Abstract
Nuclear factor-κB (NF-κB) has been reported as a critical component of signalling mechanisms involved in the pathogenesis of a number of inflammatory conditions. Previous reports have shown that anti-inflammatory agents have a protective role in experimental diabetic neuropathy. Here, we assessed whether the inhibition of NF-κB cascade via IκB kinase (IKK) exerts any neuroprotective effect in experimental diabetic neuropathy. IKK inhibitor SC-514 (1 and 3 mg/kg) was administered daily for 2 weeks starting after 6 weeks of streptozotocin-induced diabetes. Nerve conduction and blood flow were determined by Powerlab and LASER Doppler system, respectively. We evaluated the changes in NF-κB, iNOS, and COX-2 expression by Western blotting in sciatic nerve. We found that IKK inhibition with SC-514 increased nerve blood flow and conduction velocity and improved pain threshold in diabetic animals. SC-514 also reduced the expression of NF-κB and phosphorylation of IKKβ in the sciatic nerve. Treatment with SC-514 reduced the elevated levels of pro-inflammatory cytokines (TNF-α and IL-6), iNOS, and COX-2. SC-514 reduces the expression of NF-κB and its downstream inflammatory components which may be involved in the improvement in nerve functions and pain perception in diabetic neuropathy. From the data of the present study, we suggest that diminution in IKK can be exploited as a drug target to significantly reduce the development of long-term complications of diabetes, particularly neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic neuropathy, the most common complication of long-standing diabetes mellitus, is associated with clinically significant morbidities. Being multifactorial in pathogenesis, several pathways have been implicated in diabetic neuropathy such as polyol pathway, oxidative stress, neuroinflammation, mitogen-activated protein kinase (MAPK), and poly ADP ribose polymerase (PARP) overactivation and activation of transcription factors, such as nuclear factor-κB (NF-κB). Diabetes and related complications have been found to be associated with altered levels of cytokines and chemokines which suggest a strong participation of inflammation in their pathophysiology. NF-κB is considered the master regulator of inflammatory responses, and it is now clear that many of the major pathways that are involved in pathophysiology of diabetic neuropathy are related to NF-κB activation. Furthermore, NF-κB regulates the transcription of many genes like iNOS, COX, and lipoxygenase which further propagate inflammation in peripheral nerves.
Under normal physiological condition, an inhibitory protein, IκB (inhibitor of NF-κB), binds to NF-κB thus sequestering it within cytosol in inactive form. Various stimulatory signals like TNF-α, cytokines, ROS, and bacterial and viral stimulants lead to the activation of a specific IκB kinase (IKK) complex that phosphorylates IκB. Phosphorylation of IκB leads to the IκB tagging with ubiquitin by an SKp1–Cullin–F-box (SCF)-type E3 ligase [1, 2]. Polyubiquitination marks IκB for rapid proteolytic degradation by the 26S proteasome. IκB degradation result in unmasking of nuclear localization signal of NF-κB which thus translocates into the nucleus and binds to promoter regions of target proinflammatory genes, thus initiates their transcription.
Thus, phosphorylation of IκB by IKK is a triggering event in NF-κB activation. IKK has been identified as a multisubunit complex containing catalytic subunits IKKα and IKKβ and regulatory subunit IKKγ (NEMO, NF-κB essential modifier). Although IKKα and IKKβ share 50 % sequence identity, many IKKα and IKKβ knockout studies have shown that IKKβ is the prime regulator of NF-κB-dependent proinflammatory signal transduction whereas IKKα is required for activation of alternative noncanonical pathway [3-6]. Several IKKβ inhibitors have been developed and have shown therapeutic responses in animal models of different diseases and are in early clinical trials [7-9]. Recent findings demonstrate that IKK overactivity is one of the pathogenetic factors in the development of diabetes, obesity, and related complications [10, 11].
The present study explores the specific involvement of NF-κB cascade in peripheral nerve dysfunction that characterize neuropathic component of diabetes. For this, we used small molecule inhibitor SC-514 (4-Amino-[2′, 3′-bithiophene]-5-carboxamide) which is specific for a pivotal event in the NF-κB cascade, i.e., IκB phosphorylation.
