Abstract
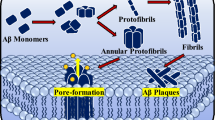
The senile plaque is a pathologic hallmark of Alzheimer's disease (AD). Amyloid-β peptide (Aβ), the main constituent of senile plaques, is neurotoxic especially in its oligomeric form. Aβ is derived from the sequential cleavage of amyloid precursor protein (APP) by β- and γ-secretases in the amyloidogenic pathway. Alternatively, APP can be cleaved by α-secretases within the Aβ domain to produce neurotrophic and neuroprotective α-secretase-cleaved soluble APP (sAPPα) in the nonamyloidogenic pathway. Since APP and α-, β-, and γ-secretases are membrane proteins, APP processing should be highly dependent on the membrane composition and the biophysical properties of cellular membrane. In this review, we discuss the role of the biophysical properties of cellular membrane in APP processing, especially the effects of phospholipases A2 (PLA2s), fatty acids, cholesterol, and Aβ on membrane fluidity in relation to their effects on APP processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
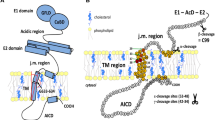
In Alzheimer's disease (AD) brains, there is an increased deposition of amyloid plaques which are mainly composed of neurotoxic amyloid-β peptide (Aβ). Recent research indicates that the soluble oligomeric form of Aβ significantly contributes to the pathogenesis of the disease [1]. In the amyloidogenic pathway, Aβ is derived from a proteolytic process of amyloid precursor protein (APP), in which APP is cleaved sequentially by β- and γ-secretases [2, 3]. Alternatively, APP can be cleaved by α-secretases between amino acids 16 and 17 within the Aβ domain and produce neurotrophic and neuroprotective soluble APP (α-secretase-cleaved soluble APP, sAPPα) in the nonamyloidogenic pathway [4]. These two pathways compete with each other, and enhancing APP processing by α-secretases has been suggested as a potential therapeutic strategy for AD [5]. Since APP and α-, β-, and γ-secretases are membrane proteins, APP processing should be affected by the local membrane environment. For example, γ-secretase activity can be modulated by membrane thickness in a cell-free system [6]. The cleavage of APP by β-secretase, the primary step to produce Aβ [7, 8], occurs preferentially in lipid rafts, which are highly ordered membrane microdomains rich in cholesterol, sphingolipids, and saturated phospholipids [9–14]. On the other hand, the activity of α-secretases is favorable in nonraft domains [15].
Phospholipases A2 (PLA2s) are responsible for the maintenance of phospholipid homeostasis in cellular membranes and implicated in AD and APP processing [16–18]. Fatty acids, the hydrolyzed products of PLA2s, alter membrane properties [19–21] which influence cellular functions. Moreover, Aβ in different forms directly bind to membrane and change its biophysical properties [22]; Aβ indirectly affects membrane properties by binding to membrane receptors and triggering downstream signaling pathways [23–26]. In this review, we discuss the evidence about the effects of PLA2s, fatty acids, cholesterol, and Aβ on membrane properties in relation to their effects on APP processing. Understanding the mechanisms leading to changes of membranes biophysics and how they result in changes in APP processing should provide insights into new therapeutic strategies for the prevention and treatment of AD.
Phospholipases A2 on Membrane Properties and APP Processing
PLA2s are ubiquitous enzymes in mammalian cells that catalyze the hydrolysis of fatty acids from sn-2 position of phospholipids. PLA2s are classified into three major families: calcium-dependent cytosolic PLA2 (cPLA2), calcium-independent PLA2 (iPLA2), and secretory PLA2 (sPLA2). These enzymes are responsible for the maintenance of phospholipid homeostasis in cellular membranes [27]. They are also important in the production of lipid mediators, such as arachidonic acid (AA), a precursor for the synthesis of eicosanoids [28]. Activation of PLA2s has been implicated in diverse cellular responses such as mitogenesis, differentiation, inflammation, and cytotoxicity, and changes in activities of PLA2s occur in many neurodegenerative diseases including AD [29–36]. For a comprehensive understanding of PLA2, see the review by Dennis et al. [27].
Immunoreactivity of cPLA2 is upregulated in reactive astrocytes in AD patient brains [37, 38]. Increases of sPLA2-IIA and cPLA2-IVA expression were also found in the hippocampus of AD patients [29, 39, 40]. In addition, Aβ has been shown to activate cPLA2 in primary rat and mouse brain endothelial cells, astrocytes, cortical neurons, and PC12 cells [41–45]. However, PLA2 activity was significantly decreased in the parietal and, to a lesser degree, in the frontal cortex of AD brains [46]. Lower PLA2 activity correlates significantly with an earlier onset of the disease, higher counts of neurofibrillary tangles and senile plaques, and an earlier age of death, indicating a relationship between abnormally low PLA2 activity and a more severe form of the illness [47].
PLA2s play key roles in the modulation of membrane properties under pathological and physiological conditions. PLA2 activation affects membrane fluidity, which characterizes an average lateral motion of phospholipid molecules within the lipid bilayer, and APP processing [17, 18]. In AD brains, there is evidence for reduced membrane fluidity coupled with decreased PLA2 activity [47, 48]. Similarly, inhibition of PLA2 activity in rat hippocampus has been shown to reduce membrane fluidity and impair the formation of short- and long-term memory [18, 49]. In addition, nonspecific PLA2 inhibitor partially suppressed muscarinic receptor-stimulated increases in sAPPα secretion in human neuroblastoma cells (SH-SY5Y) [50]. Our study showed that sPLA2-III increased membrane fluidity and sAPPα secretion and decreased levels of Aβ in SH-SY5Y cells and primary neurons [51]. Moreover, AA increased fluidity of membranes in cultured cerebral endothelial cells [52, 53], SH-SY5Y cells [51], and hippocampal neurons in vivo [54]. Another hydrolyzed product of PLA2, docosahexaenoic acid (DHA), has also been demonstrated to increase membrane fluidity and sAPPα secretion in human embryonic kidney (HEK) 293 cells and overexpressing APP cells [55]. Therefore, the effects of PLA2 on membrane fluidity and APP processing may partially attribute to its hydrolyzed products, fatty acids, which will be reviewed in the following section. Interestingly, compounds capable of altering membrane fluidity also modulate sAPPα production. Benzyl alcohol (C6H5OH) increases, whereas pluronic F68 (PF68) decreases, membrane fluidity and sAPPα secretion [22]. In turn, Aβ itself accelerates the amyloidogenic processing of APP by reducing membrane fluidity [22]. The study by Kojro et al. [56] showed that treatment with methyl-β-cyclodextrin (MβCD) to reduce cellular cholesterol increased membrane fluidity, APP accumulation at the cell surface, and sAPPα secretion. Our study also showed that sPLA2-III and AA treatment increased the accumulation of APP at cell surface [51]. These results are consistent with the notion that Aβ production mainly occurs in endosomes [57–62]. Increased membrane fluidity partially impairs the endocytosis of APP and subsequently increases sAPPα production. Since PLA2 increases membrane fluidity and nonamyloidogenic cleavage of APP, PLA2 activity modulation can be considered as a potential target for AD treatment.
