Abstract
The objective of this study was to explore the association between the P2X7 purinergic receptor (P2X7R) and neuroinflammation using a preclinical model of acute bipolar mania. We analyzed the modulatory effects of P2X7R agonist (3′-O-(4-benzoyl)benzoyl-adenosine 5′-triphosphate, BzATP) and antagonists (brilliant blue, BBG and 3-[[5-(2,3 dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride, A438079) on assessments related to behavior (locomotor activity), neuroinflammation (interleukin-1 beta, IL-1β; tumor necrosis factor alpha, TNF-α; and interleukin- 6, IL-6), oxidative stress (thiobarbituric acid reactive substances, TBARS) and neuroplasticity (brain-derived neurotrophic factor, BDNF) markers in a pharmacological model of mania induced by acute and chronic treatment with D-amphetamine (AMPH) (2 mg/kg) in mice. An apparent lack of responsiveness to AMPH was observed in terms of the locomotor activity in animals with blocked P2X7R or with genetic deletion of P2X7R in knockout (P2X7R−/−) mice. Likewise, P2X7R participated in the AMPH-induced increase of the proinflammatory and excitotoxic environment, as demonstrated by the reversal of IL-1β, TNF-α, and TBARS levels caused by P2X7R blocking. Our results support the hypothesis that P2X7R plays a role in the neuroinflammation induced by AMPH in a preclinical model of mania, which could explain the altered behavior. The present data suggest that P2X7R may be a therapeutic target related to the neuroinflammation reported in bipolar disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The precise mechanisms underlying the pathophysiology of bipolar disorder (BD) remain unknown. Recent reports suggest a role of neuroinflammation in the pathophysiology of BD [1, 2]. For instance, evidence points out to increased levels of the proinflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin-1beta (IL-1β) both in plasma and in postmortem frontal cortex of patients with BD [3, 4, 2]. In addition, increased excitotoxicity has been found in the postmortem frontal cortex of patients with BD [2], including increased oxidative stress [5]. Similarly, several data suggest that lower levels of brain-derived neurotrophic factor (BDNF) [6] may play a role in the reduced neuroplasticity observed in BD [7].
The development of animal models has been an important tool in the investigation of BD neurobiology and of new drugs for its treatment. In this sense, both acute and chronic uses of psychostimulants such as amphetamine (AMPH) have been widely used as animal models of mania [8–10].
The P2X7 purinergic receptor (P2X7R) has been increasingly implicated in the pathophysiology of medical conditions of the central nervous system [11]. P2X7R is an adenosine 5′-triphosphate (ATP)-binding ligand-gated ion channel that is activated by high concentrations of extracellular ATP [12]. It plays a key role in the modulation of inflammatory response mainly in the IL-1β, TNF-α, and IL-6 release, as well as an important action on the pathological activation of glial cells [13, 12]. Moreover, it has the ability to mediate cell death, making a critical contribution in mediating excitotoxicity [13]. Together, these functions have raised the potential link between P2X7R and the pathophysiology of BD. Additionally, it has been shown that the gene coding for P2X7R is located on a susceptibility locus associated with BD [14]. However, to date, few studies have been conducted to study a possible role of P2X7R as a molecular target in BD [15–17]. Thus, the objectives of the present work were to explore the modulatory effects of P2X7R on behavior and on markers of neuroinflammation, oxidative stress, and neuroplasticity in a preclinical pharmacological model of mania induced by acute and chronic treatment with D-amphetamine (AMPH).
Methods
Animals
Fifty male wild-type C57BL/6 (age 6–8 weeks; weight 18–25 g) divided by five to six animals per group were used in all experiment conducted. For the behavior experiments, we also used 20 male P2X7R knockout mice (P2X7R−/−) (age 6–8 weeks; weight: 18–25 g) and 24 male wild-type C57BL/6 as controls, in this case reported as P2X7R+/+, also divided by five to six animals per group. C57BL/6 mice were obtained from Universidade Federal de Pelotas (UFPEL), Pelotas, Brazil. P2X7R−/− mice were donated by Dr. Robson Coutinho-Silva from Universidade Federal do Rio de Janeiro (UFRJ), Brazil, and were generated by the method developed by Dr. James Mobley (PGRD, Pfizer Inc., Groton, CT, USA), originally from The Jackson Laboratory, USA, stock number 005576; whereas, P2X7R−/− mice were C57BL/6 inbred.
