induced by pathogenic A53T α-synuclein overexpression. We observed that T10 significantly reduced both A53T and WT α-synuclein level in neuronal cell line, as well as in primary cultured cortical neurons. Excluding the changes of syntheses, secretion, and aggregation of α-synuclein, we further added autophagy inhibitor or proteasome inhibitor with T10, and we noticed that T10 promoted the clearance of α-synuclein mainly by the autophagic pathway. Lastly, we observed increased autophagy marker LC3-II expression and autophagosomes by GFP-LC3-II accumulation and ultrastructural characterization. However, the lysosome activity and cell viability were not modulated by T10. Our study revealed that T10 could induce autophagy and promote the clearance of both WT and A53T α-synuclein in neurons. These results provide evidence of T10 as a promising mean to treat PD and other neurodegenerative diseases by reducing pathogenic proteins in neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is an aging-associated neurodegenerative disease, characterized by the progressive loss of midbrain dopaminergic neurons in the substantia nigra (SN). The hallmark of PD patients is the presence of Lewy bodies, which are the intracytoplasmic inclusions containing α-synuclein [1].
α-Synuclein is a 140 amino acid protein encoded by the SNCA gene, localized at presynaptic or cytoplasm. The aberrant level of α-synuclein in PD is mainly caused by the abnormal metabolism of α-synuclein [2]. In physiological conditions, α-synuclein is recognized and cleared via the ubiquitin-proteasome system (UPS) and chaperone-mediated autophagy (CMA) pathways [3–5]. In the pathological state, misfolding or mutation of α-synuclein leads to the formation of pathologically modified species that ultimately package into Lewy body. In early stage of PD, the UPS and CMA cannot fully clear these aggregated species. Therefore, the autophagy-lysosome pathway (ALP), macroautophagy in particular, is recruited as an alternative strategy to degrade α-synuclein [6].
Autophagy is a cellular process that mediates the lysosome-dependent degradation of cytoplasmic-damaged components and organelles, thus contributes to maintenance of cellular homeostasis and adaptation to environmental challenges. Autophagic dysfunction is involved with common human diseases [7]. Autophagy can be detected in the neurons of the substantia nigra and peripheral blood of PD patients [8, 9]. Several studies have reported successful neuroprotection aimed at increasing autophagy both in vitro and in vivo [10–12]. Identification of specific and safe compounds that boost the ALP could be a successful strategy in PD.
Triptolide (T10) is a monomeric compound isolated from a traditional Chinese herb, tripterygium wilfordii Hook.f. (TWHF). T10 has been identified as a major component in TWHF to exert immunosuppressive, anti-inflammatory, antifertility, and antitumor activities, as well as neurotrophic and neuroprotective effects [13, 14]. Our group demonstrated for the first time that T10 possesses potent neuroprotective properties both in vitro and in vivo PD models. And, T10 also showed effectiveness in treating other aggregation-prone protein-associated neurodegenerative diseases such as Alzheimer’s disease [15]. This study explored a new activity of T10 as a potent autophagy inducer to promote the clearance of both wild-type (WT) and A53T mutant (A53T) α-synuclein.
Materials and Methods
Neuronal Cell Line Culture
MN9D is the cell line closest to primary mesencephalic dopamine neurons. It was generated by fusion of rostral mesencephalic neurons from embryonic C57BL/6 J (embryonic day 14) mice with N18TG2 neuroblastoma cells [16]. MN9D cells were cultured in DMEM/F12 (GIBCO, Carlsbad, CA, USA) with 10 % FBS (HyClone, Logan, Utah, USA), supplemented with 100 U/ml penicillin–streptomycin (HyClone, Logan, Utah, USA), and maintained at 37 °C with 5 % CO2. The cells were seeded in 100 μg/ml poly-l-lysine (Sigma)-coated 6-well plates and grown to 80 % confluence prior to plasmid transfection.
Rat Primary Neuron Culture
Rat primary neuron cultures were prepared from brains of embryonic day 14 Sprague–Dawley (SD) rats. The tissues of ventral mesencephalons, with meninges carefully removed, were dissociated with gentle mechanical trituration. Cells were seeded at 1 × 106/well to 6-well culture plates precoated with poly-l-lysine (100 μg/ml) and cultured in the neurobasal medium containing 2 % B27, 1 % l-glutamine (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin–streptomycin, maintaining at 37 °C with 5 % CO2. The same volume of the fresh culturing medium was added to each well on 3 days after the initial seeding. Cultures were maintained 7 days for neuron maturation and drug treatment. The experimental procedures in this study were approved by the Committee on Animal Care and Usage (Capital Medical University), and efforts were engaged to minimize the number of animal usage and suffering.