Fatty Acids on Membrane Properties and APP Processing
Fatty acids are important ingredients in various dietary sources. They are essential components of cellular membrane and play a pivotal role in the normal development and function of the brain [63, 64]. Long-chain ω-3 and ω-6 polyunsaturated fatty acids (PUFAs), the major polyunsaturated fatty acids in the central nervous system [65], are essential for prenatal brain development and normal brain functions [64, 66, 67]. Diets rich in long-chain ω-3 PUFAs (e.g., DHA) have been shown to modulate gene expression for brain function, improve synaptic and neurotransmitter functions of neurons, enhance learning and memory performances, and display neuroprotective properties [67–71]. Animals with diets deficient in ω-3 fatty acids have reduced visual acuity and impaired learning ability [16, 67]. AA, another abundant fatty acid in the brain, is a second messenger [72] and a precursor for the synthesis of eicosanoids [28]. The presence of PUFAs in neuronal cells influences cellular function both directly through their effects on membrane properties and indirectly by acting as precursors for lipid-derived messengers [19, 20].
Disturbed metabolism of fatty acids is associated with AD [73–76]. For example, lower levels of DHA were reported in serum samples taken from an AD patient [77], while greater consumption of DHA significantly reduced the likelihood of developing AD [78]. DHA and curcumin have been shown to suppress Aβ-induced phosphorylation of tau tangles and the inactivation of insulin receptors in primary rat neurons [79]. Recently, reduced expression of the neuronal sortilin-related receptor SorLA/LR11, a sorting protein that regulates APP trafficking to β- and γ-secretases, was identified as a probable genetic risk factor for late-onset AD [80]. DHA, in turn, has been found to increase LR11 expression in primary rat neurons, human neuronal line, and aged nontransgenic and DHA-depleted APPsw AD transgenic mice [44]. In 15-month-old APP/presenilin-1 mice, DHA supplementation improved spatial memory, decreased Aβ deposition, and slightly increased relative cerebral blood volume, indicating that a DHA-enriched diet can diminish AD-like pathology [81]. One plausible explanation is that ω-3 PUFAs enhance phagocytosis of Aβ by microglia and decrease inflammation [82]. In addition, dietary ω-3 PUFA depletion has been shown to activate caspases and decrease NMDA receptors in the brain of a transgenic mouse model of AD [83].
PUFAs in neuronal cells influence cellular functions through their ability to integrate into cell membrane and change their physical properties [19, 20]. Not only can PUFAs be incorporated into membrane phospholipids but also are they able to associate with cellular membrane as free fatty acids. The ability of fatty acids to modulate membrane properties and functions [18, 70, 84–88] depends on both the saturation degree of the fatty acids and the trans/cis ratio of the unsaturated fatty acids [21, 89, 90]. For example, diets rich in PUFAs, including DHA and AA, have been shown to increase membrane fluidity of neurons and other cells [54, 69, 91, 92]. DHA is capable of counteracting a cholesterol-induced decrease in platelet membrane fluidity and modulating platelet hyperaggregation [91]. In contrast, membrane incorporation of saturated fatty acids led to decreased membrane fluidity [87, 90, 93, 94]. However, the fatty acids with short chain length (e.g., length = 10) increase α-secretase activity [95]. Trans fatty acids accumulate in the cellular membrane and increase Aβ production and oligomerization [96]. Many other membrane properties including molecular order, compressibility, and permeability are also affected by PUFA [97].
It has been reported that an increase in membrane fluidity leads to an increase in nonamyloidogenic cleavage by α-secretase to produce sAPPα [22, 56]. Consistently, enrichment of cell membranes with PUFAs increases membrane fluidity and, subsequently, promotes nonamyloidogenic processing of APP [21]. A typical Western diet (with 40 % saturated fatty acids and 1 % of cholesterol) fed to transgenic APP/PS1 mice increases Aβ, while diets supplemented with DHA decrease Aβ levels [98]. Similarly, DHA decreases the amount of vascular Aβ deposition [99] and reduces cortical Aβ burden [100] in the aged mouse model of AD. In this model, DHA modulates APP processing by decreasing both α- and β-APP C-terminal fragment products and full-length APP [100]. DHA stimulates nonamyloidogenic APP processing resulting in reduced Aβ levels in cellular models of AD [101]. Meanwhile, our study of the effects of fatty acids on cell membrane fluidity and sAPPα secretion in relation to degrees of unsaturation has suggested that not all unsaturated fatty acids but only those with four or more double bonds, such as AA (20:4), eicosapentaenoic acid (EPA, 20:5), and DHA (22:6), increased membrane fluidity and led to an increase in sAPPα secretion, while stearic acid (SA, 18:0), oleic acid (OA, 18:1), linoleic acid (LA, 18:2), and α-linolenic acid (ALA, 18:3) did not [21]. Moreover, another study indicated that treatment of PSwt-1 CHO cells with oleic acid and linoleic acid increased γ-secretase activity and Aβ production [102]. These studies suggest that modulation of PUFAs content in cellular membrane is essential in enhancing sAPPα production partially due to their effects on membrane fluidity.
Cholesterol on Membrane Properties and APP Processing
Cholesterol is an essential component of cellular membrane and plays a vital role in the regulation of membrane functions. Distribution of cholesterol within plasma membrane is not even: cholesterol is mostly condensed in lipid rafts, which are more tightly packed than nonlipid raft domains due to intermolecular hydrogen bonding involving sphingolipid and cholesterol [103, 104]. Cholesterol distribution correlates with altered APP processing in mice treated with statins (3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors) [105]. Levels of membrane cholesterol can be modulated by specific inhibitors of the cellular biosynthesis such as statins, or it can be selectively extracted from plasma membrane by MβCD [56, 106]. Chronic simvastatin treatment decreases cholesterol levels in mouse brains and affects cholesterol distribution within synaptosomal membranes [107]. Simvastatin also significantly increases the levels of insoluble Aβ but reduces levels of soluble Aβ in the brain [107]. The content of cholesterol in phospholipid bilayers affects many biophysical parameters of lipid bilayers, such as thickness, thermomechanical properties, molecular packing, conformational freedom of phospholipid acyl chains and water, molecular oxygen permeability, membrane hydrophobicity, membrane excitability in neurons, internal dipolar potential, and membrane fluidity [104, 108–114].