Materials and Methods
Unless otherwise stated, all chemicals were of reagent grade and were purchased from Sigma (St Louis, Missouri, USA).
Induction of Diabetes and Experimental Design
All animal experiments were performed in accordance with Committee for the Purpose of Control and Supervision on Experimentation on Animals (CPCSEA) guidelines. Experimental protocols were approved by the Institute Animal Ethics Committee (approval number IAEC/10/71) of NIPER SAS Nagar, Punjab, India. Male Sprague Dawley rats (250–270 g) were used and fed on standard rat diet and water ad libitum. Diabetes was induced by single dose of streptozotocin (STZ, 55 mg/kg, i.p.). Blood samples were collected 48 hours after STZ administration. Rats with plasma glucose level more than 250 mg/dl were considered as diabetics and were further considered for the study. The experimental groups comprised of nondiabetic (ND) control rats, diabetic control rats (STZ-D), and diabetic rats treated with SC-514 (D + SC 1 and D + SC 3, respectively for 1 and 3 mg/kg, i.p., in 2 % DMSO in saline). Each group contained six to eight animals. The treatment was started 6 weeks after diabetes induction and was continued for 2 weeks. The functional experiments were performed and nerves were isolated for biochemical and protein expression studies 24 h after administration of last dose.
Nerve Function Studies
Nerve Blood Flow
Nerve blood flow (NBF) was measured using LASER Doppler system (Perimed, Jarfalla, Sweden) [12]. The sciatic nerve was exposed by giving incision on the left flank, and the LASER Doppler probe was applied just in contact with an area of sciatic trunk free from epi or perineurial blood vessels. Flux measurement was obtained from the same part of the nerve and for the same time period (over a 10-min period). The blood flow was reported in arbitrary perfusion units (PU).
Motor Nerve Conduction Velocity
Motor nerve conduction velocity (MNCV) was determined in the sciatic-posterior tibial conducting system using the Power Lab 8sp system (ADInstruments, Bellaviata, NSW, Australia) as previously reported [13]. Briefly, animals were anesthetized by 4 % halothane in a mixture of nitrous oxide and oxygen and anesthesia was maintained with 1 % halothane, using gaseous anesthesia system (Harvard apparatus, Kent, UK). Body temperature was monitored using a rectal probe and maintained with a homeothermic blanket throughout the experiment. The sciatic nerve was stimulated with 3 V proximally at the sciatic notch and distally at the ankle via bipolar electrodes. Receiving electrodes were placed on the foot muscle. The latencies of the compound muscle action potentials were recorded via bipolar electrodes from the first interosseous muscle of the hind paw and measured from the stimulus artifact to the onset of the negative M-wave deflection. MNCV was calculated by dividing the distance between the stimulating and recording electrode with the difference obtained by subtracting the distal latency from the proximal latency and expressed in meters per second.
Behavioral Studies
Thermal Hyperalgesia
Thermal hyperalgesia was assessed in the rat hind paws using a Hargreaves apparatus (Ugo Basile, Como, Italy) [14]. Rats were placed into individual transparent plexiglass testing chamber. Rats were acclimatized to their environments for at least 30 min before any testing was carried out. A heat stimulus was delivered using a 0.5-cm-diameter radiant heat source situated under the plantar surface of the paw. The radiant heat source was adjusted to result in basal latencies of 12–14 s. We used a cutoff time of 15 s. The trials were performed three times at 5-min intervals, and the paw withdrawal latencies were averaged.
Mechanical Hyperalgesia
Sensitivity to noxious mechanical stimuli was determined by quantifying the withdrawal threshold of the hind paw in response to mechanical stimulation using a von Frey anesthesiometer (IITC Life Science, California, USA) as described [15]. The force causing the withdrawal response was recorded in grams. The test was repeated four to five times at ~5-min intervals on each animal, and the mean value was calculated.
Biochemical Parameters
Blood was collected from tail vein and plasma was separated at 5,000 rpm for 5 min at 4 °C. Plasma glucose level was estimated using GOD–POD kit (Accurex, Mumbai, India) as per manufacturer’s instructions.