Plasmid Transfection and Viral Infection
The pcDNA3.1 plasmid expressing human WT or A53T mutant α-synuclein (Prof. Glenda Halliday) was transfected using Lipofectamine LTX and Plus reagent (Invitrogen, Carlsbad, CA, USA). Plasmids encoding the pcDNA3.1 vector alone were transfected into cells as controls using the same protocol. The transfected cells were harvested at 48 h, or subjected further intervention after 24 h.
Lentiviral vectors expressing human wild-type α-synuclein (Lv-syn) were purchased from Obio Technology Co., Ltd., Shanghai, China. The multiplicity of infection (MOI) is 5 when infecting primary cultured cortical neurons.
Cell Viability Assay
The cytotoxicity of T10 or α-synuclein was determined by using a cell proliferation assay (CellTiter 96 AQ, Proliferation Assay; Promega). Cells (1.5 × 104) were plated per well in 96-well plates. After different treatments, the medium was removed from cells and replaced by 100-μl mixture of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2Htetrazolium) with phenazine ethosulfate diluted in culture medium (1:10). Cells were incubated in a 5 % CO2 incubator at 37 °C for 2 h with protection from light. The optical density was determined at 490 nm using a microplate reader (ELX800, BIOTEK, VT, USA).
Western Blot Analysis
Following various treatments, cellular proteins were extracted using RIPA buffer (Beyotime, Beijing, China) containing protease and phosphatase inhibitor cocktails (Roche, Shanghai, China) on ice. After that, sonication at 40 % max power in 8-s bursts was performed and samples were separated by centrifugation at 12,000 rpm for 15 min at 4 °C. Protein concentrations were determined using a BCA kit (Thermo Fisher Scientific, Rockford, IL, USA). Protein samples (20 μg) were loaded and separated with 4–12 % Bis-Tris gel in 2-(N-morpholino) ethanesulfonic acid (MES) running buffer (Invitrogen, Carlsbad, CA, USA), and then transferred to polyvinylidene difluoride (PVDF) filters (Millipore, Bedford, MA). After blocking with 5 % non-fat milk, the membranes were probed with primary antibodies against α-synuclein (BD Transduction Laboratories, San Diego, CA, USA), LC3 (Sigma, St. Louis, MO, USA), p62 (Sigma), and incubated overnight at 4 °C. Membranes were then washed with 0.5 % Tween-20 in phosphate-buffered saline (PBST), incubated with IRDye 800-labeled secondary antibody (1:10,000, Rockland Immunochemicals, Gilbertsville, PA, USA), and scanned with an Odyssey infrared imaging system (LI-COR instrument, Lincoln, NE, USA). GAPDH (Sigma) or β-actin (Sigma) was used as a loading control. The band density was quantified using the ImageJ program and normalized to that of the control group.
Detergent solubility was performed by adding Triton X-100 to total cell lysates (final concentration 1 %) and incubating for 30 min on ice followed by centrifugation (15,000g, 60 min, 4 °C). The supernatant was designated Triton X-100 soluble fraction, and the pellet was redissolved in 2 % SDS-containing lysis buffer and sonicated for 10 s (Triton X-100 insoluble fraction).
Immunofluorescence and Confocal Microscopy
MN9D cells were seeded at 2 × 105/well to 24-well culture plates precoated with poly-l-lysine (100 μg/ml) and maintained at 37 °C with 5 % CO2. A53T or WT α-synuclein plasmid were transfected with Lipofectamine LTX and Plus reagent (Invitrogen, Carlsbad, CA, USA) for 48 h. Then, cells were fixed with 4 % paraformaldehyde at room temperature for 30 min followed by permeabilization in 0.03 % Triton X-100 (Beyotime, Beijing, China) at room temperature for 30 min. After fixation and permeabilization, cells were incubated with a mouse anti-α-synuclein antibody (BD) in phosphate-buffered saline (Beyotime, Beijing, China) containing 10 % normal goat serum (ZSJQ, Beijing, China) overnight at 4 °C. After washing with PBS, cells were incubated with Alexa488-conjugated goat anti-rabbit IgG (ZSJQ, Beijing, China) at room temperature for 1 h. Hoechst 33258 staining (Molecular Probes, H-1398; 1 μg/ml) was performed for 5 min at room temperature. Samples were observed under a Leica TCS SP5 confocal system.