Intracellular cholesterol homeostasis regulates APP processing [115]. In membrane compartmentalization model, APP presents in two cellular pools, one is associated with the cholesterol-enriched lipid rafts, where Aβ is generated, and the other is outside of rafts (i.e., nonlipid raft domains), where α-cleavage occurs [10, 116]. It was reported that membrane cholesterol depletion decreased the content of APP in cholesterol and sphingolipid-rich membrane microdomains and subsequently inhibited the amyloidogenic pathway to produce Aβ [56, 117]. DHA decreases cholesterol de novo synthesis, shifts its distribution from raft to nonraft domains, and decreases β- and γ-secretase activity [118]. In contrast, cholesterol accumulation in Niemann-Pick type C (NPC) model cells has been shown to shift APP localization to lipid rafts [119]. Consistent with the membrane compartmentalization model, cellular cholesterol depletion leads to increased membrane fluidity [56, 120–122]. An increase in membrane fluidity shifts APP processing to nonamyloidogenic cleavage by α-secretase [119–121, 123, 124]. The removal of cholesterol with MβCD or treatment with lovastatin increased membrane fluidity, which resulted in higher expression of the α-secretase and impaired internalization of APP [56]. The increased membrane fluidity also correlates with a redistribution of cholesterol, sphingomyelin, and proteins involved in APP processing between raft and nonraft domains and enhances sAPPα production [125]. Local cholesterol increase triggers APP-BACE1 (β-secretase) clustering in lipid rafts and rapid endocytosis [126]. Actually, APP has a flexible transmembrane domain and binds to cholesterol [127]. Titration of C99 fragment of APP reveals a binding site for cholesterol, providing a mechanistic insight into how cholesterol promotes APP accumulation in lipid raft and amyloidogenesis [127].
Meanwhile, cholesterol enrichment has been shown to reduce membrane fluidity [91, 128]. Exposure of cholesterol to astrocytes, primary neurons, and glial cultures inhibited the secretion of sAPPα and reduced cell viability [123, 124, 129]. Furthermore, some studies showed that cholesterol levels in the membranes were positively correlated with β-secretase activity [130], while lovastatin enhanced α-secretase activity [124]. Cholesterol enrichment that impeded membrane fluidity may lower sAPPα production by hindering the interaction of the substrate with its proteases [131]. Interestingly, substitution of cholesterol by the steroid 4-cholesten-3-one induces a minor change in membrane fluidity and reduces sAPPα secretion, whereas substitution of cholesterol by lanosterol increases membrane fluidity and sAPPα secretion [56]. These results suggest reversible effects of cholesterol on the α-secretase activity depending on membrane fluidity.
Many studies support the notion that Aβ production occurs in endosomes [58–62]. APP internalization from plasma membrane is regulated by key regulators of endocytosis, such as Rab5, and this process enhances APP cleavage by β-secretase to increase Aβ levels [132]. In contrast, APP, lacking its cytoplasmic internalization motif, accumulates at the plasma membrane and undergoes cleavage by α-secretase [7, 8]. Cholesterol increases clathrin-dependent APP endocytosis in a dose-dependent and linear manner [133]. Moreover, alterations in cholesterol transport from late endocytotic organelles to the endoplasmic reticulum had important consequences for both APP processing and the localization of γ-secretase-associated presenilins [134]. It has been suggested that cholesterol increase in AD could be responsible for the enhanced internalization of clathrin-dependent endocytosis of APP and the overproduction of Aβ [133]. Alternatively, APP internalization could be reduced by lowering cholesterol, which leads to an increase in membrane fluidity, APP accumulation on the cell surface, and increased sAPPα secretion [56].
Aβ on Membrane Properties and APP Processing
Many studies showed direct interactions of Aβ with components of the plasma membrane, which disrupts the membrane properties consequentially [135–144]. Several types of Aβ-membrane interactions were suggested. Aβ peptide can be retained in a membrane upon APP cleavage and thus be prevented against release and aggregation [145]. Aβ can also be released as soluble monomers into the extracellular environment and then be removed [145, 146]. After releasing, on the other hand, Aβ can reinsert into a membrane and form ion-conducting pores or undergo accelerated aggregation on a membrane surface and form nonspecific structures, which causes thinning and deformation to the membrane [145, 147–151]. A simulation study showed that a highly asymmetric cholesterol distribution which is depleted on the exofacial leaflet but enhanced on the cytofacial leaflet of the model lipid membrane thermodynamically favors membrane retention of a fully embedded Aβ peptide [152]. However, in the case of cholesterol redistribution that increases concentration of cholesterol on the exofacial layer, typical of aging or AD, the free energy favors peptide extrusion of the highly reactive N-terminus into the extracellular space that may be vulnerable to aggregation, oligomerization, or deleterious oxidative reactivity [152]. The insertion of the peptide into the artificial membrane bilayers alters membrane lipid packing and induces molecular disorder (more water molecules were partitioned into the membrane core), as shown by the fluorescence microscopy of the environmentally sensitive probe laurdan [153–155]. The membranes of immortalized rat astrocytes become more molecularly ordered upon incubation with Aβ in a time-dependent manner, which is due to the signaling pathway involving NADPH oxidase and cPLA2 triggered by Aβ [153]. The incorporation of Aβ into the membranes and formation of cation-selective channels lead to the alteration of membrane permeability and electrical conductance [138, 151, 156–164]. It has been suggested that Aβ-induced membrane depolarization and increased ion influx in neurons were not just due to forming of cation-selective pores but rather resulted from downstream pathways involved with metabotropic glutamate receptor and G-proteins [164].
Aβ has been shown to reduce membrane fluidity and accelerate the amyloidogenic processing of APP [22, 140, 165–169]. Aβ stimulates the amyloidogenic processing of APP by reducing membrane fluidity and complexing with GM-1 ganglioside [22]. This dynamic action of Aβ may start a vicious circle, where endogenous Aβ stimulates its own production [22]. Interestingly, DHA has protective effect against impaired learning in Aβ-infused rats, which is associated with increased synaptosomal membrane fluidity [170]. It was shown that, in vivo, Aβ administration caused a decrease in membrane fluidity of synaptosomes isolated from frontal and hypothalamic neurons of 3-month-old mice [168]. In a model system of liposomes, decreased fluidity reduced membrane permeabilization [171]. By using in situ atomic force microscopy and fluorescence spectroscopy, randomly structured Aβ has been reported to decrease membrane fluidity of planar bilayers composed of total brain lipids, and this effect is cholesterol-content dependent: the most dramatic effect has been seen for cholesterol-rich samples [166]. DPH (1,6-diphenyl-1,3,5-hexatriene) fluorescence study has shown a similar effect of Aβ on membrane fluidity of unilamellar liposomes with a strong correlation to Aβ aggregation state and pH [167]. Unaggregated peptides at pH 7 do not affect membrane fluidity, while aggregated Aβ at pH 6 or 7 decreased membrane fluidity in a time- and dose-dependent manner [167]. Studies of SH-SY5Y human neuroblastoma cells have shown some contradictory results. In this study, Aβ monomers increased fluidity of cell membranes, and Aβ-aluminum complex promoted even a greater effect [172]. Another study showed Aβ significantly increased annular and bulk fluidity in synaptic plasma membranes (SPM) of rat cerebral cortex and hippocampus, while Aβ had no effect on fluidity of SPM of cerebellum [173]. The differences in the effects of Aβ on fluidity could result from the tissue source and preparation, the amounts of cholesterol and phospholipid, whether Aβ is soluble or aggregated, and the age of the organism. The differences could also be due to the different locations of fluorescent probes in the membrane environment and the lifetime of the fluorescent probes.
