Abstract
Sepsis results in unfettered inflammation, tissue damage, and multiple organ failure. Diffuse brain dysfunction and neurological manifestations secondary to sepsis are termed sepsis-associated encephalopathy (SAE). Extracellular nucleotides, proinflammatory cytokines, and oxidative stress reactions are associated with delirium and brain injury, and might be linked to the pathophysiology of SAE. P2X7 receptor activation by extracellular ATP leads to maturation and release of IL-1β by immune cells, which stimulates the production of oxygen reactive species. Hence, we sought to investigate the role of purinergic signaling by P2X7 in a model of sepsis. We also determined how this process is regulated by the ectonucleotidase CD39, a scavenger of extracellular nucleotides. Wild type (WT), P2X7 receptor (P2X7−/−), or CD39 (CD39−/−) deficient mice underwent sham laparotomy or CLP induced by ligation and puncture of the cecum. We noted that genetic deletion of P2X7 receptor decreased markers of oxidative stress in murine brains 24 h after sepsis induction. The pharmacological inhibition or genetic ablation of the P2X7 receptor attenuated the IL-1β and IL-6 production in the brain from septic mice. Furthermore, our results suggest a crucial role for the enzyme CD39 in limiting P2X7 receptor proinflammatory responses since CD39−/− septic mice exhibited higher levels of IL-1β in the brain. We have also demonstrated that P2X7 receptor blockade diminished STAT3 activation in cerebral cortex and hippocampus from septic mice, indicating association of ATP-P2X7-STAT3 signaling axis in SAE during sepsis. Our findings suggest that P2X7 receptor might serve as a suitable therapeutic target to ameliorate brain damage in sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the major cause of admission to intensive care units and cause of death worldwide. This severe clinical condition is characterized by an uncontrolled, excessive, and systemic inflammation that impacts multiple organs, including the brain [1, 2]. As a result, septic patients may present neurological symptoms, ranging from delirium, confusion, to coma. The detection of these neurological manifestations characterizes the sepsis-associated encephalopathy (SAE) [3–5].
SAE have been detected in up to 70 % of patients with severe systemic infection, and a substantial cognitive deficit has been reported in survivors [3, 6]. In addition, impairment in learning and memory has been observed in mammalian models of sepsis, although these are poorly defined [7–9]. Indeed, pathogenetic mechanisms underlying these neurological manifestations still remain poorly understood.
Increasing evidence suggest that neuroinflammation [10, 11], oxidative damage [12], caspase activation, and cellular death [13] are phenomena related to the pathophysiology of SAE. Proinflammatory cytokines, such as IL-1β and IL-6, also play a key role in the SAE pathophysiology [9, 11]. Activated microglia secretes IL-1β inducing transient synaptic deficits, which are strongly associated with sepsis-induced memory impairment [11]. In addition, blocking of IL-1β signaling attenuates cognitive deficits after sepsis [14]. Interestingly, the IL-1β release classically depends on a secondary signal, such as the extracellular adenosine triphosphate (ATP) that triggers the assembly of the inflammasome and subsequent caspase activation that in turn processes pro-IL-1β to its mature form. In this context, the extracellular ATP (eATP) might play an important role in the IL-1β release during SAE [15–17].
ATP is secreted and accumulates extracellularly in the central nervous system (CNS) as a result of cellular damage or inflammation [18]. This eATP has been considered a “danger” signal in the CNS and induces microglia activation and migration to the sites of injury, through the activation of P2 receptors [17–19]. The P2 receptor family comprises P2Y (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14) G-protein coupled receptors and P2X (P2X1–7) ligand-gated ion channels [20, 21]. The P2X7 receptor plays a key role in the microglial activation and proliferation [22, 23].
P2X7 receptor activation also induces maturation and release of proinflammatory cytokines, such as IL-1β and IL-18 [15], and the production of IL-6 [24] and reactive nitrogen and oxygen species in microglial cells [25–27]. Furthermore, the P2X7 receptor is involved in phospholipase and caspase activation, as well as in apoptosis induction [28–30], suggesting that P2X7 receptor may contribute to the progression of neuroinflammatory diseases.
The ATP-dependent proinflammatory signaling mediated by the P2X7 receptor can be regulated by cell surface ectonucleotidases expressed in microglial cells, such as CD39/ectonucleoside triphosphate diphosphohydrolase 1 (E-NTPDase 1) and CD73/ecto-5′-nucleotidase [31–33]. CD39/E-NTPDase1 hydrolyzes extracellular tri- and diphosphonucleosides to monophosphonucleosides, whereas the CD73/ecto-5′-nucleotidase is responsible for AMP hydrolysis, generating adenosine [32, 33]. This enzymatic cascade for degradation of extracellular ATP is important to reduce activation of phagocytic cells [34] and modulate the microglial migration and phagocytosis [35, 36].
Since the ATP-P2X7 receptor signaling responses induce oxidative stress and the maturation and release of proinflammatory cytokines, such as IL-1β, in the present study, we sought to investigate the role of ATP proinflammatory signaling in sepsis-associated brain dysfunction by inducing polymicrobial sepsis in P2X7−/− and CD39−/− mice, and in mice treated with the P2X7 specific antagonist brilliant blue G (BBG). We show that eATP acting through P2X7 receptor contributes to inflammation and oxidative stress in the brain during systemic polymicrobial infections, pointing P2X7 receptor as a new target for SAE management and treatment.