Animals were randomized and housed in groups of four per cage and maintained under controlled temperature (22 ± 2 °C) and humidity (60–70 %), at a 12/12-h light-dark cycle, with food and water ad libitum. Animals were acclimatized to the laboratory for at least 1 h before testing and were used only once throughout the experiment. All tests were performed between 7:00 a.m. and 7:00 p.m. P2X7R+/+ mice were used in all experimental procedures and P2X7R−/− mice were used only in the behavior assessment, as indicated. The experimental procedures reported in this manuscript were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals [18] and the Brazilian College of Animal Experimentation and were approved by the Animal Ethics Committee of the institution where the study was carried out (protocol no. 10/00206).
Drugs and Treatment
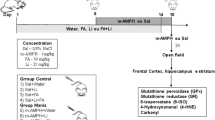
D-amphetamine sulfate salt (AMPH) and all other drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless indicated otherwise. Mice received intraperitoneal (i.p.) injections of AMPH (2 mg/kg) or vehicle (saline, 0.9 % NaCl). P2X7R agonists and antagonists were delivered to animals by intracerebroventricular (i.c.v.) microinjection at 2-μl volume in the following concentrations: potent P2X7R agonist 3′-O-(4-benzoyl)benzoyl-adenosine 5′-triphosphate (BzATP), 10.5 nmol; non-selective P2X7R antagonist brilliant blue G (BBG), 20 nmol; and high-affinity and selective P2X7R antagonist 3-[[5-(2,3 dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride (A438079), 1.75 nmol or vehicle (saline, 0.9 % NaCl). A438079 was obtained from Tocris Biosciences (Ellisville, MO, USA).
Treatment protocols were adapted from previous studies [19, 20] and performed as follows: in the acute treatment with AMPH, animals received i.c.v. microinjection of BzATP, A438079 or vehicle (saline, 0.9 % NaCl) followed by a single i.p. injection of AMPH or vehicle (saline, 0.9 % NaCl). Time interval between the i.c.v. and i.p. drug administrations was 15 min. In the chronic treatment with AMPH, mice received the i.p. injection of either AMPH or vehicle (saline, 0.9 % NaCl) once a day for 7 days. No behavioral test was performed between days 1 and 6. On the seventh day of treatment, animals received a single i.c.v. microinjection of BzATP, BBG, A438079 or vehicle (saline, 0.9 % NaCl). In all cases, both in acute and in chronic AMPH treatment, animals were subjected to the behavioral test (open field) immediately after administration of the last injection of AMPH or vehicle (saline, 0.9 % NaCl). Again, time interval between the i.c.v. and i.p. drug administrations was 15 min. The doses were chosen based on literature data [19, 21, 8] and preliminary tests.
Open Field Test
Locomotor activity was assessed using the open field test. All animals were behaviorally evaluated. Experiments were conducted in a sound-attenuated room under low-intensity light. Each animal was placed individually in the periphery of the arena of an acrylic box (40 × 60 × 50 cm) and left free for 60 min [22]. The animal’s behavior was recorded and analyzed using the ANY-Maze video-tracking system (Stoelting Co., Wood Dale, IL, USA). The overall distance traveled by the animal over 60 min of observation was quantified and was represented in meters (m) [23]. The apparatus was cleaned with ethanol 70 % after each trial.
Preparation of Samples
Immediately after the behavioral test, animals were euthanized and different brain structures isolated, namely, the striatum, prefrontal cortex and hippocampus. Brain tissue samples were homogenized (w/v, 1:10) with ice-cold 0.1M phosphate buffer (pH 7.4) with the addition of protease inhibitor cocktail. Homogenates were centrifuged at 2000g for 5 min, and aliquots of supernatants were separated and stored at −80 °C until further analysis.
Biochemical Determinations
Proinflammatory Cytokine Levels
Cytokine concentrations were determined via flow cytometry using the BD™ Cytometric Bead Array (CBA) assay, with the Mouse TNF-α, IL-6 and IL-1β Enhanced Sensitivity Flex Sets (BD Biosciences, San Diego, CA, USA), according to manufacturer’s instructions. Enhanced sensitivity flow cytometry is one of the main methods of choice for the assessment of such low-expressing molecules and the assays employed in this study have extremely low sensitivity (quantitation range of 0.274–200 pg/ml), which makes them ideal for these analyses. A further exploratory analysis of other inflammatory markers was later performed with BD CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences). The results were expressed as fg/ml.