Nerves were homogenized in PBS buffer containing phenyl methane sulphonyl fluoride (PMSF) and protease inhibitor cocktail. After homogenization, it was sonicated and detergent was added to the homogenate. Homogenate was kept in ice-cold water for 30 min and then sonicated. The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C, and the supernatant was used for estimations. Commercially available ELISA kits from eBiosciences (USA) for assaying TNF-α and IL-6 proteins were used. TNF-α and IL-6 levels were expressed as picograms per milligram of protein [16].
Immunohistochemistry
Sciatic nerves were fixed in 10 % buffered formaldehyde, dehydrated through graded concentrations of ethanol, embedded in paraffin, and sectioned. Sections (5-μm thick) were mounted on slides, cleared, and hydrated. Sections were treated with a buffered blocking solution (5 % goat serum). Then, sections were co-incubated with primary rabbit polyclonal IgG for NF-κB p65 subunit (rabbit polyclonal; Cell Signaling Technology) at a dilution of 1:400 at 4 °C for 12 h. Sections were washed with Tris–HCl buffer 0.5 M, pH 7.6, and incubated with horse radish peroxidase conjugated anti-rabbit secondary antibody, at room temperature for 1 h. Thereafter, sections were washed as before. Bound antibody was visualized by incubating the microsections with 3, 3-o-diaminobenzidine chromogen substrate solution in the dark, at room temperature for 10 min. Sections were washed with Tris–HCl buffer, counterstained with hematoxylin according to standard protocols and observed under light microscope (Leica, Solms, Germany) [13].
Western Blotting
Protein lysates were obtained by homogenizing sciatic nerves with lysis buffer containing 1 % Triton X-100, 150 mM NaCl, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 20 mM Tris (pH 7.5). Equal amounts of proteins were separated by SDS–PAGE and transferred to a nitrocellulose membrane (Pall Life Sciences, Florida, USA). After blocking with 3 % bovine serum albumin, membranes were incubated with primary rabbit polyclonal IgG NF-κB (p65 subunit), IKKβ, phospho-IKKβ, iNOS (Cell Signaling Technology, Massachusetts, USA) (1:1,000), and COX-2 (Santa Cruz Biotechnologies, California, USA) (1:400) for 12 h at 4 °C. After washing, membranes were incubated with horse radish peroxidase/alkaline phosphatase conjugated secondary antibody (1:2,000) and bound antibody was visualized by enhanced chemilumniscence or by using a colored reaction with BCIP-NBT. The relative band densities were quantified by densitometry. Equal loading of protein was confirmed by measuring β-actin expression [17].
Statistical Analysis
Data were expressed as mean ± SEM. Data were first analyzed by normality test to ascertain normal distribution. For comparing the differences between the two groups Student t test was used. For multiple comparisons, analysis of variance (ANOVA) was used. If ANOVA test showed significant difference, post hoc Tukey or Dunnet test was applied. Significance was defined as p < 0.05. All statistical analysis was performed using Jandel Sigma Stat 2, statistical software (Jandel Scientific, Erkrath, Germany).
Results
Body Weight and Plasma Glucose
STZ-injected rats displayed symptoms characteristic of type 1 diabetes like hyperglycemia and weight loss. Eight weeks post-STZ administration, diabetic rats had significantly lower body weight and higher plasma glucose levels as compared to nondiabetic rats (p < 0.001). IKK inhibition with SC-514 did not bring any significant changes in body weight or plasma glucose levels in treated diabetic group (Table 1).
Nerve Function Parameters
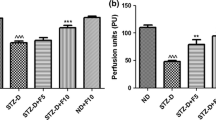
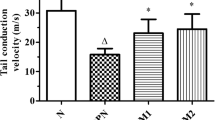
Eight weeks of diabetes resulted in a significant reduction in NBF (p < 0.001) and MNCV (p < 0.01) in the sciatic-tibial nerve conducting system of rats. Nerve function studies with SC-514 demonstrated that in SC-514 (3 mg/kg) treated diabetic group, the reduction in NBF and slowing of MNCV was reversed as compared to untreated diabetic rats (p < 0.05). The lower dose of 1 mg/kg, however, did not produce significant improvement in these parameters (Fig. 1).