Materials and Methods
Reagents and Antibodies
Brilliant Blue G (BBG) was purchased from Sigma-Aldrich (MO, USA). Rabbit Phospho-Stat3 (Tyr705) (#9131) was obtained from Cell Signaling Technology (Danvers, MA, USA); mouse β-actin (AC-15, #ab6276) was obtained from Abcam (Cambridge, MA, USA); HRP-conjugated goat anti-mouse and donkey anti-rabbit IgG and the SuperSignal West Femto Maximum Sensitivity Substrate reagents (#PI-34096) were obtained from Thermo Scientific (Rockford, IL, USA).
Animals
Male, 8–10-week-old wild type (WT), P2X7 receptor deficient (P2X7−/−) C57BL/6 mice (originally from the Jackson Laboratory, USA) or CD39 deficient mice (CD39−/−) C57BL/6 weighing ∼25 g were used in this study. Animals were housed at a ratio of five mice per cage, with water and food ad libitum, on a 12-h light/dark cycle (lights on at 7:00 am), and at a temperature of 22 ± 1 °C. The procedures for the care and use of animals were according to the guidelines of the Brazilian College of Animal Experimentation (COBEA) and to the Guide for the Care and Use of Laboratory Animals (National Research Council, USA). All efforts were made to minimize animal suffering and to reduce the number of animals used in this study. All experiments were approved by the Commission for the Ethical Use of Research Animals (CEUA) from the Federal University of Rio de Janeiro (UFRJ) (approved protocol number 115/15) and by the Institutional Animal Care and Use Committees (IACUC) of Beth Israel Deaconess Medical Center (approved protocol number 019-2015).
Sepsis Induction by Cecal Ligation and Puncture (CLP)
CLP-induced polymicrobial sepsis was performed as previously described [37]. Briefly, mice anesthetized by intraperitoneal injection of 80 mg/kg ketamine and 5 mg/kg xylazine were subjected to laparotomy followed by extracorporeal cecum mobilization and ligation. After double enterotomy with a 21-G needle, a small amount of stool was gently squeezed out, to induce polymicrobial peritonitis, and the peritoneal cavity and abdominal wall were closed by suture. Control animals were subjected to a “sham” operation, consisting of laparotomy and intraperitoneal replacement, without ligation and puncture. After surgery, mice were injected subcutaneously with 1 mL of 0.9 % isotonic NaCl solution to compensate for third-spacing that occurred during the procedure. The brain samples were collected 24 h after surgery. For pharmacological inhibition of the P2X7 receptor in vivo, mice were injected intraperitoneally with 45.5 mg/kg of the P2X7 receptor specific antagonist Brilliant Blue G (from Sigma-Aldrich, MO, USA) or with vehicle control (PBS), 24 h before sepsis induction, as previously described [38].
Tissue Preparation and Blood Collection
Mice were anesthetized and blood samples were obtained from the inferior vena cava using tubes for blood collection with sodium citrate (5 %). The blood samples were centrifuged at 2000×g for 10 min, at 4 °C, and the recovered plasma was stored at −20 °C until further use. For determination of oxidative stress parameters, the cerebral cortex and hippocampus tissue was homogenized in 1:5 w/v of 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl. The homogenate was centrifuged at 750×g for 10 min at 4 °C. The pellet was discarded and the supernatant was immediately separated and used for the measurements of oxidative stress. For cytokine assay, the cerebral cortex samples were homogenized 1:4 (w/v) and hippocampus samples were homogenized 1:2 (w/v) in PBS buffer. The homogenate was centrifuged at 800×g for 5 min at 4 °C and the supernatant was used in the analysis. The Bio-Rad (Hercules, CA, USA) protein assay kit was used to determine the protein concentrations.
Quantitative Real-Time PCR (RT-qPCR)
Total RNA was isolated from brain tissue (harvested 24 h after sepsis induction) using TRIzol® reagent (Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s instructions. Total RNA was quantified using a ND-1000 spectrophotometer (NanoDrop), and cDNA was synthesized from 500 ng of total RNA using the High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Thermo Fisher Scientific, Rockford, IL, USA). The SYBR® Select Master Mix (Applied Biosystems, Foster City, CA, USA) was used for RT-qPCR to detect double-stranded DNA synthesis. Reactions were carried out in a final volume of 10 μL, using 2 μL of diluted cDNA (1:10) and 300 nM each of reverse and forward primers. The following primers were used for RT-qPCR: for P2rx7, 5′-AATCGGTGTGTTTCCTTTGG-3′ (forward) and 5′-CCGGGTGACTTTGTTTGTCT-3′ (reverse); and for Actb, 5′-TATGCCAACACAGTGCTGTCTGG-3′ (forward) and 5′-TACTCCTGCTTGCTGATCCACAT-3′ (reverse). Reactions were performed in a 7500 Fast Real-Time System (Applied Biosystems, Foster City, CA, USA). Relative expression levels were determined using the Sequence Detection Software v.2.0.5 (Applied Biosystems). P2rx7 to Actb relative amount was calculated using the comparative cycle threshold (Ct) method (ΔΔCt) and normalized to the level of sham-operated group.
2′,7′-Dichlorofluorescein (H2DCF) Oxidation Assay
The reactive oxygen species (ROS) and nitrogen reactive species (RNS) production was measured through 2′,7′-dichlorofluorescein (H2DCF) oxidation method [39]. Briefly, cerebral cortex or hippocampus supernatants (60 μL) were incubated for 30 min at 37 °C in the dark with 240 μL of 100 μM 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) solution in a 96-well plate. H2DCFDA is cleaved by cellular esterases and form H2DCF that is oxidized by ROS and RNS present in the sample producing a fluorescent compound, dichlorofluorescein (DCF). DCF oxidation was measured fluorimetrically by using a 488 nm excitation and 525 nm emission wavelength. A standard curve, using standard DCF (0.25–10 mM), was performed in parallel with the samples, and the results were represented by nanomoles of DCF per milligram of protein.