TBARS Levels
Lipid peroxidation levels were measured using the commercial thiobarbituric acid reactive substances (TBARS) assay kit. The protocol was adapted [24] according to manufacturer’s instructions (Cayman, Ann Arbor, MI, USA). Results were expressed as μM of malondialdehyde (MDA).
BDNF Levels
Tissue concentrations of BDNF were measured using sandwich ELISA with monoclonal antibodies specific for BDNF. The protocol was adapted [25] according to manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Total protein content was measured using the Bradford method [26] and results were expressed as pg/μg of protein.
Statistical Analysis
The behavioral effects of subsequent administration of AMPH on P2X7R−/− mice were analyzed using two-way analysis of variance (ANOVA). The model includes two fixed factors each with two levels corresponding to P2X7R−/− mice (yes versus no) and subsequent AMPH administration (yes versus no). All other data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test for unequal samples. Significance was set at p < 0.05. Statistical analyses and graphics were performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 for Windows and the GraphPad Prism software version 5.0 for Windows (GraphPad, San Diego, CA, USA), respectively. All data are presented as mean ± standard error of mean (SEM).
Results
P2X7R Acts on Behavior in a Pharmacological Model of Mania Induced by Acute and Chronic AMPH Treatment
Figure 1a represents the pharmacological modulation of P2X7R associated with acute and chronic AMPH treatment in P2X7R+/+ mice. Both acute and chronic treatments with AMPH significantly increased locomotor activity when compared to control animals (p = 0.03 and p = 0.02 for acute and chronic AMPH treatment, respectively), even when AMPH was administered along with the P2X7R agonist BzATP (p = 0.01 and p = 0.03 for acute and chronic AMPH treatment, respectively). Conversely, acute AMPH treatment had no significant effect on locomotor activity when administered along with A438079 (p = 0.08). Similarly, treatment with the antagonists BBG and A438079 blocked the action of chronic treatment with AMPH, given that both had no significant effect on locomotor activity (p = 0.66 and p = 0.99 in comparison with vehicle/vehicle group, respectively). Finally, treatment with A438079 significantly reduced locomotor activity to control levels when compared with the vehicle/AMPH and BzATP/AMPH groups (p = 0.03 and p = 0.04, respectively) in the chronic AMPH treatment.
Modulatory effects of P2X7R on locomotor behavior in the model of mania induced by acute and chronic AMPH treatment. Effect of single i.c.v. administration of the potent P2X7R agonist BzATP (10.5 nmol), the non-selective P2X7R antagonist BBG (20 mmol), and the selective P2X7R antagonist A438079 (1.75 nmol) on locomotor activity, demonstrated as distance traveled (m) by a mouse model of mania induced by acute (2 mg/kg, i.p., single injection) and chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment (a). Behavioral changes in P2X7R−/− and P2X7R+/+ mouse models of mania induced by acute (2 mg/kg, i.p., single injection) and chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment (b). Two-way ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001. Data expressed as means ± SEM of five to six P2X7R+/+ or P2X7R−/− animals per group
Figure 1b shows the acute and chronic AMPH responses in P2X7R+/+ and P2X7R−/− animals. Two-way ANOVA revealed a significant interaction between the effects of genotype and AMPH administration in the locomotor activity of chronic AMPH treatment (F = 4.814; df = 1; p = 0.042), but not in the locomotor activity of acute AMPH treatment (F = 4.328; df = 1; p = 0.052). On the other hand, we found an acute AMPH-induced effect on locomotor activity (F = 14.257; df = 1; p = 0.001). As expected, post hoc analysis has indicated that AMPH increased locomotor activity in P2X7R+/+ animals in comparison to the P2X7R+/+/vehicle control group, in both the acute (p = 0.002) and chronic (p = 0.001) AMPH treatment groups. AMPH had no effects in P2X7R−/− animals when compared to P2X7R−/−/vehicle controls, neither in the acute (p = 0.66) nor in the chronic (p = 0.62) AMPH treatment groups. Moreover, the P2X7R+/+/AMPH groups showed a significant increase in locomotor activity compared to P2X7R−/−/vehicle groups in both acute (p = 0.001) and chronic (p = 0.001) AMPH treatments and also compared to P2X7R−/−/AMPH groups, again both in acute (p = 0.018) and chronic (p = 0.009) AMPH treatments.