For quantitative analysis of fluorescence intensity, a maximum of ten randomly selected transduced cells in gray matter in all sections of one complete series were manually outlined and the mean fluorescence intensity was measured using the ImageJ program.
Immunofluorescence of Autophagy Detection
MN9D cells were seeded at 2 × 105/well to 24-well culture plates precoated with poly-l-lysine (100 μg/ml) and maintained at 37 °C with 5 % CO2. GFP-LC3 plasmids (Prof. Wei Ding) were transfected with Lipofectamine LTX and Plus reagent (Invitrogen, Carlsbad, CA, USA) for 48 h. MN9D cells expressing GFP-LC3 were treated with 50 nM T10 for 6 or 24 h. Then, cells were fixed with 4 % paraformaldehyde at room temperature for 30 min. After fixation, the samples were observed under a Leica TCS SP5 confocal system. The GFP-LC3II puncta in each cell were manually counted, and 55–60 cells were randomly selected for counting in each group. The data presented were from one representative experiment of at least three independent experiments.
Lysotracker Staining and High Content Screening Assay
For measurement of lysosomal activity, cells were incubated with 1 μM Lysosensor Green (Molecular probes, L7535) for 30 min at 37 °C at 24 h after T10 treatment. Subsequently, cells were fixed in 4 % paraformaldehyde for 15 min and washed twice with PBS. Fluorescent images were recorded using a fluorescence microscope or confocal microscope.
To quantify the fluorescence intensity, cells were harvested, stained with DAPI, and detected by ArrayScan XTI HCS Reader (Thermo Fisher Scientific, Rockford, IL, USA). Ten views of cells in each group were selected randomly to read the fluorescence intensity of individual cells and identify total cell numbers. The mean fluorescence intensity was determined to estimate lysosomal activity.
Transmission Electron Microscopy
After T10 treatment, MN9D cells were collected by digestion and centrifugation. The precipitate was fixed in ice-cold 3 % glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h. The pellets were washed in 0.1 M PB buffer, postfixed in Osmium tetroxide (OsO4), dehydrated by serial dilutions in EtOH, and embedded. Ultrathin sections (70 nm) were stained and photographed on a JEM-1400 plus electron microscope with 80-kV acceleration voltages.
Real-Time Polymerase Chain Reaction
The total RNA from cells was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). The ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA, USA) was used for cDNA synthesis at 1 μg total RNA per reaction. The primers to detect human α-synuclein were designed as 5′-ACAGCAGTAGCCCAGAAGACAG-3′ and 5′-GGCTTCAGGTTCGTAGTCTTGATA-3’; for GAPDH, the primers were 5′-TCTGGAAAGCTGTGGCGTG-3′ and 5′-CCAGTGAGCTTCCCGTTCAG-3′. SYBR Green-based quantitative PCR (Agilent Technologies, Santa Clara, CA, USA) was performed with Mx3000P systems and the reaction conditions were as follows: 1 cycle at 95 °C for 30 s; 40 cycles at 55 °C for 60 s; and 72 °C for 30 s; followed by 72 °C for 5 min.
ELISA of Secretory α-Synuclein Detection
MN9D cells were transfected with A53T or WT α-synuclein plasmid for 24 h, and then treated with different concentrations of T10 (0, 5, 50 nM) or rapamycin 0.5 μm for 24 h. Primary cultured cortical neurons were infected with lentivirus carrying WT α-synuclein for 48 h, and then treated with different concentrations of T10 (0, 0.5, 5, 50 nM) or rapamycin 0.5 μm for 24 h. The culture medium was collected and centrifuged (1000g, 5 min, 4 °C). The supernatant was subjected to detect the concentration of α-synuclein by ELISA according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA).
Macroautophagy and Proteasome Inhibition
Autophagy inhibitor 3-methyladenine (3-MA) of 1 mM (Sigma, St. Louis, MO, USA) or the proteasome inhibitor lactacystin of 2.5 μM (Sigma, St. Louis, MO, USA) was added to the medium 1 h before T10 treatment. Twenty-four hours later, cell lysates were collected for western blotting.
Statistical Analysis
All results were expressed as the means ± standard deviation (SD) of at least three independent experiments performed in duplicate. The statistical analyses were performed via the Student’s t test (for the comparison between two parallel data sets) or one-way ANOVA followed by the Newman–Keuls test (for multigroup comparisons) using Prism 4.0 software. Data were presented as the mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 were considered statistically significant.