Superoxide Dismutase Assay (SOD)
The SOD activity assay is based on the capacity of pyrogallol to autoxidize, a process highly dependent on superoxide, a substrate for SOD. The inhibition of autoxidation of this compound occurs in the presence of SOD, whose activity was then indirectly assayed at 420 nm [40]. A calibration curve was performed with purified SOD as standard in order to calculate the activity of SOD present in the samples. SOD activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50 %, which is equal to 1 unit. The data were expressed as units per milligram of protein.
Catalase Assay (CAT)
CAT activity was assayed according to Aebi [41] by measuring the absorbance decrease at 240 nm in a reaction medium containing 20 mM H2O2, 0.1 % Triton X-100, and 10 mM potassium phosphate buffer, pH 7.0. One CAT unit is defined as 1 μmol of hydrogen peroxide consumed per minute, and the specific activity is reported as units per milligram of protein. The data were expressed as units per milligram of protein.
Cytokine Determination
The concentrations of the cytokines IL-6, IL-1β, and IL-10 in brain extract samples were measured using commercially available ELISA kits, as recommended by the manufacturer (R&D Systems, Minneapolis, MN, USA).
Western Blotting
The brain samples were then lysed in ice-cold modified-RIPA buffer (50 mM Tris-HCl, pH 7.4; 1 % NP-40; 0.25 % sodium deoxycholate; 150 mM NaCl) supplemented with Pierce™ Protease Inhibitor Mini Tablets (Thermo Fisher Scientific, MA, USA; #88665) and Phosphatase Inhibitor Cocktails (Sigma-Aldrich, MO, USA; #P5726). The lysates were sonicated briefly on ice and centrifuged at 14,000 rpm for 10 min at 4 °C. The measurement of protein concentrations and detailed procedures of immunoblotting were described previously [42].
Statistical Analysis
Results are expressed as means ± standard error of mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by Tukey multiple range tests. Differences between groups were considered statistically significant when p <0.05.
Results
P2X7 Receptor Expression Increase in the Brain from Septic Mice
Considering the role of P2X7 receptor in inflammatory diseases, we evaluated the gene expression profile of this receptor in the mice brain 24 h after sepsis induction. We detected a significant increase in P2X7 receptor expression in hippocampus (p > 0.05; Fig. 1b), but no significant difference was observed in the cerebral cortex (p > 0.05; Fig. 1a), suggesting that this receptor might contribute differentially to sepsis-associated brain inflammation in specific brain areas.
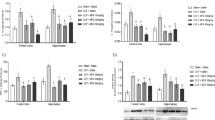
P2X7 receptor gene expression in cerebral cortex and hippocampus from septic mice. P2X7 gene expression was analyzed by RT-qPCR in cerebral cortex (a) and hippocampus (b) from septic mice 24 h after surgery. Data are expressed as mean ± SEM (n = 5 for both sham and CLP groups). Statistically significant differences between sham and CLP groups are represented by asterisks (*p < 0.05)
Genetic Deletion of the P2X7 Receptor Reduces Sepsis-Induced Oxidative Stress in Mice Brain
Since oxidative stress have been related with sepsis-induced brain dysfunction, we evaluated the production of reactive oxygen species (ROS) and nitrogen reactive species (RNS) through the 2′,7′-DCF oxidation and the antioxidant status measuring the activities of superoxide dismutase (SOD) and catalase (CAT) in WT and P2X7 deficient mice (P2X7−/−). Our results show an increase in the DFC oxidation in cerebral cortex (*p < 0.05; Fig. 2a, left) and hippocampus from WT septic mice (*p < 0.05; Fig. 2b, left), confirming sepsis-induced oxidative stress. The P2X7 deletion significantly reduced the oxidation of DCF in the hippocampus from CLP-induced mice (#p < 0.05; Fig. 2b, right), but no difference was found in the cerebral cortex (p > 0.05; Fig. 2a, right), suggesting that P2X7 receptor may be important for the production of reactive species in the brain. Corroborating this assumption, analysis of SOD and CAT in the hippocampus showed a significant increase of these enzymes activities only in samples from P2X7−/− septic mice (#p < 0.05; Fig. 3b, d), but not in the samples from WT mice (p > 0.05; Fig. 3b, d). Indeed, despite SOD and CAT activities are increased in cerebral cortex of both P2X7 deficient and WT septic mice (*p < 0.05; Fig. 3a, c), a significantly higher activity of these enzymes were seen in P2X7−/− septic mice when compared to WT group (#p < 0.05; Fig. 3a, c). Altogether, these results indicate that P2X7 receptor corroborates to the sepsis-induced oxidative stress in mice brain.