Aβ also alters composition of cellular membrane lipids [174], causes oxidative lipid damage [175], increases membrane fusion [176], impairs membrane redox system [177], stimulates trafficking of cholesterol from plasma membrane to the Golgi complex in mouse primary astrocytes [178], reduces the cell membrane roughness [179], and disrupts membrane trafficking of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction [180]. These perturbing effects may contribute to amyloidogenic processing of APP. Metal ions, pH, fatty acids, and cholesterol affect interactions of Aβ with membrane lipid and membrane insertion of Aβ and potentially inhibit fibril formation and the membrane perturbing effects of Aβ [170, 181–183]. Aβ polymers have a higher affinity for cholesterol than phosphatidylcholine or saturated fatty acids [184]. Aggregated Aβ may affect lipid transport between cells or remove specific lipids from membranes, and such effects could contribute to neuronal dysfunction. Actually, in C99/APP, membrane-buried GXXXG motifs (G, Gly; X, any amino acid) play a key role in cholesterol binding [127]. Association of C99/APP with cholesterol may favor partitioning of the protein into membrane domains rich in the proteases of the amyloidogenic pathway [127]. The linear fragment 22-35 of Aβ is a functional cholesterol-binding domain that could promote the insertion of β-amyloid peptides or amyloid pore formation in cholesterol-rich membrane domains [185]. Molecular dynamic simulations suggest that cholesterol induces a tilted α-helical topology of Aβ22-35. This facilitates the establishment of an interpeptide hydrogen bond network involving Asn-27 and Lys-28, a key step in the octamerization of Aβ22-35 which proceeds gradually until the formation of a perfect annular channel in a phosphatidylcholine membrane [186].
Conclusion
An increasing amount of evidence demonstrates that a lot of cellular processes in AD are intimately associated with physical properties and organization of membranes. The primary step in Aβ accumulation, the amyloidogenic cleavage of APP, is affected by the membrane properties such as membrane fluidity and can be modulated by removal of cholesterol and manipulation of membrane lipid composition. PLA2s and their hydrolyzed products, such as AA and DHA and other fatty acids, play important roles in the modulation of membrane properties in relation to their effects on APP processing. Aβ-membrane interactions, in turn, affect biophysical membrane properties and accelerate the amyloidogenic processing of APP. We review the role of the biophysical properties of cellular membrane in APP processing, especially the effects of PLA2s, fatty acids, cholesterol, and Aβ on membrane fluidity in relation to their effects on APP endocytosis and processing, which are summarized in Table 1. Understanding how membrane properties and organization are related to cellular pathways including APP processing in AD should provide insights into the mechanisms of AD pathogenesis.
References
Larson ME, Lesne SE (2012) Soluble Abeta oligomer production and toxicity. J Neurochem 120(Suppl 1):125–139
Vassar R (2004) BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci 23:105–114
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2:a006270
Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C (2006) Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res 1094:38–46
Cheng H, Vetrivel KS, Gong P, Meckler X, Parent A, Thinakaran G (2007) Mechanisms of disease: new therapeutic strategies for Alzheimer's disease—targeting APP processing in lipid rafts. Nat Clin Pract Neurol 3:374–382
Winkler E, Kamp F, Scheuring J, Ebke A, Fukumori A, Steiner H (2012) Generation of Alzheimer disease-associated Abeta42/43 by gamma-secretase can directly be inhibited by modulation of membrane thickness. J Biol Chem 287:21326–21334
Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ (1993) Beta-amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem 268:3021–3024
Koo EH, Squazzo SL (1994) Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 269:17386–17389
Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ (2003) Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A 100:11735–11740
Ehehalt R, Keller P, Haass C, Thiele C, Simons K (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160:113–123
Kaether C, Haass C (2004) A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. J Cell Biol 167:809–812
Marlow L, Cain M, Pappolla MA, Sambamurti K (2003) Beta-secretase processing of the Alzheimer's amyloid protein precursor (APP). J Mol Neurosci 20:233–239
Tun H, Marlow L, Pinnix I, Kinsey R, Sambamurti K (2002) Lipid rafts play an important role in A beta biogenesis by regulating the beta-secretase pathway. J Mol Neurosci 19:31–35
Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC et al (2004) Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279:44945–44954
Reid PC, Urano Y, Kodama T, Hamakubo T (2007) Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. J Cell Mol Med 11:383–392
Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aid S et al (2004) Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev 44:509–538
Liu MS, Ghosh S, Yang Y (1983) Change in membrane lipid fluidity induced by phospholipase A activation: a mechanism of endotoxic shock. Life Sci 33:1995–2002
Schaeffer EL, Bassi F Jr, Gattaz WF (2005) Inhibition of phospholipase A2 activity reduces membrane fluidity in rat hippocampus. J Neural Transm 112:641–647
Hibbeln JR, Umhau JC, George DT, Shoaf SE, Linnoila M, Salem N Jr (2000) Plasma total cholesterol concentrations do not predict cerebrospinal fluid neurotransmitter metabolites: implications for the biophysical role of highly unsaturated fatty acids. Am J Clin Nutr 71:331S–338S
Sinclair AJ, Begg D, Mathai M, Weisinger RS (2007) Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr 16(Suppl 1):391–397
Yang X, Sheng W, Sun GY, Lee JC (2011) Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem Int 58:321–329
Peters I, Igbavboa U, Schutt T, Haidari S, Hartig U, Rosello X, Bottner S et al (2009) The interaction of beta-amyloid protein with cellular membranes stimulates its own production. Biochim Biophys Acta 1788:964–972
Giuffrida ML, Tomasello F, Caraci F, Chiechio S, Nicoletti F, Copani A (2012) Beta-amyloid monomer and insulin/IGF-1 signaling in Alzheimer's disease. Mol Neurobiol 46:605–613
Demuro A, Parker I, Stutzmann GE (2010) Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem 285:12463–12468
Yamin G (2009) NMDA receptor-dependent signaling pathways that underlie amyloid beta-protein disruption of LTP in the hippocampus. J Neurosci Res 87:1729–1736
Xie CW (2004) Calcium-regulated signaling pathways: role in amyloid beta-induced synaptic dysfunction. Neuromol Med 6:53–64
Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111:6130–6185
Zhou L, Nilsson A (2001) Sources of eicosanoid precursor fatty acid pools in tissues. J Lipid Res 42:1521–1542
Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY (2006) Secretory PLA2-IIA: a new inflammatory factor for Alzheimer's disease. J Neuroinflammation 3:28
Farooqui AA, Ong WY, Horrocks LA (2006) Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev 58:591–620
Sun GY, Horrocks LA, Farooqui AA (2007) The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem 103:1–16
Yedgar S, Cohen Y, Shoseyov D (2006) Control of phospholipase A2 activities for the treatment of inflammatory conditions. Biochim Biophys Acta 1761:1373–1382
Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A et al (2005) Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol 31:27–41
Desbene C, Malaplate-Armand C, Youssef I, Garcia P, Stenger C, Sauvee M, Fischer N et al (2012) Critical role of cPLA2 in Abeta oligomer-induced neurodegeneration and memory deficit. Neurobiol Aging 33(1123):e1117–e1129
Sun GY, He Y, Chuang DY, Lee JC, Gu Z, Simonyi A, Sun AY (2012) Integrating cytosolic phospholipase A(2) with oxidative/nitrosative signaling pathways in neurons: a novel therapeutic strategy for AD. Mol Neurobiol 46:85–95
Gentile MT, Reccia MG, Sorrentino PP, Vitale E, Sorrentino G, Puca AA, Colucci-D'Amato L (2012) Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer's disease pathogenesis. Mol Neurobiol 45:596–604
Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA (1996) Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer's disease brain. Neurobiol Dis 3:51–63
Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J et al (1999) Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia 27:110–128
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002) Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosc Res 70:462–473
Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cisse M et al (2008) Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci 11:1311–1318
Kriem B, Sponne I, Fifre A, Malaplate-Armand C, Lozac'h-Pillot K, Koziel V, Yen-Potin FT et al (2005) Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. Faseb J 19:85–87
Yang X, Askarova S, Sheng W, Chen JK, Sun AY, Sun GY, Yao G et al (2010) Low energy laser light (632.8 nm) suppresses amyloid-beta peptide-induced oxidative and inflammatory responses in astrocytes. Neuroscience 171:859–868
Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC (2006) Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci 26:11111–11119
Chalimoniuk M, Stolecka A, Cakala M, Hauptmann S, Schulz K, Lipka U, Leuner K et al (2007) Amyloid beta enhances cytosolic phospholipase A2 level and arachidonic acid release via nitric oxide in APP-transfected PC12 cells. Acta Biochim Pol 54:611–623
Askarova S, Yang X, Sheng W, Sun GY, Lee JC (2011) Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience 199:375–385
Gattaz WF, Cairns NJ, Levy R, Forstl H, Braus DF, Maras A (1996) Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 246:129–131
Gattaz WF, Maras A, Cairns NJ, Levy R, Forstl H (1995) Decreased phospholipase A2 activity in Alzheimer brains. Biol Psychiatry 37:13–17
Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer's disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70:786–793
Forlenza OV, Schaeffer EL, Gattaz WF (2007) The role of phospholipase A2 in neuronal homeostasis and memory formation: implications for the pathogenesis of Alzheimer's disease. J Neural Transm 114:231–238
Cho HW, Kim JH, Choi S, Kim HJ (2006) Phospholipase A2 is involved in muscarinic receptor-mediated sAPPalpha release independently of cyclooxygenase or lypoxygenase activity in SH-SY5Y cells. Neurosci Lett 397:214–218
Yang X, Sheng W, He Y, Cui J, Haidekker MA, Sun GY, Lee JC (2010) Secretory phospholipase A2 type III enhances alpha-secretase-dependent amyloid precursor protein processing through alterations in membrane fluidity. J Lipid Res 51:957–966
Villacara A, Spatz M, Dodson RF, Corn C, Bembry J (1989) Effect of arachidonic acid on cultured cerebromicrovascular endothelium: permeability, lipid peroxidation and membrane “fluidity”. Acta Neuropathol 78:310–316
Beck R, Bertolino S, Abbot SE, Aaronson PI, Smirnov SV (1998) Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ Res 83:923–931
Fukaya T, Gondaira T, Kashiyae Y, Kotani S, Ishikura Y, Fujikawa S, Kiso Y et al (2007) Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol Aging 28:1179–1186
Eckert GP, Chang S, Eckmann J, Copanaki E, Hagl S, Hener U, Muller WE et al (2011) Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim Biophys Acta 1808:236–243
Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci U S A 98:5815–5820
von Arnim CA, von Einem B, Weber P, Wagner M, Schwanzar D, Spoelgen R, Strauss WL et al (2008) Impact of cholesterol level upon APP and BACE proximity and APP cleavage. Biochem Biophys Res Commun 370:207–212
Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G et al (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58:42–51
Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT (2003) Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci 116:3339–3346
Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P et al (2008) Efficient inhibition of the Alzheimer's disease {beta}-secretase by membrane targeting. Science 320:520–523
Schobel S, Neumann S, Hertweck M, Dislich B, Kuhn PH, Kremmer E, Seed B et al (2008) A novel sorting nexin modulates endocytic trafficking and alpha-secretase cleavage of the amyloid precursor protein. J Biol Chem 283:14257–14268
Small SA, Gandy S (2006) Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron 52:15–31
Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A (2010) Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr 169:149–164
Dyall SC, Michael-Titus AT (2008) Neurological benefits of omega-3 fatty acids. Neuromol Med 10:219–235
Bazan NG, Scott BL (1990) Dietary omega-3 fatty acids and accumulation of docosahexaenoic acid in rod photoreceptor cells of the retina and at synapses. Ups J Med Sci Suppl 48:97–107
Holman RT, Johnson SB, Ogburn PL (1991) Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc Natl Acad Sci U S A 88:4835–4839
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001) Essential fatty acids in visual and brain development. Lipids 36:885–895
Carrie I, Abellan Van Kan G, Rolland Y, Gillette-Guyonnet S, Vellas B (2009) PUFA for prevention and treatment of dementia? Curr Pharm Des 15:4173–4185
Horrocks LA, Farooqui AA (2004) Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fat Acids 70:361–372
Heinrichs SC (2010) Dietary omega-3 fatty acid supplementation for optimizing neuronal structure and function. Mol Nutr Food Res 54:447–456
Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y et al (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats. J Neurochem 81:1084–1091
Khan WA, Blobe GC, Hannun YA (1995) Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell Signal 7:171–184
Rapoport SI (2008) Arachidonic acid and the brain. J Nutr 138:2515–2520
Leskovjan AC, Kretlow A, Miller LM (2010) Fourier transform infrared imaging showing reduced unsaturated lipid content in the hippocampus of a mouse model of Alzheimer's disease. Anal Chem 82:2711–2716
Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS (2011) Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer's disease prefrontal cortex. J Alzheimers Dis 24:507–517
Eckert GP, Lipka U, Muller WE (2013) Omega-3 fatty acids in neurodegenerative diseases: focus on mitochondria. Prostaglandins Leukot Essent Fat Acids 88:105–114
Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D et al (2003) Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr 89:483–489
Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL et al (2006) Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 63:1545–1550
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP et al (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29:9078–9089
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T et al (2007) The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39:168–177
Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM et al (2009) DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis 33:482–498
Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y et al (2013) Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J Alzheimers Dis 35:697–713
Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N Jr, Frautschy SA et al (2005) Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci 22:617–626
Pepe S (2007) Dietary polyunsaturated fatty acids and age-related membrane changes in the heart. Ann N Y Acad Sci 1114:381–388
Shaikh SR, Edidin M (2008) Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem Phys Lipids 153:24–33
Aricha B, Fishov I, Cohen Z, Sikron N, Pesakhov S, Khozin-Goldberg I, Dagan R et al (2004) Differences in membrane fluidity and fatty acid composition between phenotypic variants of Streptococcus pneumoniae. J Bacteriol 186:4638–4644
Cader AA, Butterfield DA, Watkins BA, Chung BH, Hennig B (1995) Electron spin resonance studies of fatty acid-induced alterations in membrane fluidity in cultured endothelial cells. Int J Biochem Cell Biol 27:665–673
Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR (2005) Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 45:559–579
Loffhagen N, Hartig C, Babel W (2004) Pseudomonas putida NCTC 10936 balances membrane fluidity in response to physical and chemical stress by changing the saturation degree and the trans/cis ratio of fatty acids. Biosci Biotechnol Biochem 68:317–323
Zavodnik IB, Zaborowski A, Niekurzak A, Bryszewska M (1997) Effect of free fatty acids on erythrocyte morphology and membrane fluidity. Biochem Mol Biol Int 42:123–133
Hashimoto M, Hossain S, Shido O (2006) Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol Cell Biochem 293:1–8
McLauren Dorrance A, Graham D, Dominiczak A, Fraser R (2000) Inhibition of nitric oxide synthesis increases erythrocyte membrane fluidity and unsaturated fatty acid content. Am J Hypertens 13:1194–1202
Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA (1994) Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J 300(Pt 2):509–518
Kitagawa S, Kotani K, Kametani F (1990) Inhibitory mechanism of cis-polyunsaturated fatty acids on platelet aggregation: the relation with their effects on Ca2+ mobilization, cyclic AMP levels and membrane fluidity. Biochim Biophys Acta 1054:114–118
Grimm MO, Haupenthal VJ, Rothhaar TL, Zimmer VC, Grosgen S, Hundsdorfer B, Lehmann J et al (2013) Effect of different phospholipids on alpha-secretase activity in the non-amyloidogenic pathway of Alzheimer's disease. Int J Mol Sci 14:5879–5898
Grimm MO, Rothhaar TL, Grosgen S, Burg VK, Hundsdorfer B, Haupenthal VJ, Friess P et al (2012) Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP). J Nutr Biochem 23:1214–1223
Stillwell W, Wassall SR (2003) Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 126:1–27
Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L et al (2006) Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis 23:563–572
Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM et al (2007) Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol Dis 28:16–29
Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr et al (2005) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci 25:3032–3040
Sahlin C, Pettersson FE, Nilsson LN, Lannfelt L, Johansson AS (2007) Docosahexaenoic acid stimulates non-amyloidogenic APP processing resulting in reduced Abeta levels in cellular models of Alzheimer's disease. Eur J Neurosci 26:882–889
Liu Y, Yang L, Conde-Knape K, Beher D, Shearman MS, Shachter NS (2004) Fatty acids increase presenilin-1 levels and [gamma]-secretase activity in PSwt-1 cells. J Lipid Res 45:2368–2376
Barenholz Y (2004) Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell Biochem 37:167–215
Fantini J, Yahi N (2010) Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: common mechanisms in neurodegenerative diseases. Expert Rev Mol Med 12:e27
Burns MP, Igbavboa U, Wang L, Wood WG, Duff K (2006) Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. Neuromol Med 8:319–328
Eckert GP, Kirsch C, Leutz S, Wood WG, Muller WE (2003) Cholesterol modulates amyloid beta-peptide's membrane interactions. Pharmacopsychiatry 36(Suppl 2):S136–S143
Eckert GP, Reik C, Muller WE (2013) Simvastatin alters membrane cholesterol distribution and beta-amyloid levels in brains of female APP751SL mice. Pharmazie 68:590–594
Arrais D, Martins J (2007) Bilayer polarity and its thermal dependency in the l(o) and l(d) phases of binary phosphatidylcholine/cholesterol mixtures. Biochim Biophys Acta 1768:2914–2922
Halling KK, Ramstedt B, Slotte JP (2008) Glycosylation induces shifts in the lateral distribution of cholesterol from ordered towards less ordered domains. Biochim Biophys Acta 1778:1100–1111
Chen Q, Amaral J, Biancani P, Behar J (1999) Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology 116:678–685
Dumas D, Latger V, Viriot ML, Blondel W, Stoltz JF (1999) Membrane fluidity and oxygen diffusion in cholesterol-enriched endothelial cells. Clin Hemorheol Microcirc 21:255–261
Socaciu C, Jessel R, Diehl HA (2000) Competitive carotenoid and cholesterol incorporation into liposomes: effects on membrane phase transition, fluidity, polarity and anisotropy. Chem Phys Lipids 106:79–88