Results
T10 Protected Dopaminergic Cells From α-Synuclein-Induced Cytotoxicity
Both in vivo and in vitro studies indicated that overexpression of α-synuclein led to dopaminergic neuron degeneration [17–19]. MN9D cells were fusion of neuroblastoma with mice embryonic ventral mesencephalic cells and were commonly used as dopaminergic neuron model to study PD and other neurodegenerative diseases [20–22]. We first transfected the cells with wild-type (WT), A53T mutant (A53T) α-synuclein or pcDNA3.1 vector plasmids for 24 h and treated with or without 50 nM T10 for another 24 h. A marked expression of α-synuclein was observed in WT and A53T-transfected group (Fig. 1a–d), and the number of cells decreased in both WT and A53T α-synuclein-transfected group compared to vector control group according to the DAPI staining (Fig. 1a, c, e), but increased after T10 treatment (Fig. 1b, d). To quantify the effect of T10 on α-synuclein-induced neurotoxicity, we tested the cell viability by MTS assay. We observed that after A53T α-synuclein overexpression, the cell viability significantly decreased to 67.8 % compared to the vector control group, but T10 increased it to 81.8 %. After WT α-synuclein overexpression, the cell viability significantly decreased to 87.8 % compared to the vector control group, and T10 had no significant effect (Fig. 1f). These results indicated that T10 attenuated the cytotoxicity induced by pathogenic A53T α-synuclein overexpression.
T10 attenuates the cytotoxicity induced by α-synuclein overexpression. MN9D cells were overexpressed with WT or A53T α-synuclein and then exposed to 50 nM T10 for 24 h. a–e Immunofluorescent staining of α-synuclein (green), as well as the nuclear stain DAPI are shown (scale bar, 100 μm). f Cell viability was assessed by MTS assay. pcDNA3.1 vector plasmid was transfected as vector control (n = 6)
T10 Promoted the Clearance of α-Synuclein in Neuronal Cells
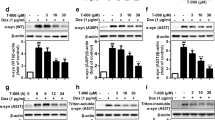
The neurotoxicity of α-synuclein is related to its expression level, so we next investigated the effect of T10 on the α-synuclein expression level in neuronal cells. We first transfected MN9D cells with WT or A53T α-synuclein plasmids for 24 h, and then added 50 nM T10 into the medium for another 24 h. By α-synuclein immunostaining, we found that α-synuclein expression level in single cell decreased significantly in T10-treated groups compared to WT or A53T α-synuclein-transfected group (Fig. 2a–e). To further confirm that T10 decreased α-synuclein expression level, we added various concentrations of T10, including 0.5, 5, 25, and 50 nM into the medium of WT or A53T α-synuclein overexpression cells. The α-synuclein expression level was determined by western blot. Rapamycin is an mTOR inhibitor and can induce autophagy in neurons. It is used as a positive control to promote α-synuclein clearance by autophagic pathway in this experiment. We observed that T10 significantly reduced WT α-synuclein level by 29.7 % (P < 0.05) at 50 nM, compared to no T10 treated group, which was more than rapamycin treatment (9.3 %) (Fig. 2f, g), and reduced A53T mutant α-synuclein level by 29.1 % (P < 0.05) at 5 nM and 37 % (P < 0.05) at 50 nM, which were more than rapamycin treatment group (21.8 %) (Fig. 2h, i). As a control, T10 did not reduce GFP levels (Fig. 2j).