P2X7 receptor deletion attenuates sepsis-induced production of reactive oxygen species (ROS) and nitrogen reactive species (RNS) in mice brain. WT or P2X7−/− mice underwent sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). Brain samples were collected 24 h after surgery. The production ROS and RNS was assessed through the 2′,7′-DCF oxidation in cerebral cortex (a) and hippocampus (b). Data are expressed as mean ± SEM of two independent experiments (n = 6 for both sham and CLP groups). Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
P2X7 receptor deletion increases the superoxide dismutase (SOD) and catalase (CAT) activities in brain from septic mice. WT or P2X7−/− mice underwent sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). SOD (a, b) and CAT activities (c, d) were assessed in cerebral cortex (a, c) and hippocampus (b, d) 24 h after surgery. Data are expressed as mean ± SEM of two independent experiments (n = 6 for both sham and CLP groups). Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
Genetic Deletion of the P2X7 Receptor Decreases IL-1β and IL-6 Production in Cerebral Cortex and Hippocampus from Septic Mice
ATP signaling through the P2X7 receptor is involved in the maturation and release of proinflammatory cytokines involved in the sepsis-induced cognitive impairment [11, 15]. To explore the potential role for P2X7 receptor-induced cytokine production in the brain during sepsis, we evaluated the amount of IL-1β and IL-6 present in cerebral cortex and hippocampus from WT and P2X7−/− septic mice. As depicted in Fig. 4, both IL-1β and IL-6 are increased in the cerebral cortex and hippocampus from WT septic mice when compared to the sham group (*p < 0.05; Fig. 4a–d, left), confirming sepsis-induced brain inflammation. P2X7 genetic deletion attenuated the IL-1β production in cerebral cortex (#p < 0.05; Fig. 4a) and completely blocked the increase of this cytokine in the hippocampus after 24 h of CLP induction (#p < 0.05; Fig. 4b), reinforcing P2X7 greater activity in controlling inflammation on the later tissue. Indeed, P2X7 ablation leads to a significant decrease in the IL-6 levels both in the cerebral cortex and hippocampus (#p < 0.05; Fig. 4c, d) despite sepsis induction, suggesting that P2X7 receptor is important not only for IL-1β maturation but also for IL-6 production and release during sepsis.
Genetic deletion of P2X7 receptor reduces the sepsis-induced IL-1β and IL-6 production in mice brain. WT or P2X7−/− mice underwent sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). The IL-1β (a, b) and IL-6 production (c, d) were measured in cerebral cortex (a, c) and hippocampus (b, d) from septic mice 24 h after surgery. Cytokine levels are expressed as picograms per milligram of protein. Data are expressed as mean ± SEM of two independent experiments (n = 4 for sham groups and n = 6 for CLP groups). Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
CD39 Genetic Deletion Enhances IL-1β Production in Cerebral Cortex and Hippocampus from Septic Mice
Since eATP concentrations are precisely regulated through the action of ectoenzymes, such as CD39, we have evaluated the cytokine production in cerebral cortex and hippocampus from CD39 deficient septic mice. We detected an increase in IL-1β, IL-6, and IL-10 production in cerebral cortex (*p < 0.05; Fig. 5a–e) and hippocampus (*p < 0.05; Fig. 5b–f) from both WT and CD39−/− septic mice. The IL-1β levels were significantly higher in CD39−/− septic mice when compared to WT septic mice (#p < 0.05; Fig. 5a, b), while IL-6 levels showed no difference between these groups (p > 0.05; Fig. 5c, d). Differently, IL-10 levels were lower in the hippocampus from CD39−/− septic mice in comparison to WT septic mice (#p < 0.05; Fig. 5f). Altogether, these results reinforce the eATP role on the control and/or exacerbation of sepsis-induced inflammation, mainly concerning to IL-1β production and release, and highlight CD39 role in limiting this processes.
P2X7 receptor blockade inhibits the sepsis-induced cytokine production in cerebral cortex and hippocampus from WT and CD39−/−. WT and CD39−/− mice were injected i.p. with the P2X7 antagonist Brilliant Blue G (BBG, 45.5 mg/kg) or with vehicle control (PBS), 24 h before sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). Brain samples were collected 24 h after surgery. ELISA kits were used to measure the levels of and IL-1β (a, b), IL-6 (c, d), and IL-10 (e, f) in the cerebral cortex (a, c, e) and hippocampus (b, d, f). Cytokine levels are expressed as picograms per milligram of protein. Data are expressed as mean ± SEM of two independent experiments (n = 4 for sham groups and n = 8 for CLP groups). Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
Pharmacological Inhibition of P2X7 Receptor Reduces Cytokine Production in the Blood and Brain from WT and CD39−/− Septic Mice
Pharmacological inhibition of P2X7 receptor with BBG decreased the CLP-induced increase in IL-1β and IL-10 in the cerebral cortex and hippocampus from WT and CD39−/− septic mice (#p > 0.05 when compared to the sham groups; Fig. 5a–f). However, BBG pre-treatment abrogated the sepsis-induced increase in IL-6 production only in the brain samples from WT septic mice (#p > 0.05; when compared to the sham group), but not in samples from CD39−/− mice (p < 0.05; Fig. 5c, d), suggesting that BBG was not able to overcome ATP accumulation and IL-6 induction in this later group.
Despite this difference, we could detect significant decreases in IL-6 levels in hippocampus from CD39−/− septic mice treated with BBG when compared to non-treated CD39−/− septic mice (#p < 0.05, Fig. 5d). These data suggest that BBG and therefore P2X7 blockade may reduce cytokine formation and release, thus having a protective effect against sepsis-induced inflammation and brain damage.
Similar to our brain data, we found a significant increase in the IL-1β (p < 0.05; Fig. 6a) and IL-6 (p < 0.05; Fig. 6b) levels in the blood from WT and CD39−/− septic mice. The BBG pre-treatment decreased the sepsis-induced increase in IL-1β and IL-6 levels in the blood from septic mice (#p < 0.05; Fig. 6a, b), except the IL-6 levels in CD39 deficient mice, which remained elevated (p > 0.05).