Wang D, Schreurs BG (2010) Dietary cholesterol modulates the excitability of rabbit hippocampal CA1 pyramidal neurons. Neurosci Lett 479:327–331
Hao M, Mukherjee S, Sun Y, Maxfield FR (2004) Effects of cholesterol depletion and increased lipid unsaturation on the properties of endocytic membranes. J Biol Chem 279:14171–14178
Burns MP, Rebeck GW (2010) Intracellular cholesterol homeostasis and amyloid precursor protein processing. Biochim Biophys Acta 1801:853–859
Colell A, Fernandez A, Fernandez-Checa JC (2009) Mitochondria, cholesterol and amyloid beta peptide: a dangerous trio in Alzheimer disease. J Bioenerg Biomembr 41:417–423
Eckert GP, Kirsch C, Muller WE (2003) Brain-membrane cholesterol in Alzheimer's disease. J Nutr Health Aging 7:18–23
Grimm MO, Kuchenbecker J, Grosgen S, Burg VK, Hundsdorfer B, Rothhaar TL, Friess P et al (2011) Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem 286:14028–14039
Kosicek M, Malnar M, Goate A, Hecimovic S (2010) Cholesterol accumulation in Niemann Pick type C (NPC) model cells causes a shift in APP localization to lipid rafts. Biochem Biophys Res Commun 393:404–409
Luneva OG, Brazhe NA, Maksimova NV, Rodnenkov OV, Parshina EY, Bryzgalova NY, Maksimov GV et al (2007) Ion transport, membrane fluidity and haemoglobin conformation in erythrocyte from patients with cardiovascular diseases: role of augmented plasma cholesterol. Pathophysiology 14:41–46
Colell A, Garcia-Ruiz C, Lluis JM, Coll O, Mari M, Fernandez-Checa JC (2003) Cholesterol impairs the adenine nucleotide translocator-mediated mitochondrial permeability transition through altered membrane fluidity. J Biol Chem 278:33928–33935
Rog T, Stimson LM, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M (2008) Replacing the cholesterol hydroxyl group with the ketone group facilitates sterol flip-flop and promotes membrane fluidity. J Phys Chem B 112:1946–1952
Galbete JL, Martin TR, Peressini E, Modena P, Bianchi R, Forloni G (2000) Cholesterol decreases secretion of the secreted form of amyloid precursor protein by interfering with glycosylation in the protein secretory pathway. Biochem J 348(Pt 2):307–313
Xiu J, Nordberg A, Qi X, Guan ZZ (2006) Influence of cholesterol and lovastatin on alpha-form of secreted amyloid precursor protein and expression of alpha7 nicotinic receptor on astrocytes. Neurochem Int 49:459–465
Clement AB, Gimpl G, Behl C (2010) Oxidative stress resistance in hippocampal cells is associated with altered membrane fluidity and enhanced nonamyloidogenic cleavage of endogenous amyloid precursor protein. Free Radic Biol Med 48:1236–1241
Marquer C, Devauges V, Cossec JC, Liot G, Lecart S, Saudou F, Duyckaerts C et al (2011) Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. Faseb J 25:1295–1305
Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ et al (2012) The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 336:1168–1171
Buffone MG, Verstraeten SV, Calamera JC, Doncel GF (2009) High Cholesterol Content and Decreased Membrane Fluidity in Human Spermatozoa Are Associated With Protein Tyrosine Phosphorylation and Functional Deficiencies. J Androl 30:552–558
Racchi M, Baetta R, Salvietti N, Ianna P, Franceschini G, Paoletti R, Fumagalli R et al (1997) Secretory processing of amyloid precursor protein is inhibited by increase in cellular cholesterol content. Biochem J 322(Pt 3):893–898
Liu WW, Todd S, Coulson DT, Irvine GB, Passmore AP, McGuinness B, McConville M et al (2009) A novel reciprocal and biphasic relationship between membrane cholesterol and beta-secretase activity in SH-SY5Y cells and in human platelets. J Neurochem 108:341–349
Bodovitz S, Klein WL (1996) Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem 271:4436–4440
Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP et al (2003) Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem 278:31261–31268
Cossec JC, Simon A, Marquer C, Moldrich RX, Leterrier C, Rossier J, Duyckaerts C et al (2010) Clathrin-dependent APP endocytosis and Abeta secretion are highly sensitive to the level of plasma membrane cholesterol. Biochim Biophys Acta 1801:846–852
Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T (2002) Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci 22:1679–1689
Friedman R, Pellarin R, Caflisch A (2009) Amyloid aggregation on lipid bilayers and its impact on membrane permeability. J Mol Biol 387:407–415
Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K (2002) Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 41:7385–7390
Terzi E, Holzemann G, Seelig J (1997) Interaction of Alzheimer beta-amyloid peptide(1-40) with lipid membranes. Biochemistry 36:14845–14852
Sokolov Y, Kozak JA, Kayed R, Chanturiya A, Glabe C, Hall JE (2006) Soluble amyloid oligomers increase bilayer conductance by altering dielectric structure. J Gen Physiol 128:637–647
Buchsteiner A, Hauss T, Dante S, Dencher NA (2010) Alzheimer's disease amyloid-beta peptide analogue alters the ps-dynamics of phospholipid membranes. Biochim Biophys Acta 1798:1969–1976
Eckert GP, Wood WG, Muller WE (2010) Lipid membranes and beta-amyloid: a harmful connection. Curr Protein Pept Sci 11:319–325
Murray IV, Sindoni ME, Axelsen PH (2005) Promotion of oxidative lipid membrane damage by amyloid beta proteins. Biochemistry 44:12606–12613
Yao JK, Wengenack TM, Curran GL, Poduslo JF (2009) Reduced membrane lipids in the cortex of Alzheimer's disease transgenic mice. Neurochem Res 34:102–108
Williamson R, Usardi A, Hanger DP, Anderton BH (2008) Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. Faseb J 22:1552–1559
Ambroggio EE, Kim DH, Separovic F, Barrow CJ, Barnham KJ, Bagatolli LA, Fidelio GD (2005) Surface behavior and lipid interaction of Alzheimer beta-amyloid peptide 1-42: a membrane-disrupting peptide. Biophys J 88:2706–2713
Bokvist M, Lindstrom F, Watts A, Grobner G (2004) Two types of Alzheimer's beta-amyloid (1-40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol 335:1039–1049
Lansbury PT Jr (1999) Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci U S A 96:3342–3344
Nag S, Chen J, Irudayaraj J, Maiti S (2010) Measurement of the attachment and assembly of small amyloid-beta oligomers on live cell membranes at physiological concentrations using single-molecule tools. Biophys J 99:1969–1975
Jang H, Zheng J, Lal R, Nussinov R (2008) New structures help the modeling of toxic amyloidbeta ion channels. Trends Biochem Sci 33:91–100
McLaurin J, Chakrabartty A (1996) Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem 271:26482–26489
Widenbrant MJ, Rajadas J, Sutardja C, Fuller GG (2006) Lipid-induced beta-amyloid peptide assemblage fragmentation. Biophys J 91:4071–4080
Kawahara M, Arispe N, Kuroda Y, Rojas E (1997) Alzheimer's disease amyloid beta-protein forms Zn(2+)-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys J 73:67–75