Effect of SC-514 on nerve functions (nerve blood flow, NBF and motor nerve conduction velocity, MNCV) in diabetic rats. ND nondiabetic, STZ-D diabetic, D + SC 1 and D + SC 3 diabetic group treated with SC-514 1 and 3 mg/kg, respectively. Results are expressed as mean ± SEM; ## p < 0.01 and ### p < 0.001 vs. ND; *p < 0.05 vs. STZ-D (n = 6)
Nociceptive Parameters
After 8 weeks of diabetes, rats developed significant thermal (p < 0.01) and mechanical hyperalgesia (p < 0.001) compared with the control group. Both thermal and mechanical hyperalgesia in the hind paw was significantly attenuated with SC-514 treatment at the dose level 3 mg/kg (p < 0.05) (Fig. 2).
TNF-α and IL-6 Levels
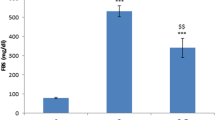
As TNF-α and IL-6 play central role in the inflammatory response in immediate, acute, and chronic inflammation, their levels were measured in this study by sandwich ELISA. Nerve homogenate from diabetic animals contained significantly higher amount of TNF-α (31.9 ± 2.4 pg/mg of protein, p < 0.05) and IL-6 (34.5 ± 1.8 pg/mg of protein, p < 0.01) compared to normoglycemic animals (TNF-α, 14.8 ± 3.2 pg/mg of protein and IL-6, 18.7 ± 1.1 pg/mf of protein). With SC-514 treatment, there was a significant reduction (p < 0.05) in pro-inflammatory cytokines levels (TNF-α 18.1 ± 2.5 and 17.4 ± 3.7 with 1 and 3 mg/kg respectively; IL-6 24.4 ± 2.5 and 22.4 ± 2.6 with 1 and 3 mg/kg respectively) (Fig. 3).
NF-κB Expression
Increased expression of NF-κB in sciatic nerves of diabetic animals indicates an overwhelmed NF-κB pathway which is subsequently associated with inflammatory neuronal death. We therefore investigated the neuroprotective potential of NF-κB inhibition in diabetic neuropathy. Western blot analysis in sciatic nerve homogenate revealed an increased NF-κB expression (p < 0.001) in untreated diabetic rats, while SC-514-treated diabetic group displayed far less NF-κB levels (D + SC 1 p < 0.01 and D + SC 3 p < 0.05) (Fig. 4a).
Effect of SC-514 on NF-κB in diabetic rats measured by Western blotting (a). Equal loading was confirmed by β-actin. Change in the expression of NF-κB as observed by immunohistochemistry (b). Arrows indicate NF-κB-positive staining corresponding to the magnification images × 40. Micron bar shows a length of 20 μm. ND nondiabetic, STZ-D diabetic, D + SC 1 and D + SC 3 diabetic group treated with SC-514 1 and 3 mg/kg, respectively. Results are mean ± SEM of three independent experiments. ### p < 0.001 vs. ND; *p < 0.05 and **p < 0.01 vs. STZ-D
Immunohistochemical analysis of sciatic nerve sections supports the immunoblotting studies as NF-κB-positive cells were more pronounced in the sciatic nerve microsections of diabetic rats than in nondiabetic control group (p < 0.001). Similar to the Western blot results, 2-week treatment with SC-514 was able to reduce the percentage of NF-κB-positive cells (p < 0.05) (Fig. 4b).
IKK Phosphorylation
Next, we analyzed the phosphorylation of IKKβ, which was increased significantly (p < 0.01) in diabetic group indicating that it is activated to a greater extent in diabetes. Analogous to NF-κB, IKKβ phosphorylation was inhibited by SC-514 in treated groups (p < 0.05) (Fig. 5).
Effect of SC-514 on IKK phosphorylation in diabetic rats measured by Western blotting. Equal loading was confirmed by β-actin. ND nondiabetic, STZ-D diabetic, D + SC 1 and D + SC 3 diabetic group treated with SC-514 1 and 3 mg/kg, respectively. Results are mean ± SEM of three independent experiments. ## p < 0.01 vs. ND; *p < 0.05 vs. STZ-D
iNOS and COX-2 Expression
iNOS is NF-κB target gene that regulates inflammation and plays a role in neurodegeneration by generating a highly reactive species peroxynitrite. COX-2, another NF-κB target, has been shown to be a key enzyme in nerve injury in diabetes. Thus, to assess the effect of NF-κB inhibition on diabetes-associated inflammatory stress, we examined two enzyme markers of inflammation in the sciatic nerve. Diabetes resulted in a significant increase (p < 0.001) in the expression of iNOS and COX-2. Treating diabetic rats with SC-514 significantly prevented the diabetes-induced increase in iNOS and COX-2 (p < 0.01) levels in comparison with untreated diabetic rats (Fig. 6).