P2X7 receptor blockade decreases the sepsis-induced increase in cytokine levels in the blood from WT and CD39−/−. WT and CD39−/− mice were injected i.p. with the P2X7 antagonist Brilliant Blue G (BBG, 45.5 mg/kg) or with vehicle control (PBS), 24 h before sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). Blood samples were collected 24 h after surgery. ELISA kits were used to measure the levels of and IL-1β (a) and IL-6 (b). Cytokine levels are expressed as picograms per milliliter. Data are expressed as mean ± SEM of two independent experiments (n = 4 for sham and n = 8 for CLP groups). Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
P2X7 Receptor Blockade Attenuates STAT3 Activation in the Brain from WT and CD39−/− Septic Mice
Oxidative stress and cytokines, such as IL-6, can activate the Signal Transducer and Activator of Transcription 3 (STAT3), which is involved in mechanisms underlying neuroinflammation and brain damage. In this way, our next step was to evaluate the intracellular pathway involved in the ATP-P2X7 controlling sepsis inflammation in the brain, focusing on STAT3 activation. Our results show an increase in STAT3 phosphorylation in the cerebral cortex (Fig. 7a, c) and hippocampus (Fig. 7b, d) from both WT and CD39 deficient mice 24 h after sepsis induction. P2X7 pharmacological inhibition with BBG prevented the sepsis-induced STAT3 activation in the brain samples from WT mice, but not in samples from CD39−/− mice (Fig. 7a–d). However, a slight but significant reduction in p-STAT3 levels could be observed in the hippocampus from CD39−/− septic mice treated with BBG when compared to non-treated CD39−/− septic mice (#p < 0.05; Fig. 7d). Considering that IL-6 leads to STAT3 activation, these results are in agreement with those showed above (Fig. 5c, d) and predict that the downstream pathway involved in ATP-P2X7 activation by sepsis involves IL-6 production and STAT-3 activation in the brain.
Pharmacological inhibition of P2X7 receptor attenuates STAT3 activation in cerebral cortex and hippocampus from septic mice. WT and CD39−/− mice were injected i.p. with the P2X7 antagonist Brilliant Blue G (BBG, 45.5 mg/kg) or with vehicle control (PBS), 24 h before sepsis induction by cecal ligation and puncture (CLP), or to sham laparotomy without puncture (Sham). Brain samples were collected 24 h after surgery. Representative Western blots (a, b) and quantification (c, d) of p-STAT3 in cerebral cortex (a, c) and hippocampus (b, d) from septic mice. Data are expressed as mean ± SEM of three independent experiments performed with a protein pool of 3 mice/experiment. Statistically significant differences between sham and CLP, and between CLP groups (CLP WT versus P2X7−/−) are represented by asterisks (*p < 0.05) and by the number sign (#p < 0.05), respectively
Discussion
Sepsis and multiple organ failure are major causes of death in intensive care units, despite advances in the supportive therapy over the last decades [1, 43]. Early diagnosis and intervention are essential for reversal of the clinical condition [44]. The survivors exhibit neuropsychological sequelae, which includes a substantial cognitive dysfunction [3, 4].
Excessive inflammation, oxidative stress, disruption in blood-brain barrier, and severe glial activation have been described as the mechanism underlying to sepsis-induced cognitive impairment [10–12]. Nevertheless, there is no effective treatment or strategy to prevent the onset of these sepsis-related neurological symptoms, and few studies have been conducted to identify potential therapeutic mechanisms.
Taking into account the broadly known ATP proinflammatory actions mediated through P2X7 receptor, we explored roles for purinergic signaling in sepsis-associated brain dysfunction. We show that genetic deletion of P2X7 receptor reduced the oxidative stress in mice brain 24 h after sepsis induction, with a pronounced effect on the hippocampus. Pharmacological inhibition or genetic ablation of the P2X7 receptor attenuates the cytokine production in the brain from septic mice. Furthermore, our results suggest a crucial role for the enzyme CD39 in limiting the P2X7 receptor proinflammatory responses since CD39−/− septic mice exhibited higher levels of IL-1β in the brain. Finally, we show that P2X7 receptor blockade in WT and CD39 deficient mice reduced the STAT3 activation in cerebral cortex and hippocampus, suggesting that ATP-P2X7 signaling may control intracellular pathways that could be involved with brain damage and inflammation during sepsis.
ATP-gated P2X7 receptor is thought to contribute to the development of the exacerbated inflammatory response in sepsis [45]. In the brain, this receptor has been described to be involved in the microglial activation and proliferation as well as in the ROS and RNS production by these cells under inflammatory conditions [23, 25, 26]. ROS and RNS are produced under physiological conditions as a result of normal cellular metabolism, and the enzymatic (SOD, CAT, and GSH-Px) and non-enzymatic (vitamins, carotenoids, glutathione, and others) natural antioxidant defenses maintain the balance between production and elimination of these reactive species [46, 47]. The oxidative stress occurs due to an imbalance between the ROS/RNS production and the antioxidant defenses negatively affecting cellular components (i.e., lipids, proteins, carbohydrates, and DNA) and leading to cell damage.