Modulatory Effects of P2X7R on Proinflammatory Cytokines and TBARS Levels Induced by Acute AMPH Treatment
There was a significant increase in IL-1β levels in the striatum (p = 0.025) after acute AMPH injection when compared to control animals (Table 1). Striatal TNF-α levels were decreased in the A438079/AMPH group when compared with the vehicle/AMPH group (p = 0.026). Acute AMPH treatment also increased TBARS production in the striatum when compared with the vehicle/vehicle control group (p < 0.001). TBARS levels were reversed with the administration of A438079 (p = 0.005) pointing to a possible decrease of AMPH-induced oxidative stress by P2X7R blocking. We also evaluated the levels of IL-6 and BDNF in the acute AMPH model, but no significant differences were found (data not shown).
P2X7R is Implicated in the Increase of IL-1β and TNF-α Levels Observed in Response to Chronic AMPH Treatment
There was a significant increase in the levels of IL-1β in the striatum (p = 0.003) of mice subjected to chronic AMPH treatment when compared to the control group, which was reversed by treatment with BBG and A438079 (p = 0.005 and p = 0.005, respectively) (Fig. 2a). In the hippocampus, IL-1β levels were increased in the BzATP/AMPH group vs. the vehicle/vehicle control group (p = 0.025) and reversed with treatment with A438079 (p = 0.023) (Fig. 2a). In the same line, there was a significant increase in the levels of TNF-α (p = 0.018) in the hippocampus of animals in the BzATP/AMPH group when compared to the control group, a finding that was reversed by treatment with A438079 (p = 0.044) (Fig. 2b). We have also evaluated the levels of IL-6, but no significant differences were found between the groups (data not shown). An exploratory analysis was later performed to assess other inflammatory markers (IL-2, IL-6, IFN-γ, IL-4, IL-10, IL-17) in the same samples. Results for IL-2, IL-6, IFN-γ, and IL-4 were mostly below the detection limit of the respective standard curves and were not able to be statistically analyzed. As for IL-10 and IL-17, we found no significant differences between groups (p > 0.05 for all comparisons, data not shown).
Modulatory effects of P2X7R on proinflammatory cytokine response in the model of mania induced by chronic AMPH treatment. Effect of single i.c.v. administration of the potent P2X7R agonist BzATP (10.5 nmol), the non-selective P2X7R antagonist BBG (20 mmol) and the selective P2X7R antagonist A438079 (1.75 nmol) on IL-1β (a) and TNF-α (b) levels in the prefrontal cortex, striatum, and hippocampus of a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05, **p < 0.01. Data expressed as means ± SEM of five to six P2X7R+/+ animals per group
P2X7R Seems to Modulate TBARS Levels in the Model of Mania Induced by Chronic AMPH Treatment
TBARS levels are considered here as an indicator of oxidative stress. There was an increase in TBARS levels in the prefrontal cortex in the BzATP/AMPH group (p = 0.037) when compared to the control group, which was reversed by the treatment with A438079 (p = 0.01) (Fig. 3). In the hippocampus, chronic AMPH treatment increased TBARS levels (p = 0.022) when compared to the control group, and treatment with BBG and A438079 reversed that finding (p = 0.016 and p = 0.016, respectively) (Fig. 3).
Modulatory effects of P2X7R on TBARS levels in the model of mania induced by chronic AMPH treatment. Effect of single i.c.v. administration of the potent P2X7R agonist BzATP (10.5 nmol), the non-selective P2X7R antagonist BBG (20 mmol), and the selective P2X7R antagonist A438079 (1.75 nmol) on lipid peroxidation levels in the prefrontal cortex, striatum, and hippocampus of a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05. Data expressed as means ± SEM of five to six P2X7R+/+ animals per group
P2X7R is not Involved in BDNF Decrease Induced by Chronic AMPH Treatment
The last assessment focused on the BDNF levels. There was a significant decrease in BDNF levels in the prefrontal cortex of animals treated with chronic AMPH vs. those treated with vehicle/vehicle (p = 0.037) which was not modulated by P2X7R agonist or antagonist (Fig. 4).
Modulatory effects of P2X7R on BDNF levels in the model of mania induced by chronic AMPH treatment. Effect of single i.c.v. administration of the potent P2X7R agonist BzATP (10.5 nmol), the non-selective P2X7R antagonist BBG (20 mmol) and the selective P2X7R antagonist A438079 (1.75 nmol) on BDNF levels in the prefrontal cortex, striatum and hippocampus of a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05. Data expressed as means ± SEM of five to six P2X7R+/+ animals per group
Discussion
The results of the present study suggest an association between P2X7R and AMPH-induced hyperactivity, as demonstrated by a lack of responsiveness to AMPH in animals with blocked or absent P2X7R. Our study is consistent with previous reports [15, 17] and moves forward in the investigation of possible mechanisms involved in the neuroinflammation induced by AMPH. The results presented herein, and summarized in Table 2, reinforce the involvement of P2X7R as a mediator of behavioral and biochemical changes induced by AMPH in an animal model of mania.