T10 reduces the expression of α-synuclein in MN9D cells and cortical neurons. a–d MN9D cells were overexpressed with WT or A53T α-synuclein and then exposed to 50 nM T10 for 24 h. Immunofluorescent staining of α-synuclein (green), as well as the nuclear stain DAPI are shown (scale bar, 25 μm). e Summary of results demonstrating the mean intensity per cell from maximum of ten randomly selected transduced cells in gray matter per section from four independent experiments (n = 4). f, h MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h and then treated with different concentrations of T10 (0, 0.5, 5, 25, 50 nM) or rapamycin (0.5 μm) for 24 h. The cells were lysed for α-synuclein expression by western blot. g, i The protein levels were quantified relative to GAPDH levels and normalized to fold of control (n = 3 for WT and n = 4 for A53T). j MN9D cells were transfected with GFP plasmids for 24 h and then treated with 50 nM T10 for 24 h. The cells were lysed for GFP expression by western blot. k Primary cultured cortical neurons were infected with lentivirus carrying WT α-synuclein for 48 h, and then treated with different concentrations of T10 (0, 0.5, 5, 50 nM) for another 24 h. The cells were lysed for α-synuclein expression by western blot. l The protein levels were quantified relative to GAPDH levels and normalized to fold of control. (n = 4)
Since neurons are non-dividing cells, they are different from cell lines. We further confirmed the results in primary cultured cortical neurons. We infected neurons with lentivirus carrying WT α-synuclein for 48 h, and then added 0, 0.5, 5, 25, and 50 nM T10, as well as rapamycin into the medium for another 24 h. We observed that WT α-synuclein expression decreased by 19.0 % at 0.5 nM, 27.9 % at 5 nM, and 29.7 % at 50 nM T10 treatment, compared to no T10 treated group. As a control group, α-synuclein expression decreased by 21.8 % by rapamycin treatment (Fig. 2k, l).
Changes of syntheses, aggregation, clearance, and secretion of α-synuclein may all lead to the reduced level of α-synuclein after T10 treatment. It is reported that T10 inhibited RNA polymerase II-mediated transcription [23]; therefore, we first investigated the mRNA level of α-synuclein after T10 treatment. We transfected MN9D cells with A53T or WT α-synuclein plasmids for 24 h, and then added 50 nM T10 into the medium for another 24 h. We observed that T10 did not change the transcription level of either A53T or WT α-synuclein (Fig. 3a, b). Next, to examine the aggregation level of α-synuclein, we investigated the α-synuclein in Triton X-100-insoluble fractions. However, we did not observe any α-synuclein expression in the Triton X-100-insoluble fractions (data not shown). This may be due to the short time and limited amount of α-synuclein by transient transfection. Lastly, we observed the secretion of α-synuclein in MN9D cells and primary cultured neurons by collecting the medium of the transfected cells or neurons after Lv-syn infection and T10 treatment. We found that T10 did not significantly change the secretion of α-synuclein (Fig. 3c, d, e). Data from these studies indicated that T10 promoted the clearance of α-synuclein.
T10 does not change the syntheses, secretion, or proteasomal degradation of α-synuclein in MN9D cells. a, b MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h and then treated with 50 nM T10 for 24 h. The syntheses of α-synuclein were detected by q-PCR (n = 3). c, d, e MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h, and then treated with different concentrations of T10 (0, 5, 50 nM) or rapamycin 0.5 μm for another 24 h. Primary cultured cortical neurons were infected with lentivirus carrying WT α-synuclein for 48 h, and then treated with different concentrations of T10 (0, 0.5, 5, 50 nM) or rapamycin 0.5 μm for another 24 h. The medium was subjected to ELISA to detect the concentration of secretory α-synuclein, and the data was normalized by total protein (n = 3). f, h MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h, and 50 nM T10 with or without 2.5 uM lactacystin were added to the medium for 24 h. The cells were lysed for α-synuclein expression by western blot. g, i Quantification of α-synuclein/GAPDH ratio was described and normalized to fold of control (n = 3)
To examine whether the ubiquitin-proteasome system (UPS) is responsible for α-synuclein degradation by T10, we added lactacystin, a proteasome inhibitor with T10. We observed that lactacystin did not change the effect of T10. In WT α-synuclein overexpression cells, the α-synuclein level decreased to 74.5 % in T10 and lactacystin-treated group, compared to no T10 treated group, which is similar to 77.3 % in T10-treated group (Fig. 3f, g). In A53T α-synuclein overexpression cells, the α-synuclein level decreased to 62.8 % in T10 and lactacystin-treated group, compared to no T10-treated group, which is even lower than 90.6 % in T10-treated group (Fig. 3h, i). These results indicated that UPS was not the main pathway in T10 promoting the clearance of α-synuclein.
T10 Promoted the Clearance of α-Synuclein in Neuronal Cells Via Inducing Autophagy
α-Synuclein is degraded by both the UPS and ALP. We added 3-MA, an autophagy inhibitor with T10, and observed that 3-MA totally blocked the effect of T10 on α-synuclein degradation. In WT α-synuclein overexpression cells, the α-synuclein level significantly increased to 1.53-fold in T10 and 3-MA-treated group, compared to T10-treated group. In A53T α-synuclein overexpression cells, the α-synuclein level significantly increased to 1.44-fold in T10 and 3-MA-treated group, compared to T10-treated group (Fig. 4a–d). These results indicated that T10 promoted the clearance of α-synuclein mainly by the autophagic pathway.