Effect of SC-514 on iNOS and COX-2 in diabetic rats measured by Western blotting. Equal loading was confirmed by β-actin. ND nondiabetic, STZ-D diabetic, D + SC 1 and D + SC 3 diabetic group treated with SC-514 1 and 3 mg/kg, respectively. Results are mean ± SEM of three independent experiments. ### p < 0.001 vs. ND; **p < 0.01 vs. STZ-D
Discussion
In the recent years, experimental, epidemiological, and clinical data have suggested that inflammation is a determinant factor in the initiation and progression of diabetic neuropathy. There is a mounting consensus that hyperglycemia-induced NF-κB activation and subsequent events initiate as well as propagate the inflammatory changes in diabetes [18]. In the present study, we sought to delineate the role of IKK (a critical component of NF-κB activation cascade) in diabetic neuropathy, which can form novel target for drugs directed against inflammatory stress in various diabetic complications. IKK inhibitor, SC-514, improved nerve conduction and perfusion and normalized the altered sensory perception, thermal and mechanical hyperalgesia.
As discussed in previous sections, dissociation of inhibitory protein IκB from NF-κB is a prerequisite for its activation and its nuclear translocation. A number of detailed studies have shown that meeting point for the majority of NF-κB activating stimuli is multisubunit IKK which carries out the phosphorylation of IκB [19, 20]. SC-514 reduced the expression of NF-κB in our study in the sciatic nerve of treated animals. This concurs with other reports on various other disease models. Selective inhibition of IKK by SC-514 abrogates the phosphorylation and activation of p65 subunit of NF-κB [21, 22]. It not only inhibits the degradation of IκB but also promotes the export of nuclear NF-κB back to cytosol [23]. Thus, SC-514 not only reduces the p65 translocation into the nucleus but also serves to reduce the transcriptional effects of the NF-κB which has already reached the nucleus.
IKK is one of the first kinases activated in NF-κB cascade following exposure to activating stimuli. In vitro kinase assays in Ikkβ+/−cells showed that IKK activity is rate limiting in NF-κB activation as marginal diminution of kinase activity resulted in significant decline in IκBα degradation and NF-κB activation [24]. IKK activation depends on its phosphorylation which brings about a conformational change [2]. In our study, we found that SC-514 inhibited the IKK phosphorylation in the treated group thereby preventing it from further phosphorylating IκBα.
Recently, considerable progress has been made in dissecting out the potential role of NF-κB in various nerve pathophysiologies. Particular stress has been placed on the involvement of NF-κB in neurodegeneration in the central nervous system. Brain tissues from patients and in vitro studies with cultured neurons have revealed increased NF-κB activity in cells undergoing neurodegeneration [25-27]. Although much less information is available on its role in diabetic insult to peripheral nervous system, NF-κB has been extensively studied for its role in nociceptive alterations. NF-κB activation is one of the earliest events occurring in sciatic nerve injury model and is critical for hyperalgesia. We found that hyperalgesia develops 8 weeks after induction of experimental diabetes in rats. This coincides with the reduction of nerve blood flow and motor nerve conduction velocity. NF-κB inhibition with SC-514 suppressed thermal and mechanical hyperalgesia and also improved the nerve function by increasing nerve perfusion. In other studies as well SC-514 has been shown to modulate pain perception via NF-κB inhibition. In ceramide-induced inflammatory pain in rats, SC-514 blocked the development of thermal hyperalgesia in a dose-dependent manner [28]. A recent study concluded that IKK is involved in the development of inflammatory hyperalgesia as IKK knockout mice exhibited a decreased pain [29]. Proinflammatory mediators TNF-α and IL-6 which are regulated by NF-κB have been strongly correlated to the peripheral nerve injury and pain [30]. Thus, we quantified the levels of proinflammatory mediators. SC-514 treatment diminished the increase in the levels of IL-6 and TNF-α in the treated group thus decreasing altered nociceptive perception and improvement in nerve perfusion and conduction.