Our study is in agreement with earlier data that showed that the blockade/deletion of P2X7R abrogates AMPH-induced hyperactivity [17]. In fact, a relationship between P2X7R and BD had already been suggested by genetic linkage and association studies, given that the P2X7R gene has been described to be located in the 12q23–24 chromosome region, a susceptible locus for BD and major depression [27]. Moreover, a recent study also showed that a selective P2X7R antagonist attenuated AMPH-induced hyperactivity in rats, which shows that this receptor might be a potential target to BD treatment [15]. Interestingly, previous studies from our laboratory showed that acute (in vitro) and chronic (in vivo) treatment with lithium and valproate (two known mood stabilizers) prevented ATP-induced cell death [28], probably via P2X7R, suggesting that a modulation of this receptor may also play a key role in the mechanism of action of such drugs.
The results obtained for the proinflammatory cytokines IL-1β and TNF-α suggest that P2X7R may play a central role in the AMPH-induced increase of the proinflammatory environment, as demonstrated by the reversal caused by P2X7R blocking. Of note, treatment with methamphetamine, a psychostimulant that shares a nearly identical chemical structure with AMPH [29], has been associated with increasing levels of proinflammatory cytokines in brain tissue [30]. We observed the same proinflammatory response after chronic treatment with AMPH and, to a lesser extent, after acute AMPH treatment as well. Since elevated levels of proinflammatory cytokines have been repeatedly demonstrated in patients with acute mania [31], the proinflammatory response to AMPH further validates the use of this animal model of acute mania. It is well known that P2X7R plays a central role in neuroinflammation [32], mainly due to its role in regulating the production and release of IL-1β, TNF-α, and IL-6 [33, 34] and in processing and releasing IL-1β [35]. Indeed, mice deficient in P2X7R have been shown to present decreased inflammatory responses [36], confirming the previously suggested relationship between neuroinflammation and P2X7R [37]. Interestingly, we found no significant differences in IL-6, which might be explained by its dual pro- and anti-inflammatory properties [38]. Accordingly, further studies should be performed to clarify the possible involvement of IL-6 and its receptors, as well as IL-10 and IL-17, in the P2X7R-modulating effects of AMPH in the periphery and also in other brain regions of interest.
In the present study, we suggest the involvement of P2X7R in the general increase of TBARS levels induced by acute and chronic AMPH administration. Lipid peroxidation is a particularly important consequence of oxidative stress [39] and is also a hallmark of excitotoxicity [40]. Previous studies had already shown increased lipid peroxidation levels in an AMPH-induced model of mania [41]. Similarly, several lines of evidence suggest that increased oxidative stress plays a prominent role in the progressive brain changes observed in patients with BD [7, 42]. P2X7R activation has also been related to enhanced oxidative stress [43, 44], justifying the responses observed when P2X7R was blocked. Additionally, our results confirm those of previous studies describing a decrease in BDNF levels after chronic AMPH treatment [45]—apparently, P2X7R modulation is not involved in that decrease. It is known that the P2X4 receptor (P2X4R) can cause the release of BDNF from microglia [46], and in vitro experiments suggest that P2X4R could play an important role in promoting BDNF production and release by activation of p38-MAPK and/or by TrkB phosphorylation [46, 47]. These data support our finding that the antagonism of P2X7R did not result in any action on BDNF levels, suggesting that this receptor is not directly involved in this process.