The brain is particularly vulnerable to oxidative damage due to its high metabolic rate. In addition, different cell types produce the reactive species in response to microbial products, including glial cells [48, 49]. In this context, authors have shown an increase in the production of reactive species (ROS, NO, and ONOO−) as well as in the oxidative damage to lipids and proteins in the brain from septic mice [9, 12, 50]. Our results are in accordance with these reports since we observed an increase in the DFC oxidation in cerebral cortex and hippocampus from WT septic mice. Interestingly, P2X7 receptor deletion prevents the increase in reactive species production in hippocampus from septic mice. In addition, SOD and CAT activities were significantly higher in P2X7−/− septic mice in comparison with WT septic mice in both brain structures, suggesting that P2X7 deficient mice present an enhanced adaptive capacity to sepsis-induced oxidative stress in the brain. In accordance with our data, Deng et al. [51] showed a significant decrease in ROS production and a significant increase in SOD activity in the brain after P2X7 blockade with BBG in a model of hypoxia. Therefore, our results support the notion that P2X7 receptor activation can contribute to the oxidative damage in neuroinflammatory diseases.
Under inflammatory conditions, P2X7 receptor activation is also a co-stimulus or a second signal for the inflammasome activation, promoting caspase-1 activation and the cleavage of pro-IL-1β into its mature form IL-1β [52, 53]. Interestingly, we observed a significant increase in P2X7 receptor expression in the hippocampus but not in the cerebral cortex from septic mice. The increased expression of this receptor could explain the pronounced inhibitory effects in IL-1β and ROS production observed in hippocampus in comparison to those observed in cerebral cortex from P2X7 deficient or BBG-treated septic mice. In the same line of evidence, Moraes and colleagues [11] verified that the IL-1β levels increase in mice hippocampus 24 h after sepsis induction, but not in the cerebral cortex, suggesting that the hippocampus is more sensible to sepsis-induced responses. The same authors showed that IL-1β induces a transient synaptic deficit, which is strongly associated with sepsis-induced memory impairment [11]. Moreover, a single dose of IL-1βra—IL-1β receptor antagonist—significantly attenuated the cognitive deficits after sepsis induction as well as the production of other cytokines in the brain, such as IL-6 and TNF-α [14]. IL-1β can induce IL-6 production via MyD88/NF-κB in neurons and glial cells, and the synergistic interaction between IL-1β and IL-6 can enhance the cognitive impairment [54–57]. In this context, here we showed that genetic deletion or pharmacological inhibition of P2X7 receptor significantly reduced the IL-1β and IL-6 production in mice brain after sepsis induction, suggesting that eATP acting via P2X7 receptor effectively contributes to proinflammatory cytokine production in the brain and, consequently, to sepsis-associated brain dysfunction.
Microglial cells secrete ATP in response to inflammatory stimuli [58]. Extracellular ATP levels are regulated during inflammatory processes by the action of ectonucleotidases such as CD39, the major ectonucleotidase expressed in immune cells [33]. CD39 has a protective role during sepsis, attenuating the systemic inflammation [59]. The activity of this enzyme increases in lymphocytes and macrophages after stimulation by bacterial products [34, 60]. Bulavina et al. [36] reported that the expression of CD39 is crucial for the ATP and ADP degradation in cultured microglia. They showed that CD39 deletion decreases the ATP degradation to about 75 % of the values measured in the brain slices from WT mice [36]. In addition, CD39−/− deficient microglia also showed and increased phagocytic activity, which was decreased by the pharmacological blockage of P2 receptors, suggesting that this enzyme is important to limit the activation of microglia cells by reducing the availability of P2 receptor ligands (i.e., ATP). Moreover, Lévesque et al. [61] have already reported that CD39 activity might limit in vitro cellular responses triggered by P2X7 receptor activation, such as apoptosis and IL-1β and IL-18 release, by comparing cells from CD39 deficient and WT mice. In accordance with these reports, we detected a higher production of IL-1β in the brain from CD39 deficient septic mice. These data support the hypothesis that CD39 deficiency increases ATP availability in the extracellular medium and, consequently, potentiates the P2X7 receptor responses, such as IL-1β secretion. Hence, P2X7 receptor blockade in CD39−/− septic mice results in significant decreases in IL-1β production in the cerebral structures analyzed.
Shieh et al. [24] showed that P2X7 receptor activation by ATP or BzATP (a specific P2X7 agonist) induced the cytokine and chemokine production in cultured mouse primary microglia from WT mice, but not in cells from P2X7 deficient mice. In addition, they show that various selective P2X7 receptor antagonists blocked the P2X7-dependent cytokine release by microglial cells, including the BBG. In the same line of evidence, Choi et al. [22] reported that P2X7 inhibition blocks the proinflammatory cytokine production in cultured human microglia stimulated with LPS. P2X7 receptor stimulates the IL-6 production in microglial cells in a Ca2+-dependent mechanism. P2X7−/− microglia, however, is able to produce IL-6 after the stimulation with LPS, suggesting that additional mechanisms could promote the production of this cytokine in the brain under inflammatory conditions [22, 24]. Here, the IL-6 production into the cerebral cortex and the hippocampus stimulated by CLP-induced polymicrobial sepsis was completely blocked by BBG treatment (WT mice) or P2X7 ablation (P2X7−/− mice). However, a slight decrease on this cytokine production was seen in the hippocampus from CD39−/− septic mice pre-treated with BBG. Possibly, the reduced degradation of tri- and diphosphonucleosides in CD39−/− mice potentiates the activations of other P2 receptors that have been described to be involved in IL-6 production, such as P2Y6, P2Y11, and P2Y13 [62–64].
The reduced IL-10 levels in the hippocampus from CD39−/− septic mice could be explained by the fact that in CD39−/− septic mice, the eATP degradation can be reduced as well as the adenosine generation. CD39 is crucial to hydrolyze ATP and ADP to produce AMP for adenosine generation through the action of CD73. High ATP levels can inhibit the CD73 activity reducing the adenosine production [65]. Adenosine, in turn, is an important anti-inflammatory compound that stimulates IL-10 production via A2B receptor activation [66].