T10 promotes the clearance of α-synuclein in MN9D cells mainly through autophagic pathway. a–d MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h, and 50 nM T10 with or without 1 mM 3-MA was added to the medium for 24 h. The cells were lysed for α-synuclein expression by western blot. Quantification of α-synuclein/GAPDH ratio was described and normalized to fold of control (n = 3). e–h MN9D cells were transfected with WT or A53T α-synuclein plasmids for 24 h and treated with different concentrations of T10 (0, 0.5, 5, 25, 50 nM) for 24 h. The cells were lysed for p62 expression by western blot. Quantification of p62/GAPDH ratio was described and normalized to fold of control (n = 3 for WT and n = 4 for A53T)
The p62 protein is a component of protein aggregates. On binding with LC3, p62 localized to the autophagic compartment and constantly degraded by the autophagy-lysosome system [24, 25]. The total expression of p62 inversely correlates with autophagic activity [26]. To provide further support of the involvement of autophagic pathway in the degradation of α-synuclein by T10 treatment, we examined the p62 level, as well as α-synuclein level after T10 treatment. We found that p62 level declined with α-synuclein expression after 50 nM T10 treatment by 29.2 % (P < 0.05) of WT α-synuclein and 29.2 % (P < 0.05) of A53T α-synuclein (Fig. 4e–h). These results suggested T10 promoted the degradation of α-synuclein in neuronal cells via inducing autophagy.
T10 Induced Autophagy in Neuronal Cells
To clarify that T10 is an autophagy inducer in neuronal cells, we first investigated LC3-II levels in MN9D cells after T10 treatment. We found 50 nM T10 significantly increased LC3-II levels by 31.5 % (P < 0.01), compared to no T10-treated group (Fig. 5a, b). In neurons, we also found 50 nM T10 increased LC3-II levels by 25.5 % at 24 h, compared to no T10-treated group (Fig. 5c). Similarly, GFP-LC3-II forms were accumulated by 2.3-fold at 24 h following T10 treatment in MN9D cells transfected with GFP-LC3 (Fig. 6a–c). The ultrastructural characterization of increased autophagic vesicles after 50 nM T10 treatment in MN9D cells further confirmed the increased autophagosome in MN9D cells after T10 treatment (Fig. 6d–g).
T10 induces LC3-II expression in MN9D cells and primary cultured cortical neurons. a MN9D cells were treated with different concentrations of T10 (0, 0.5, 5, 25, 50 nM) for 24 h. The cells were lysed for LC3 expression by western blot. b Quantification of LC3-II/LC3-I ratio was described and normalized to fold of control (n = 3). c Primary cultured cortical neurons were treated with different concentrations of T10 (0, 0.5, 5, 25, 50 nM) for 24 h, and the cells were lysed for LC3 expression by western blot
T10 induces autophagy in MN9D cells. a, b GFP-LC3 plasmid was transfected into MN9D cells for 24 h and then 0 or 50 nM T10 was added in medium for 24 h. Cells were fixed in 4 % paraformaldehyde and analyzed under a confocal microscope. c GFP-LC3 puncta number in each GFP positive neuron was counted and 55–60 neurons in each group were counted. Data were presented of one representative experiment from three independent experiments. d–g Transmission electron microscopy showed the characteristic ultrastructure of autophagy, including double membranes and autophagosomes after T10 treatment. Arrows represent autophagosomes
To examine whether the enhancement of autophagy by T10 is due to the blockage of autophagosome maturation, we investigated lysosomal activity by observing the intensity of fluorescent signals following staining with Lysosensor Green (LSG), a marker for lysosomal activity. We found that there is no change between control and T10 treatment group (Fig. 7a–c). Next, a lysosome inhibitor, chloroquine (CQ) [27], was added to the culture medium together with T10. We observed the LC3-II levels and found LC3-II increased in cells treated with T10 and CQ compared with cells treated with T10 or CQ alone, indicating T10 and CQ play different roles in accumulating LC3-II (Fig. 7d, e). Lastly, we investigated the cell viability after T10 treatment by MTS assay and found T10 at different concentrations did not change MN9D cell viability (Fig.7f). These results suggested T10 induced autophagy in neuronal cells without disturbing lysosome function or decreasing cell viability.