Overall, we report that AMPH treatment is inducing neuroinflammation and oxidative stress and that these effects are being mediated, at least in part, by the P2X7R. Microglial activation seems suitable to explain the integration of the neuroinflammatory/oxidative stress results and the P2X7R. It has been well recognized that microglial activation leads to the synthesis of proinflammatory and excitotoxity mediators, including IL-1β and TNF-α, chemokines, and reactive oxygen species, triggering tissue impairment [48, 32] and ultimately leading to the association of neurodegenerative diseases [48] and mental illnesses [2, 49, 50]. Furthermore, a recent review suggested a key role for microglial activation in BD [51]. The P2X7R, in turn, has been put forward as an essential component of the induction of microglial activation [32] and a recent review has suggested a relationship between BD, P2X7R, and microglial activation [16]. However, another recent study has reported no changes in behavior phenotype in chimeras lacking the P2X7R in their hematopoietic compartment [17]. Of note, our results do not allow us to adequately discuss the role of microglia activation in this scenario, especially when considering the so-called M1 and M2 microglia phenotypes [52]. In spite of this, since we found neither detectable levels of IL-4 in our samples nor significant differences in IL-10, two classic M2 markers [53], we could hypothesize that the environment dominated by proinflammatory cytokines in the brain regions exanimated might favor a polarization to M1 cells and inhibits a M2 switch. In these terms, future studies should investigate the real involvement of microglial activation and the switch between M1 and M2 microglial phenotypes in the AMPH-induced hyperactivity and if this involvement is subject to modulation by P2X7R.
In some analyses, such as assessments of IL-1β and TNF-α in hippocampus and TBARS levels in prefrontal cortex, we observed that only the coadministration of AMPH and BzATP led to a significant response of the corresponding biochemical parameter. However, this pattern of action was not always present, which precludes the conclusion that P2X7R agonist could increase the response to AMPH. In this vein, the absence of a BzATP/vehicle group does not allow us to confirm whether changes were induced by the agonist alone or by a synergistic effect between AMPH and BzATP. As reported previously, we found that the chronic administration of AMPH induced more significant effects when compared to acute AMPH treatment [20], indicating that the results are primarily related to a chronic exposure to AMPH.
Some limitations of the present study have to be considered. Firstly, the use of animal models for modeling psychiatric disorders has obvious limitations related to their validity [54, 55]. In this context, the animal model of mania used in the present study has been shown to present indications of face, construct and predictive validities [56, 45, 57]. In addition, we were not able to establish the specific mechanisms mediating the P2X7R participation on the AMPH-induced behavior. In this sense, our results suggest some pathways of relevance, but more studies are needed to characterize the P2X7R involvement in AMPH-induced hyperactivity.
In conclusion, our results support the hypothesis that P2X7R plays a role in the pathophysiology of acute mania, especially by mediating neuroinflammation and oxidative stress and ultimately leading to behavioral changes. This suggests that P2X7R may provide a therapeutic target for interventions related to neuroinflammation and BD. The relevance of microglial activation should also be further explored on this sense.
References
Kim YK, Jung HG, Myint AM, Kim H, Park SH (2007) Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord 104(1–3):91–95. doi:10.1016/j.jad.2007.02.018
Rao JS, Harry GJ, Rapoport SI, Kim HW (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15(4):384–392. doi:10.1038/mp.2009.47
Ortiz-Domínguez A, Hernández ME, Berlanga C, Gutiérrez-Mora D, Moreno J, Heinze G, Pavón L (2007) Immune variations in bipolar disorder: phasic differences. Bipolar Disord 9(6):596–602. doi:10.1111/j.1399-5618.2007.00493.x
Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, Drexhage HA (2010) The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother 10(1):59–76. doi:10.1586/ern.09.144
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14(1):123–130. doi:10.1017/S1461145710000805
Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, Santin A, Kapczinski F (2006) Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett 398(3):215–219. doi:10.1016/j.neulet.2005.12.085
Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PV, Amminger P, McGorry P, Malhi GS (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35(3):804–817. doi:10.1016/j.neubiorev.2010.10.001
Macêdo DS, Medeiros CD, Cordeiro RC, Sousa FC, Santos JV, Morais TA, Hyphantis TN, McIntyre RS, Quevedo J, Carvalho AF (2012) Effects of alpha-lipoic acid in an animal model of mania induced by D-amphetamine. Bipolar Disord 14(7):707–718. doi:10.1111/j.1399-5618.2012.01046.x
Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L (2007) Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett 427(1):66–70. doi:10.1016/j.neulet.2007.09.011
Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J (2006) Changes in antioxidant defense enzymes after d-amphetamine exposure: implications as an animal model of mania. Neurochem Res 31(5):699–703. doi:10.1007/s11064-006-9070-6
Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE (2009) Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res 198(1):83–90. doi:10.1016/j.bbr.2008.10.018
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82(4):1013–1067. doi:10.1152/physrev.00015.2002
Sun SH (2010) Roles of P2X7 receptor in glial and neuroblastoma cells: the therapeutic potential of P2X7 receptor antagonists. Mol Neurobiol 41(2–3):351–355. doi:10.1007/s12035-010-8120-x
Barden N, Harvey M, Gagné B, Shink E, Tremblay M, Raymond C, Labbé M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Müller-Myhsok B (2006) Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet 141B(4):374–382. doi:10.1002/ajmg.b.30303
Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, Fraser I, Nepomuceno D, Neff RA, Welty N, Lovenberg TW, Bonaventure P, Wickenden AD, Letavic MA (2013) Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol 170(3):624–640. doi:10.1111/bph.12314
Gubert C, Rodrigo Fries G, Wollenhaupt de Aguiar B, Ribeiro Rosa A, Busnello JV, Ribeiro L, Bueno Morrone F, Oliveira Battastini AM, Kapczinski F (2013) The P2X7R purinergic receptor as a molecular target in bipolar disorder. Neuropsychiatr Neuropsychol 8(1):1
Csölle C, Andó RD, Kittel Á, Gölöncsér F, Baranyi M, Soproni K, Zelena D, Haller J, Németh T, Mócsai A, Sperlágh B (2013) The absence of P2X7 receptors (P2rx7) on non-haematopoietic cells leads to selective alteration in mood-related behaviour with dysregulated gene expression and stress reactivity in mice. Int J Neuropsychopharmacol 16(1):213–233. doi:10.1017/S1461145711001933
NIH (2011) Guide for the care and use of laboratory animals—National Research Council 8th edn. The National Academies Press, Washington, DC
Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, Miras-Portugal MT, Henshall DC, Diaz-Hernandez M (2012) Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J 26(4):1616–1628. doi:10.1096/fj.11-196089
Frey BN, Andreazza AC, Ceresér KM, Martins MR, Petronilho FC, de Souza DF, Tramontina F, Gonçalves CA, Quevedo J, Kapczinski F (2006) Evidence of astrogliosis in rat hippocampus after D-amphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry 30(7):1231–1234. doi:10.1016/j.pnpbp.2006.03.016
Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM (2013) Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19(6):773–777. doi:10.1038/nm.3162
Maciel IS, Silva RB, Morrone FB, Calixto JB, Campos MM (2013) Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS One 8(9):e77227. doi:10.1371/journal.pone.0077227
Loss CM, Córdova SD, de Oliveira DL (2012) Ketamine reduces neuronal degeneration and anxiety levels when administered during early life-induced status epilepticus in rats. Brain Res 1474:110–117. doi:10.1016/j.brainres.2012.07.046
Gubert C, Stertz L, Pfaffenseller B, Panizzutti BS, Rezin GT, Massuda R, Streck EL, Gama CS, Kapczinski F, Kunz M (2013) Mitochondrial activity and oxidative stress markers in peripheral blood mononuclear cells of patients with bipolar disorder, schizophrenia, and healthy subjects. J Psychiatr Res 47(10):1396–1402. doi:10.1016/j.jpsychires.2013.06.018
Barichello T, Generoso JS, Simões LR, Ceretta RA, Dominguini D, Ferrari P, Gubert C, Jornada LK, Budni J, Kapczinski F, Quevedo J (2014) Vitamin B6 prevents cognitive impairment in experimental pneumococcal meningitis. Exp Biol Med (Maywood). doi:10.1177/1535370214535896
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Abkevich V, Camp NJ, Hensel CH, Neff CD, Russell DL, Hughes DC, Plenk AM, Lowry MR, Richards RL, Carter C, Frech GC, Stone S, Rowe K, Chau CA, Cortado K, Hunt A, Luce K, O'Neil G, Poarch J, Potter J, Poulsen GH, Saxton H, Bernat-Sestak M, Thompson V, Gutin A, Skolnick MH, Shattuck D, Cannon-Albright L (2003) Predisposition locus for major depression at chromosome 12q22–12q23.2. Am J Hum Genet 73(6):1271–1281. doi:10.1086/379978
Wilot LC, Bernardi A, Frozza RL, Marques AL, Cimarosti H, Salbego C, Rocha E, Battastini AM (2007) Lithium and valproate protect hippocampal slices against ATP-induced cell death. Neurochem Res 32(9):1539–1546. doi:10.1007/s11064-007-9348-3
Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK (1995) Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther 274(1):90–96
Gonçalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP (2008) Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann N Y Acad Sci 1139:103–111. doi:10.1196/annals.1432.043