Oxidative stress and cytokines, such as IL-6, activate the STAT3 in brain cells, which are involved in the mechanisms underlying cell death, tissue damage, and gliosis during brain injury and inflammation [67, 68]. Here, we showed that sepsis induced STAT3 activation in cerebral cortex and hippocampus from both WT and CD39−/− mice. P2X7 pharmacological inhibition with BBG prevented this intracellular pathway activation in the brain samples from WT mice. However, BBG treatment in CD39−/− septic mice was only able to attenuate STAT3 activation in the hippocampus similarly with the profile observed for IL-6 production. Therefore, both P2X7 receptor blockade and CD39 activity seems to be protective during SAE limiting the cytokine production and the activation of deleterious intracellular signaling pathways.
In summary, our data suggest a crucial role for extracellular ATP signaling via the P2X7 receptor in the pathophysiology of sepsis-associated brain dysfunction. In addition, we provide other evidence that CD39 activity is crucial in limiting P2X7 receptor inflammatory responses in the brain during sepsis. Such findings have clinical and pathophysiological relevance since supporting the notion that these signaling pathways may represent suitable therapeutic targets in the development of treatments for inflammatory diseases that compromise brain function.
Abbreviations
- ADP:
-
Adenosine 5′-diphosphate;
- AMP:
-
Adenosine 5′-monophosphate;
- ATP:
-
Adenosine 5′-triphosphate;
- BBG:
-
Brilliant blue G
- CLP:
-
Cecal ligation and puncture
- CNS:
-
Central nervous system
- eATP:
-
Extracellular adenosine 5′-triphosphate
- E-NTPDases:
-
Ecto-nucleoside triphosphate diphosphohydrolases
- IL:
-
Interleukin
- SAE:
-
Sepsis-associated encephalopathy
- STAT3:
-
Signal Transducer and Activator of Transcription 3
- TNF-α:
-
Tumor necrosis factor alpha
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR et al (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3. JAMA 315:801–810. doi:10.1001/jama.2016.0287
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8:557–566. doi:10.1038/nrneurol.2012.183
Lamar CD, Hurley RA, Hayman LA, Taber KH (2011) Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci 23:237–241. doi:10.1176/appi.neuropsych.23.3.237
Widmann CN, Heneka MT (2014) Long-term cerebral consequences of sepsis. Lancet Neurol 13:630–636. doi:10.1016/S1474-4422(14)70017-1
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–1794. doi:10.1001/jama.2010.1553
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223
Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, Heneka MT (2007) Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol 204:733–740. doi:10.1016/j.expneurol.2007.01.003
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominguini D, Steckert A et al (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59. doi:10.1016/j.bbi.2014.07.002
Michels M, Danielski LG, Dal-Pizzol F, Petronilho F (2014) Neuroinflammation: microglial activation during sepsis. Curr Neurovasc Res 11:262–270
Moraes CA, Santos G, de Sampaio e Spohr TC, D’Avila JC, Lima FR, Benjamim CF, Bozza FA, Gomes FC (2015) Activated microglia-induced deficits in excitatory synapses through IL-1β: implications for cognitive impairment in sepsis. Mol Neurobiol 52:653–663. doi:10.1007/s12035-014-8868-5
Schwalm MT, Pasquali M, Miguel SP, Dos Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J et al (2014) Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol 49:380–385. doi:10.1007/s12035-013-8526-3
Comim CM, Barichello T, Grandgirard D, Dal-Pizzol F, Quevedo J, Leib SL (2013) Caspase-3 mediates in part hippocampal apoptosis in sepsis. Mol Neurobiol 47:394–398. doi:10.1007/s12035-012-8354-x
Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall Igna DM, Ferreira GK, Silva MC, Galant LS et al (2014) IL1-β involvement in cognitive impairment after sepsis. Mol Neurobiol 49:1069–1076. doi:10.1007/s12035-013-8581-9
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883
Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8:315. doi:10.3389/fnins.2014.00315
Morandini AC, Savio LE, Coutinho-Silva R (2014) The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J 37:169–177. doi:10.4103/2319-4170.127803
Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 32:79–87. doi:10.1016/j.tins.2008.11.003
McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA et al (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366. doi:10.1126/science.1195491
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M et al (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Choi HB, Ryu JK, Kim SU, McLarnon JG (2007) Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci 27:4957–4968. doi:10.1523/JNEUROSCI.5417-06.2007
Monif M, Reid CA, Powell KL, Smart ML, Williams DA (2009) The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci 29:3781–3791. doi:10.1523/JNEUROSCI.5512-08.2009
Shieh CH, Heinrich A, Serchov T, van Calker D, Biber K (2014) P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia. Glia 62(4):592–607. doi:10.1002/glia.22628
Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, González FA, Weisman GA, Sun GY (2003) P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem 87:344–352
Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R (2003) P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 278:13309–13317
Hewinson J, Mackenzie AB (2007) P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans 35:1168–1170. doi:10.1042/BST0351168
Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K (1997) Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol 139:1635–1643
Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A et al (1999) P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Phys 276:C1139–C1147
Coutinho-Silva R, Stahl L, Raymond MN, Jungas T, Verbeke P, Burnstock G, Darville T, Ojcius DM (2003) Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity 19:403–412
Braun N, Sévigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H (2000) Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci 12:4357–4366
Zimmermann H (2001) Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res 52:44–56