T10 does not change the lysosome activity or cell viability in MN9D cells. a, b MN9D cells were treated with 0 or 50 nM T10 for 24 h, and then loaded with 1 μM Lysosensor Green (LsG) and subjected for fluorescent imaging. c The fluorescence intensity was detected by HCS (n = 3). d MN9D cells were treated with 0 or 50 nM T10, or 10 μM CQ, or T10 plus CQ. The cells were lysed for LC3 expression by western blot. e Quantification of LC3-II/LC3-I ratio was described and normalized to fold of control (n = 3). f MN9D cells were treated with different concentrations of T10 (0, 0.5, 5, 50 nM) for 24 h, and the cell viability was tested by MTS assays
Discussion
In the present study, we demonstrated that T10 promoted the clearance of both WT and A53T α-synuclein and protected dopaminergic cells against A53T α-synuclein-induced toxicity. By adding autophagy inhibitor with T10, we found that the autophagic pathway was mainly involved in the degradation of α-synuclein by T10. Furthermore, we treated neuronal cells with T10 and found T10 induced autophagy without disturbing lysosome function or decreasing cell viability. These results indicated that T10 was an autophagy inducer in neuronal cells and could help to promote the clearance of various forms of α-synuclein in neuronal cells.
PD is the most common motor neurodegenerative disease. Overexpression of WT and mutant α-synuclein in transgenic mice caused progressive locomotor defects with dopaminergic neuron loss and intracytoplasmic inclusions [28–30]. Therefore, α-synuclein clearance could be a therapeutic target for the treatment of PD and other synucleinopathies. Enhancement of proteasomal or lysosomal activity represents therapeutic possibility for increasing α-synuclein degradation. However, autophagy is highly efficient in primary cortical neurons, and autophagosomes undergo rapid clearance in healthy brains. Induction of autophagy in neurons has been shown to be more difficult than in non-neuronal cells [31]. mTOR inhibitors such as rapamycin induce only mild autophagy in neurons [32]. Our study for the first time reported that T10 induces autophagy in neuronal cells. Our previous study indicated that 50 nM T10 inhibited inflammation in LPS-treated primary microglial cells [33]. T10 at the same concentration was found effective to induce autophagy and increase α-synuclein degradation. However, LC3-II level and autophagosomes increased only slightly after T10 treatment. Firstly, autophagy is highly efficient in neurons, and autophagosomes undergo rapid clearance in healthy brains. Therefore, induction of autophagy in neurons has been shown to be more difficult than in non-neuronal cells. Secondly, T10 did not induce potent autophagy in healthy neurons, which might in turn induce apoptosis in cells. We further proved its safety by testing the lysosome function and cell viability after T10 treatment and found T10 did not influence the lysosome function or cell viability. Our results contribute to the multifunctional therapeutic activities of T10.
Both WT and A53T α-synuclein were investigated in our study. A53T mutation of α-synuclein, a major gene related to some early-onset familial forms of PD, has been shown to increase the pathogenicity and cytotoxicity [34]. WT α-synuclein mainly aggregates in the Lewy bodies of sporadic PD patients and has lower cytotoxicity than A53T mutation [35]. In the present study, we also found that the cell viability decreased to 67.8 % in A53T α-synuclein transiently transfected MN9D cells, and 87.8 % in WT α-synuclein transiently transfected MN9D cells. After T10 treatment, the cell viability increased to 81.8 % in A53T α-synuclein transiently transfected MN9D cells. The therapeutic effect of T10 was not so obvious in WT α-synuclein transiently transfected MN9D cells.
α-Synuclein can be degraded either by proteasomes or by autophagy. Following on the accumulation of intracellular α-synuclein, these pathways play some different leading roles in the physiological status or pathologic processes of PD [36]. In our experiment, by adding 3-MA, an autophagy inhibitor, or lactacystin, a proteasome inhibitor with T10, we observed that 3-MA totally blocked the effect of T10 on α-synuclein degradation compared to 3-MA-treated group. However, after adding lactacystin, T10 still reduced the synuclein level compared with lactacystin-treated group. These data indicated that T10 promoted the clearance of α-synuclein mainly by the autophagic pathway. Although T10 promoted the clearance of both A53T and WT α-synuclein, it showed stronger effects in the degradation of A53T α-synuclein, as compared to WT α-synuclein group. The similar finding was that the neuroprotective effect of T10 was observed only in A53T transfection group.