Munkholm K, Braüner JV, Kessing LV, Vinberg M (2013) Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 47(9):1119–1133. doi:10.1016/j.jpsychires.2013.05.018
Monif M, Burnstock G, Williams DA (2010) Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol 42(11):1753–1756. doi:10.1016/j.biocel.2010.06.021
Di Virgilio F (2007) Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 28(9):465–472. doi:10.1016/j.tips.2007.07.002
Skaper SD, Debetto P, Giusti P (2010) The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24(2):337–345. doi:10.1096/fj.09-138883
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176(7):3877–3883
Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M (2011) P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol 44(3):423–429. doi:10.1165/rcmb.2010-0038OC
Sperlágh B, Vizi ES, Wirkner K, Illes P (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78(6):327–346. doi:10.1016/j.pneurobio.2006.03.007
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813(5):878–888. doi:10.1016/j.bbamcr.2011.01.034
Ferrarese C, Beal MF (2004) Excitotoxicity in neurological diseases: New therapeutic challenge. Kluwer Academic Print, Boston
Blaylock R (2004) Excitotoxicity: a possible central mechanism in fluoride neurotoxicity. Fluoride 37(4):13
Frey BN, Martins MR, Petronilho FC, Dal-Pizzol F, Quevedo J, Kapczinski F (2006) Increased oxidative stress after repeated amphetamine exposure: possible relevance as a model of mania. Bipolar Disord 8(3):275–280. doi:10.1111/j.1399-5618.2006.00318.x
Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J (2010) Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem Res 35(9):1295–1301. doi:10.1007/s11064-010-0195-2
Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, Sánchez-Armass S, Olivares-Reyes JA, Diebold B, Pérez-Cornejo P, Arreola J (2013) Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta 1830(10):4650–4659. doi:10.1016/j.bbagen.2013.05.023
Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carrì MT, Cozzolino M, Volonté C, D'Ambrosi N (2013) The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol 190(10):5187–5195. doi:10.4049/jimmunol.1203262
Frey BN, Andreazza AC, Ceresér KM, Martins MR, Valvassori SS, Réus GZ, Quevedo J, Kapczinski F (2006) Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci 79(3):281–286. doi:10.1016/j.lfs.2006.01.002
Trang T, Beggs S, Wan X, Salter MW (2009) P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29(11):3518–3528. doi:10.1523/JNEUROSCI. 5714-08.2009
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155(7):1596–1609. doi:10.1016/j.cell.2013.11.030
Weitz TM, Town T (2012) Microglia in Alzheimer's disease: it's all about context. Int J Alzheimers Dis 2012:314185. doi:10.1155/2012/314185
Bayer TA, Buslei R, Havas L, Falkai P (1999) Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett 271(2):126–128
Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68(4):368–376. doi:10.1016/j.biopsych.2010.05.024
Stertz L, Magalhães PV, Kapczinski F (2013) Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatr 26(1):19–26. doi:10.1097/YCO.0b013e32835aa4b4
Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39(1):3–18. doi:10.1111/nan.12011
Cherry JD, Olschowka JA, O'Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. doi:10.1186/1742-2094-11-98
El-Mallakh RS, Decker S, Morris M, Li XP, Huff MO, El-Masri MA, Levy RS (2006) Efficacy of olanzapine and haloperidol in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 30(7):1261–1264. doi:10.1016/j.pnpbp.2006.04.003
Krishnan V, Nestler EJ (2010) Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 167(11):1305–1320. doi:10.1176/appi.ajp.2009.10030434
Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31(5):326–332
Valvassori SS, Budni J, Varela RB, Quevedo J (2013) Contributions of animal models to the study of mood disorders. Rev Bras Psiquiatr 35(Suppl 2):S121–S131. doi:10.1590/1516-4446-2013-1168
Acknowledgments
CG, GRF, and PF are recipients of scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). BP is a scholarship recipient from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This study was supported by the National Science and Technology Institute for Translational Medicine, funded by CNPq and by Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA).
Conflict of Interest
CG, GRF, BP, PF, RCS, and FBM declare no possible conflicts of interest, financial or otherwise, or grants or other forms of financial support. AMOB has received grant/research from CNPq. FK has received grant/research support from Astra-Zeneca, Eli Lilly, Janssen-Cilag, Servier, CNPq, CAPES, NARSAD, and the Stanley Medical Research Institute; has been a member of speakers boards for Astra-Zeneca, Eli Lilly, Janssen, and Servier; and has served as a consultant for Servier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gubert, C., Fries, G.R., Pfaffenseller, B. et al. Role of P2X7 Receptor in an Animal Model of Mania Induced by D-Amphetamine. Mol Neurobiol 53, 611–620 (2016). https://doi.org/10.1007/s12035-014-9031-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9031-z