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430
Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM (2013) TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 122:1935–1945. doi:10.1182/blood-2013-04-496216
Färber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, Gertz K, Endres M et al (2008) The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia 56:331–341. doi:10.1002/glia.20606
Bulavina L, Szulzewsky F, Rocha A, Krabbe G, Robson SC, Matyash V, Kettenmann H (2013) NTPDase1 activity attenuates microglial phagocytosis. Purinergic Signal 9:199–205. doi:10.1007/s11302-012-9339-y
Baker CC, Chaudry IH, Gaines HO, Baue AE (1983) Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94(2):331–335
Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF et al (2009) Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A 106:12489–12493. doi:10.1073/pnas.0902531106
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Marklund SL (1984) Pyrogallol autoxidation. In: Handbook for oxygen radical research. CRC Press, Boca Raton
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, Junger WG, Schmelzle M et al (2013) Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 57(1):205–216. doi:10.1002/hep.25989
Nguyen HB, Smith D (2007) Sepsis in the 21st century: recent definitions and therapeutic advances. Am J Emerg Med 25:564–571. doi:10.1016/j.ajem.2006.08.015
Dejager L, Pinheiro I, Dejonckheere E, Libert C (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19(4):198–208. doi:10.1016/j.tim.2011.01.001
Santana PT, Benjamim CF, Martinez CG, Kurtenbach E, Takiya CM, Coutinho-Silva R (2015) The P2X7 receptor contributes to the development of the exacerbated inflammatory response associated with sepsis. J Innate Immun 7(4):417–427. doi:10.1159/000371388
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40. doi:10.1016/j.cbi.2005.12.009
Halliwell B, Gutteridge JMC (2007) Free radical in biology and medicine, 4th edn. Oxford University Press, New York
Pawate S, Shen Q, Fan F, Bhat NR (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferon-gamma. J Neurosci Res 77:540–551
Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML (2005) Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 52:78–84
Dal-Pizzol F, Ritter C, Cassol-Jr OJ, Rezin GT, Petronilho F, Zugno AI, Quevedo J, Streck EL (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res 35(1):1–12. doi:10.1007/s11064-009-0043-4
Deng Y, Guo XL, Yuan X, Shang J, Zhu D, Liu HG (2015) P2X7 receptor antagonism attenuates the intermittent hypoxia-induced spatial deficits in a murine model of sleep apnea via inhibiting neuroinflammation and oxidative stress. Chin Med J 128(16):2168–2175. doi:10.4103/0366-6999.162495
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. doi:10.1038/nature04515
Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 105:8067–8072. doi:10.1073/pnas.0709684105
Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5:629–640
del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO (2013) A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav Immun 33:15–23. doi:10.1016/j.bbi.2013.05.011
Granger JI, Ratti P-L, Datta SC, Raymond RM, Opp MR (2013) Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior and cytokines in brain. Psychoneuroendocrinology 38:1047–1057. doi:10.1016/j.psyneuen.2012.10.010
Murray KN, Parry-Jones AR, Allan SM (2015) Interleukin-1 and acute brain injury. Front Cell Neurosci 9:18. doi:10.3389/fncel.2015.00018
Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med 185(3):579–582
Csóka B, Németh ZH, Törő G, Koscsó B, Kókai E, Robson SC, Enjyoji K, Rolandelli RH et al (2015) CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J 29:25–36. doi:10.1096/fj.14-253567
Vuaden FC, Savio LE, Bastos CM, Bogo MR, Bonan CD (2011) Adenosine A(2A) receptor agonist (CGS-21680) prevents endotoxin-induced effects on nucleotidase activities in mouse lymphocytes. Eur J Pharmacol 651:212–217. doi:10.1016/j.ejphar.2010.11.003
Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J (2010) NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol 40:1473–1485. doi:10.1002/eji.200939741
Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B (2008) Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol 74:777–784. doi:10.1124/mol.108.046904
Myrtek D, Müller T, Geyer V, Derr N, Ferrari D, Zissel G, Dürk T, Sorichter S et al (2008) Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol 181(3):2181–2188
Kawano A, Kadomatsu R, Ono M, Kojima S, Tsukimoto M, Sakamoto H (2015) Autocrine regulation of UVA-induced IL-6 production via release of ATP and activation of P2Y receptors. PLoS One 10(6):e0127919. doi:10.1371/journal.pone.0127919
Cunha RA (2001) Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem Res 26:979–991
Németh ZH, Lutz CS, Csóka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P et al (2005) Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol 175(12):8260–8270
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P et al (2013) The role of JAK-STAT signaling within the CNS. JAKSTAT 2(1):e22925. doi:10.4161/jkst.22925
Hristova M, Rocha-Ferreira E, Fontana X, Thei L, Buckle R, Christou M, Hompoonsup S, Gostelow N et al (2016) Inhibition of signal transducer and activator of transcription 3 (STAT3) reduces neonatal hypoxic-ischaemic brain damage. J Neurochem 136(5):981–994. doi:10.1111/jnc.13490
Acknowledgments
This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Núcleos de Excelência (PRONEX), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Instituto Nacional de Ciência e Tecnologia para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INPeTAm/UFRJ), and the National Institute of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Joint senior authors: Simon C. Robson and Robson Coutinho-Silva.
Rights and permissions
About this article
Cite this article
Savio, L.E.B., Andrade, M.G.J., de Andrade Mello, P. et al. P2X7 Receptor Signaling Contributes to Sepsis-Associated Brain Dysfunction. Mol Neurobiol 54, 6459–6470 (2017). https://doi.org/10.1007/s12035-016-0168-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0168-9