Further investigations are still needed to explore the mechanism of T10. For example, we did not see the aggregation of α-synuclein in our experiment, which might be due to the transient transfected cell model that we used. The proautophagic effect of T10 is still unexplored in the mammalian in vivo PD model. Collectively, our study revealed that T10 could induce autophagy and promoted the clearance of both WT and A53T α-synuclein in neurons. These results raise the possibility of T10 for treating aggregation-prone protein-associated neurodegenerative diseases such as PD.
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM (1999) Degradation of alpha-synuclein by proteasome. J Biol Chem 274:33855–33858
Ebrahimi-Fakhari D, McLean PJ, Unni VK (2012) Alpha-synuclein’s degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy 8:281–283
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M et al (1997) Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 12:25–31
Prigione A, Piazza F, Brighina L, Begni B, Galbussera A, Difrancesco JC, Andreoni S, Piolti R et al (2010) Alpha-synuclein nitration and autophagy response are induced in peripheral blood cells from patients with Parkinson disease. Neurosci Lett 477:6–10
Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci 30:1166–1175
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T et al (2009) Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci 29:13578–13588
He Q, Koprich JB, Wang Y, Yu WB, Xiao BG, Brotchie JM, Wang J (2015) Treatment with trehalose prevents behavioral and neurochemical deficits produced in an AAV alpha-synuclein rat model of Parkinson’s disease. Mol Neurobiol. doi:10.1007/s12035-015-9173-7
Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N (1995) Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: triptolide and tripdiolide. Int J Immunopharmacol 17:351–356
Zheng Y, Zhang WJ, Wang XM (2013) Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neurosci Ther 19:76–82
Wang Q, Xiao B, Cui S, Song H, Qian Y, Dong L, An H, Cui Y et al (2014) Triptolide treatment reduces Alzheimer's disease (AD)-like pathology through inhibition of BACE1 in a transgenic mouse model of AD. Dis Model Mech 7:1385–1395
Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC, Heller A (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res 552:67–76
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 22:2780–2791
Lo BC, Ridet JL, Schneider BL, Deglon N, Aebischer P (2002) alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci U S A 99:10813–10818
Decressac M, Mattsson B, Bjorklund A (2012) Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson's disease. Exp Neurol 235:306–315
Wang J, Lou H, Pedersen CJ, Smith AD, Perez RG (2009) 14-3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem 284:14011–14019
Sivasubramanian M, Kanagaraj N, Dheen ST, Tay SS (2015) Sphingosine kinase 2 and sphingosine-1-phosphate promotes mitochondrial function in dopaminergic neurons of mouse model of Parkinson's disease and in MPP+ − treated MN9D cells in vitro. Neuroscience 290:636–648
Lim J, Kim HW, Youdim MB, Rhyu IJ, Choe KM, Oh YJ (2011) Binding preference of p62 towards LC3-ll during dopaminergic neurotoxin-induced impairment of autophagic flux. Autophagy 7:51–60
Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y, Smeaton M, Demain AL et al (2011) XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol 7:182–188
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG et al (2002) Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 -- > Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 99:8968–8973
Gispert S, Del TD, Garrett L, Chen A, Bernard DJ, Hamm-Clement J, Korf HW, Deller T et al (2003) Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci 24:419–429
Sommer B, Barbieri S, Hofele K, Wiederhold K, Probst A, Mistl C, Danner S, Kauffmann S et al (2000) Mouse models of alpha-synucleinopathy and Lewy pathology. Exp Gerontol 35:1389–1403
Tsvetkov AS, Miller J, Arrasate M, Wong JS, Pleiss MA, Finkbeiner S (2010) A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci U S A 107:16982–16987
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci 28:6926–6937
Gong Y, Xue B, Jiao J, Jing L, Wang X (2008) Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J Neurochem 107:779–788
Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PJ (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A 97:571–576
Ma QL, Chan P, Yoshii M, Ueda K (2003) Alpha-synuclein aggregation and neurodegenerative diseases. J Alzheimers Dis 5:139–148
Xilouri M, Brekk OR, Stefanis L (2013) alpha-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol 47:537–551
Acknowledgments
This study was supported by the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20140514), the Projects of Beijing Municipality (TJSHG201310025006), and the Beijing Natural Science Foundation (7162019).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, G., Gong, X., Wang, L. et al. Triptolide Promotes the Clearance of α-Synuclein by Enhancing Autophagy in Neuronal Cells. Mol Neurobiol 54, 2361–2372 (2017). https://doi.org/10.1007/s12035-016-9808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9808-3