Many enzymes upregulated during inflammatory response such as iNOS and COX-2 are known transcriptional targets of NF-κB. Evidence that NF-κB regulates the transcription of various genes including COX-2 and iNOS is largely derived from indirect immunohistochemical and neuroanatomical studies [31]. However, in vitro conditions give a clear picture because two putative NF-κB motifs have been found on promoter region of both, COX-2 and iNOS gene [32, 33]. iNOS and COX-2 both have been known to be major perpetrator of nerve injury in diabetes. Studies with diabetic rats showed that inhibition of iNOS/COX-2 by administration of the selective iNOS/COX-2 inhibitors exert anti-nociceptive effect [34-36]. Western blot analysis showed that SC-514 reduced the expression of iNOS and COX-2.
In conclusion, we have demonstrated that SC-514 significantly alleviated hyperalgesia and improved nerve functions in diabetic rats, highlighting the significance of IKK as a pharmacological target which may have therapeutic benefits in patients with painful diabetic neuropathy.
References
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260. doi:10.1146/annurev.immunol.16.1.225
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663. doi:10.1146/annurev.immunol.18.1.621
Hayden MS, Ghosh S (2012) NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26(3):203–234. doi:10.1101/gad.183434.111
Karin M (1999) How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18(49):6867–6874. doi:10.1038/sj.onc.1203219
Perkins ND (2012) The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer 12(2):121–132. doi:10.1038/nrc3204
Scheidereit C (2006) IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 25(51):6685–6705. doi:10.1038/sj.onc.1209934
Gamble C, McIntosh K, Scott R, Ho KH, Plevin R, Paul A (2012) Inhibitory kappa B Kinases as targets for pharmacological regulation. Br J Pharmacol 165(4):802–819. doi:10.1111/j.1476-5381.2011.01608.x
Kim SG, Veena MS, Basak SK, Han E, Tajima T, Gjertson DW, Starr J, Eidelman O, Pollard HB, Srivastava M, Srivatsan ES, Wang MB (2011) Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res 17(18):5953–5961. doi:10.1158/1078-0432.CCR-11-1272
Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, Sakai K, Inbe H, Takeshita K, Ishimori M, Komura H, Murata T, Lowinger T, Bacon KB (2005) A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol 145(2):178–192. doi:10.1038/sj.bjp.0706176
Solinas G, Karin M (2010) JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J 24(8):2596–2611. doi:10.1096/fj.09-151340
van Diepen JA, Wong MC, Guigas B, Bos J, Stienstra R, Hodson L, Shoelson SE, Berbee JF, Rensen PC, Romijn JA, Havekes LM, Voshol PJ (2011) Hepatocyte-specific IKK-beta activation enhances VLDL-triglyceride production in APOE*3-Leiden mice. J Lipid Res 52(5):942–950. doi:10.1194/jlr.M010405
Negi G, Kumar A, Sharma SS (2011) Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res 8(4):294–304. doi:10.2174/156720211798120972
Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS (2009) Functional and biochemical evidence indicating beneficial effect of Melatonin and Nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology 58(3):585–592. doi:10.1016/j.neuropharm.2009.11.018
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88. doi:10.1016/0304-3959(88)90026-7
Negi G, Kumar A, Sharma SS (2010) Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun 391(1):102–106. doi:10.1016/j.bbrc.2009.11.010
Negi G, Kumar A, Sharma SS (2011) Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-kappaB and Nrf2 cascades. J Pineal Res 50(2):124–131. doi:10.1111/j.1600-079X.2010.00821.x
Kumar A, Negi G, Sharma SS (2011) JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: effect on neuroinflammation and antioxidant defence. Diabetes Obes Metab 13(8):750–758. doi:10.1111/j.1463-1326.2011.01402.x
Cameron NE, Cotter MA (2008) Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets 9(1):60–67. doi:10.2174/138945008783431718