Liguori N, Nerenberg PS, Head-Gordon T (2013) Embedding Abeta42 in heterogeneous membranes depends on cholesterol asymmetries. Biophys J 105:899–910
Hicks JB, Lai Y, Sheng W, Yang X, Zhu D, Sun GY, Lee JC (2008) Amyloid-beta peptide induces temporal membrane biphasic changes in astrocytes through cytosolic phospholipase A2. Biochim Biophys Acta 1778:2512–2519
Parasassi T, Gratton E, Yu WM, Wilson P, Levi M (1997) Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys J 72:2413–2429
Parasassi T, Di Stefano M, Ravagnan G, Sapora O, Gratton E (1992) Membrane aging during cell growth ascertained by Laurdan generalized polarization. Exp Cell Res 202:432–439
Arispe N, Pollard HB, Rojas E (1996) Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc Natl Acad Sci U S A 93:1710–1715
Valincius G, Heinrich F, Budvytyte R, Vanderah DJ, McGillivray DJ, Sokolov Y, Hall JE et al (2008) Soluble amyloid beta-oligomers affect dielectric membrane properties by bilayer insertion and domain formation: implications for cell toxicity. Biophys J 95:4845–4861
Alarcon JM, Brito JA, Hermosilla T, Atwater I, Mears D, Rojas E (2006) Ion channel formation by Alzheimer's disease amyloid beta-peptide (Abeta40) in unilamellar liposomes is determined by anionic phospholipids. Peptides 27:95–104
Arispe N, Diaz JC, Simakova O (2007) Abeta ion channels. Prospects for treating Alzheimer's disease with Abeta channel blockers. Biochim Biophys Acta 1768:1952–1965
Lal R, Lin H, Quist AP (2007) Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim Biophys Acta 1768:1966–1975
Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B et al (2005) Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A 102:10427–10432
Vaisid T, Kosower NS, Elkind E, Barnoy S (2008) Amyloid beta peptide toxicity in differentiated PC12 cells: calpain-calpastatin, caspase, and membrane damage. J Neurosci Res 86:2314–2325
Sepulveda FJ, Parodi J, Peoples RW, Opazo C, Aguayo LG (2010) Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS One 5:e11820
Blanchard BJ, Thomas VL, Ingram VM (2002) Mechanism of membrane depolarization caused by the Alzheimer Abeta1-42 peptide. Biochem Biophys Res Commun 293:1197–1203
Eckert GP, Wood WG, Muller WE (2001) Effects of aging and beta-amyloid on the properties of brain synaptic and mitochondrial membranes. J Neural Transm 108:1051–1064
Yip CM, Darabie AA, McLaurin J (2002) Abeta42-peptide assembly on lipid bilayers. J Mol Biol 318:97–107
Kremer JJ, Pallitto MM, Sklansky DJ, Murphy RM (2000) Correlation of beta-amyloid aggregate size and hydrophobicity with decreased bilayer fluidity of model membranes. Biochemistry 39:10309–10318
Li Y, Wang JJ, Cai JX (2007) Aniracetam restores the effects of amyloid-beta protein or ageing on membrane fluidity and intracellular calcium concentration in mice synaptosomes. J Neural Transm 114:1407–1411
Muller WE, Koch S, Eckert A, Hartmann H, Scheuer K (1995) beta-Amyloid peptide decreases membrane fluidity. Brain Res 674:133–136
Hashimoto M, Hossain S, Shimada T, Shido O (2006) Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol 33:934–939
Wong PT, Schauerte JA, Wisser KC, Ding H, Lee EL, Steel DG, Gafni A (2009) Amyloid-beta membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J Mol Biol 386:81–96
Drago D, Bettella M, Bolognin S, Cendron L, Scancar J, Milacic R, Ricchelli F et al (2008) Potential pathogenic role of beta-amyloid(1-42)-aluminum complex in Alzheimer's disease. Int J Biochem Cell Biol 40:731–746
Chochina SV, Avdulov NA, Igbavboa U, Cleary JP, O'Hare EO, Wood WG (2001) Amyloid beta-peptide1-40 increases neuronal membrane fluidity: role of cholesterol and brain region. J Lipid Res 42:1292–1297
Qi XL, Xiu J, Shan KR, Xiao Y, Gu R, Liu RY, Guan ZZ (2005) Oxidative stress induced by beta-amyloid peptide(1-42) is involved in the altered composition of cellular membrane lipids and the decreased expression of nicotinic receptors in human SH-SY5Y neuroblastoma cells. Neurochem Int 46:613–621
Murray IV, Liu L, Komatsu H, Uryu K, Xiao G, Lawson JA, Axelsen PH (2007) Membrane-mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid beta proteins. J Biol Chem 282:9335–9345
Vestergaard MC, Morita M, Hamada T, Takagi M (2013) Membrane fusion and vesicular transformation induced by Alzheimer's amyloid beta. Biochim Biophys Acta 1828:1314–1321
Hyun DH, Mughal MR, Yang H, Lee JH, Ko EJ, Hunt ND, de Cabo R et al (2010) The plasma membrane redox system is impaired by amyloid beta-peptide and in the hippocampus and cerebral cortex of 3xTgAD mice. Exp Neurol 225:423–429
Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG (2009) Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience 162:328–338
Pan HJ, Wang RL, Xiao JL, Chang YJ, Cheng JY, Chen YR, Lee CH (2014) Using optical profilometry to characterize cell membrane roughness influenced by amyloid-beta 42 aggregates and electric fields. J Biomed Opt 19:011009
Minano-Molina AJ, Espana J, Martin E, Barneda-Zahonero B, Fado R, Sole M, Trullas R et al (2011) Soluble oligomers of amyloid-beta peptide disrupt membrane trafficking of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction. J Biol Chem 286:27311–27321
Ji SR, Wu Y, Sui SF (2002) Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (A beta 1-40), which may potentially inhibit the fibril formation. J Biol Chem 277:6273–6279
Curtain CC, Ali FE, Smith DG, Bush AI, Masters CL, Barnham KJ (2003) Metal ions, pH, and cholesterol regulate the interactions of Alzheimer's disease amyloid-beta peptide with membrane lipid. J Biol Chem 278:2977–2982
Kirsch C, Eckert GP, Mueller WE (2002) Cholesterol attenuates the membrane perturbing properties of beta-amyloid peptides. Amyloid 9:149–159
Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG (1997) Lipid binding to amyloid beta-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J Neurochem 69:1746–1752
Di Scala C, Yahi N, Lelievre C, Garmy N, Chahinian H, Fantini J (2013) Biochemical identification of a linear cholesterol-binding domain within Alzheimer's beta amyloid peptide. ACS Chem Neurosci 4:509–517
Di Scala C, Troadec JD, Lelievre C, Garmy N, Fantini J, Chahinian H (2014) Mechanism of cholesterol-assisted oligomeric channel formation by a short Alzheimer beta-amyloid peptide. J Neurochem 128:186–195
Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C et al (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167:953–960
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, X., Sun, G.Y., Eckert, G.P. et al. Cellular Membrane Fluidity in Amyloid Precursor Protein Processing. Mol Neurobiol 50, 119–129 (2014). https://doi.org/10.1007/s12035-014-8652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8652-6