Israel A (2010) The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2(3):a000158. doi:10.1101/cshperspect.a000158
Karin M, Delhase M (2000) The IkB kinase (IKK) and NF-kB: key elements of proinflammatory signalling. Semin Immunol 12(1):85–98. doi:10.1006/smim.2000.0210
Gomez AB, MacKenzie C, Paul A, Plevin R (2005) Selective inhibition of inhibitory kappa B kinase-beta abrogates induction of nitric oxide synthase in lipopolysaccharide-stimulated rat aortic smooth muscle cells. Br J Pharmacol 146(2):217–225. doi:10.1038/sj.bjp.0706308
Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN (2005) A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity. J Biol Chem 280(11):10326–10332. doi:10.1074/jbc.M412643200
Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS (2003) A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem 278(35):32861–32871. doi:10.1074/jbc.M211439200
Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M (1999) The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med 189(11):1839–1845. doi:10.1084/jem.189.11.1839
Gill JS, Windebank AJ (2000) Ceramide initiates NFkappaB-mediated caspase activation in neuronal apoptosis. Neurobiol Dis 7(4):448–461. doi:10.1006/nbdi.2000.0312
Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5(5):554–559. doi:10.1038/8432
Boissiere F, Hunot S, Faucheux B, Duyckaerts C, Hauw JJ, Agid Y, Hirsch EC (1997) Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport 8(13):2849–2852
Doyle T, Chen Z, Muscoli C, Obeid LM, Salvemini D (2011) Intraplantar-injected ceramide in rats induces hyperalgesia through an NF-kappaB- and p38 kinase-dependent cyclooxygenase 2/prostaglandin E2 pathway. FASEB J 25(8):2782–2791. doi:10.1096/fj.10-178095
Moser CV, Kynast K, Baatz K, Russe OQ, Ferreiros N, Costiuk H, Lu R, Schmidtko A, Tegeder I, Geisslinger G, Niederberger E (2011) The protein kinase IKKepsilon is a potential target for the treatment of inflammatory hyperalgesia. J Immunol 187(5):2617–2625. doi:10.4049/jimmunol.1004088
J-e Y, Yuan W, Lou X, Zhu T (2012) Streptozotocin-induced diabetic hyperalgesia in rats is associated with upregulation of toll-like receptor 4 expression. Neurosci Lett 526(1):54–58. doi:10.1016/j.neulet.2012.08.012
Nadjar A, Tridon V, May MJ, Ghosh S, Dantzer R, Amedee T, Parnet P (2005) NFkappaB activates in vivo the synthesis of inducible Cox-2 in the brain. J Cereb Blood Flow Metab 25(8):1047–1059. doi:10.1038/sj.jcbfm.9600106
Sorli CH, Zhang HJ, Armstrong MB, Rajotte RV, Maclouf J, Robertson RP (1998) Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci U S A 95(4):1788–1793
Xie QW, Kashiwabara Y, Nathan C (1994) Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem 269(7):4705–4708
Ramos KM, Jiang Y, Svensson CI, Calcutt NA (2007) Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes 56(6):1569–1576. doi:10.2337/db06-1269
Bujalska M, Tatarkiewicz J, de Corde A, Gumulka SW (2008) Effect of cyclooxygenase and nitric oxide synthase inhibitors on streptozotocin-induced hyperalgesia in rats. Pharmacology 81(2):151–157. doi:10.1159/000110787
Matsunaga A, Kawamoto M, Shiraishi S, Yasuda T, Kajiyama S, Kurita S, Yuge O (2007) Intrathecally administered COX-2 but not COX-1 or COX-3 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia in rats. Eur J Pharmacol 554(1):12–17. doi:10.1016/j.ejphar.2006.09.072
Acknowledgments
This study was financially supported by the Council of Scientific and Industrial Research (CSIR, New Delhi, India) and Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India to Dr. S.S. Sharma. Ms Geeta Negi is a recipient of CSIR-NET research fellowship.
Conflict of Interest
All the authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negi, G., Sharma, S.S. Inhibition of IκB Kinase (IKK) Protects Against Peripheral Nerve Dysfunction of Experimental Diabetes. Mol Neurobiol 51, 591–598 (2015). https://doi.org/10.1007/s12035-014-8784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8784-8